Abstract
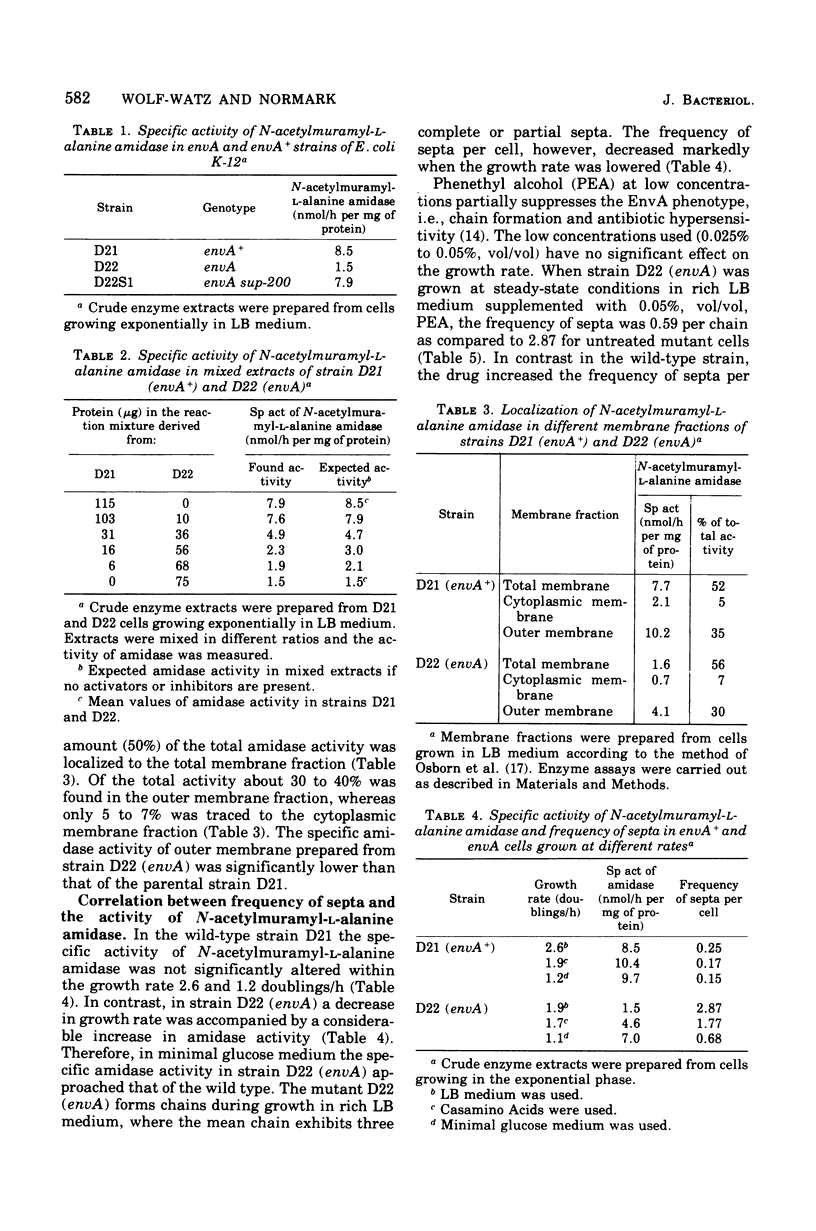
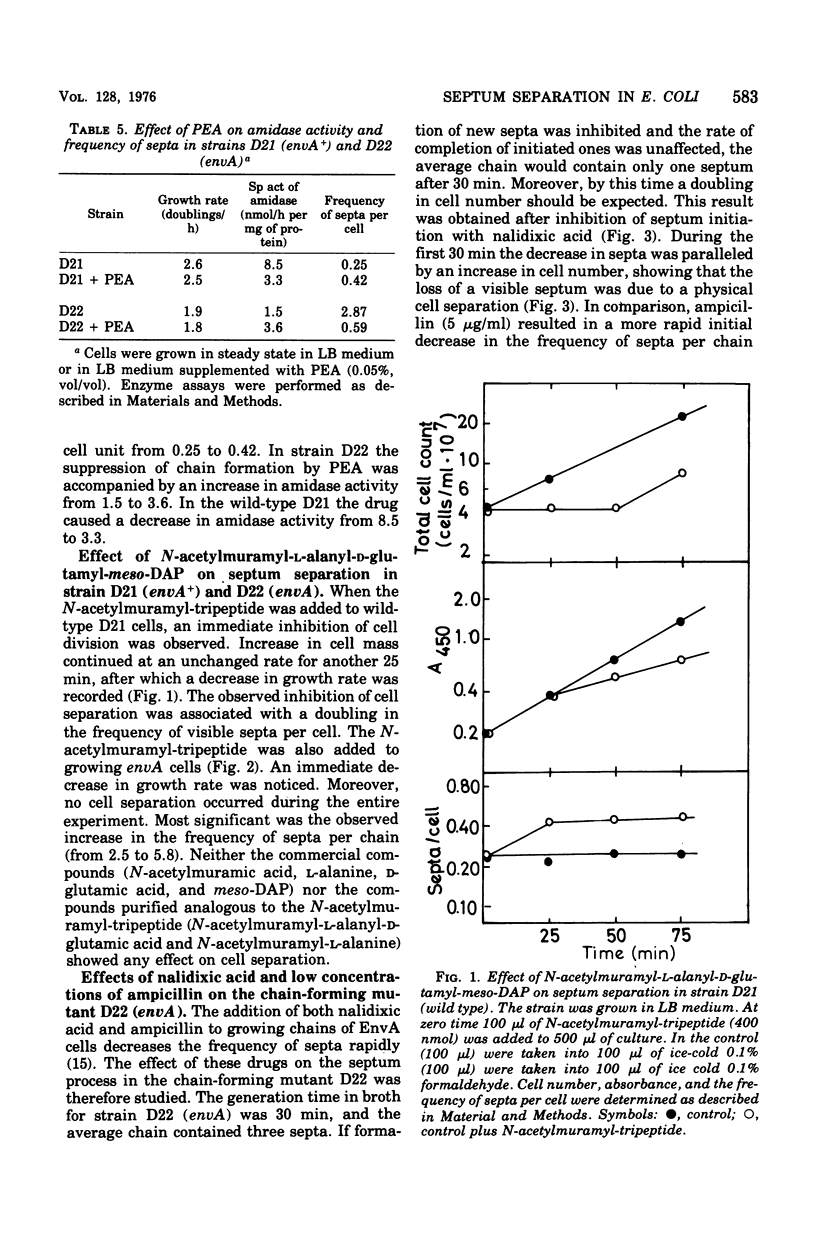
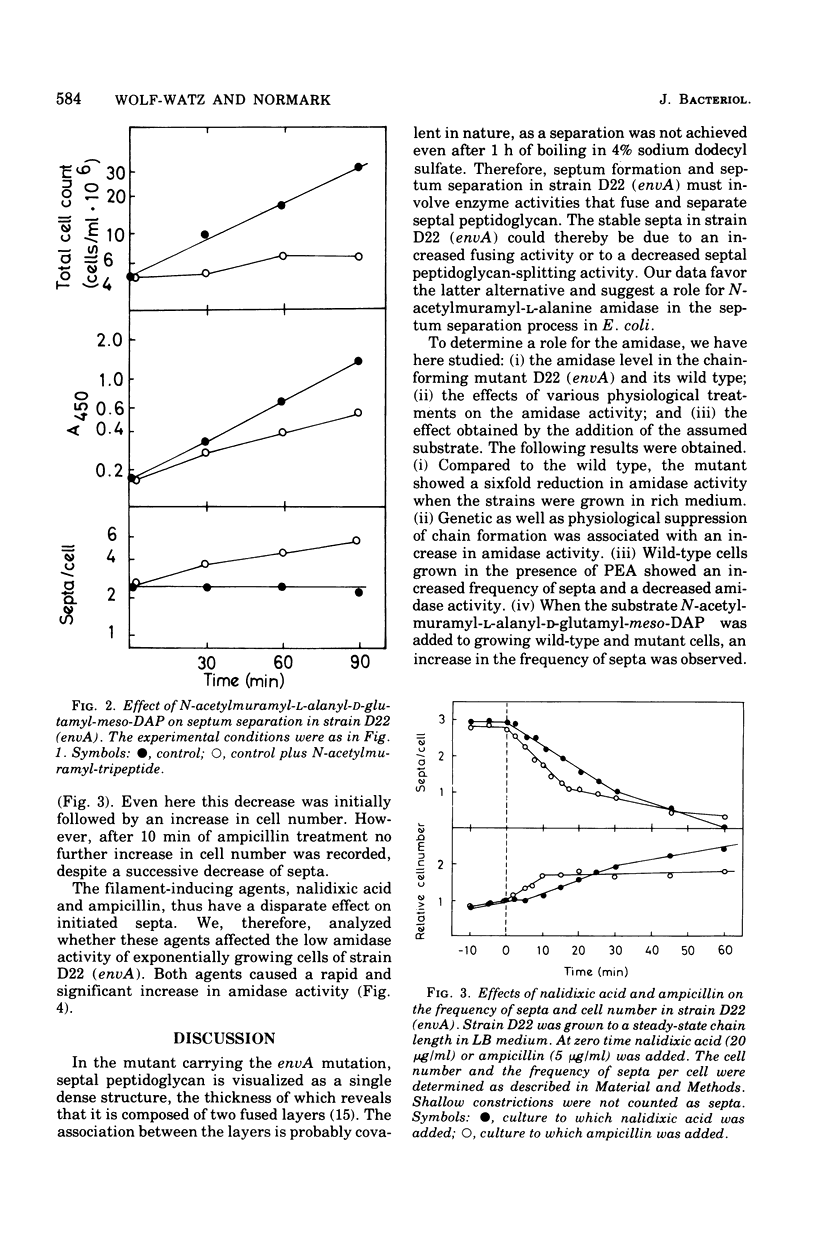
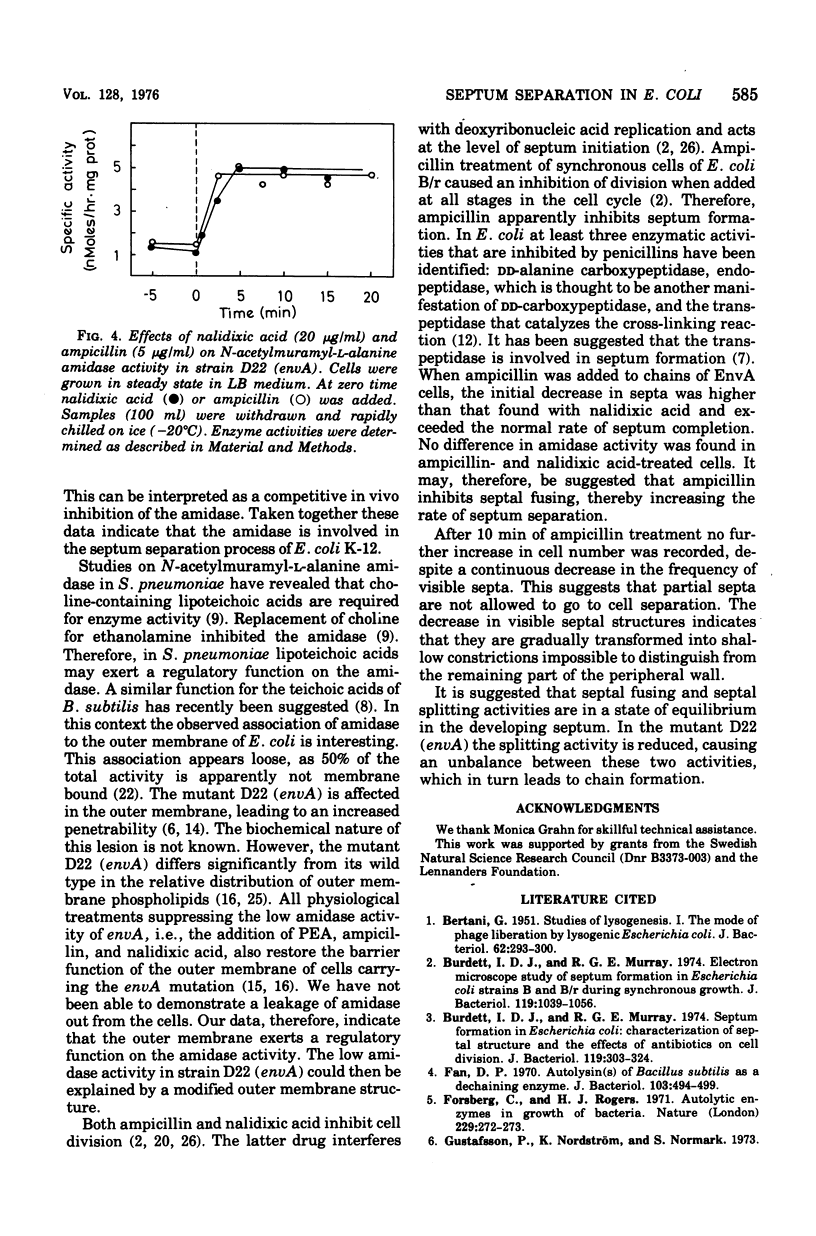
Septum formation and septum separation have been studied in a chain-forming mutant of Escherichia coli K-12 bearing the envA mutation and its parental strain. In comparison to the wild type, the mutant showed a sixfold reduction in the specific activity of the enzyme, N-acetylmuramyl-L-alanine amidase (EC 3.5.1.28), part of which was associated to the outer membrane. Genetic as well as physiological suppression of chain formation resulted in an increase in amidase activity. The addition of N-acetylmuramyl-L-alanyl-D-glutamyl-meso-diaminopimelic acid to growing wild-type cells and to cells bearing the envA mutation caused an inhibition of cell separation and an increased frequency of visible septa. The kinetics of septum formation and separation was followed in chains by the use of ampicillin and nalidixic acid. The latter drug inhibited initiation of new septa but allowed preformed ones to go to cell separation at a rate corresponding to that of steady-state growing cells. Ampicillin treatment, on the other hand, resulted in a more rapid decrease in the frequency of septa. The disparate effects of ampicillin and nalidixic acid were not explained by a difference in amidase activity but could be due to an inhibitory effect of ampicillin on a septal peptidoglycan fusing activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett I. D., Murray R. G. Electron microscope study of septum formation in Escherichia coli strains B and B-r during synchronous growth. J Bacteriol. 1974 Sep;119(3):1039–1056. doi: 10.1128/jb.119.3.1039-1056.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett I. D., Murray R. G. Septum formation in Escherichia coli: characterization of septal structure and the effects of antibiotics on cell division. J Bacteriol. 1974 Jul;119(1):303–324. doi: 10.1128/jb.119.1.303-324.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P. Autolysin(s) of Bacillus subtilis as dechaining enzyme. J Bacteriol. 1970 Aug;103(2):494–499. doi: 10.1128/jb.103.2.494-499.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C., Rogers H. J. Autolytic enzymes in growth of bacteria. Nature. 1971 Jan 22;229(5282):272–273. doi: 10.1038/229272a0. [DOI] [PubMed] [Google Scholar]
- Hartmann R., Höltje J. V., Schwarz U. Targets of penicillin action in Escherichia coli. Nature. 1972 Feb 25;235(5339):426–429. doi: 10.1038/235426a0. [DOI] [PubMed] [Google Scholar]
- Herbold D. R., Glaser L. Interaction of N-acetylmuramic acid L-alanine amidase with cell wall polymers. J Biol Chem. 1975 Sep 25;250(18):7231–7238. [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Specific recognition of choline residues in the cell wall teichoic acid by the N-acetylmuramyl-L-alanine amidase of Pneumococcus. J Biol Chem. 1975 Aug 10;250(15):6072–6076. [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. 8. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reaction in strains of Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3180–3192. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mirelman D., Yashouv-Gan Y., Schwarz U. Peptidoglycan biosynthesis in a thermosensitive division mutant of Escherichia coli. Biochemistry. 1976 May 4;15(9):1781–1790. doi: 10.1021/bi00654a001. [DOI] [PubMed] [Google Scholar]
- Normark S., Boman H. G., Bloom G. D. Cell division in a chain-forming envA mutant of Escherichia coli K12. Fine structure of division sites and effects of EDTA, lysozyme and ampicillin. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(5):651–664. doi: 10.1111/j.1699-0463.1971.tb00093.x. [DOI] [PubMed] [Google Scholar]
- Normark S. Genetics of a chain-forming mutant of Escherichia coli. Transduction and dominance of the envA gene mediating increased penetration to some antibacterial agents. Genet Res. 1970 Aug;16(1):63–78. doi: 10.1017/s0016672300002287. [DOI] [PubMed] [Google Scholar]
- Normark S. Phenethyl alcohol as a suppressor of the envA phenotype associated with the envA gene in Escherichia coli K-12. J Bacteriol. 1971 Oct;108(1):51–58. doi: 10.1128/jb.108.1.51-58.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Wolf-Watz H. Cell division and permeability of unbalanced envelope mutants of Escherichia coli K12. Ann Microbiol (Paris) 1974 Sep;125 B(2):211–226. [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Rodolakis A., Thomas P., Starka J. Morphological mutants of Escherichia coli. Isolation and ultrastructure of a chain-forming envC mutant. J Gen Microbiol. 1973 Apr;75(2):409–416. doi: 10.1099/00221287-75-2-409. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of Pneumococcus: chanin formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Van Heijenoort Y., Van Heijenoort J. Study of the N-acetylmuramyl-L-alanine amidase activity in Escherichia coli. FEBS Lett. 1971 Jun 10;15(2):137–141. doi: 10.1016/0014-5793(71)80041-8. [DOI] [PubMed] [Google Scholar]
- Wolf-Watz H., Normark S., Bloom G. D. Rapid method for isolation of large quantities of outer membrane from Escherichia coli K-12 and its application to the study of envelope mutants. J Bacteriol. 1973 Sep;115(3):1191–1197. doi: 10.1128/jb.115.3.1191-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky A., Pritchard R. H. Changes in cell size and shape associated with changes in the replication time of the chromosome of Escherichia coli. J Bacteriol. 1973 May;114(2):824–837. doi: 10.1128/jb.114.2.824-837.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heijenoort J., Parquet C., Flouret B., van Heijenoort Y. Envelope-bound N-acetylmuramyl-L-alanine amidase of Escherichia coli K 12. Purification and properties of the enzyme. Eur J Biochem. 1975 Oct 15;58(2):611–619. doi: 10.1111/j.1432-1033.1975.tb02412.x. [DOI] [PubMed] [Google Scholar]


