Abstract
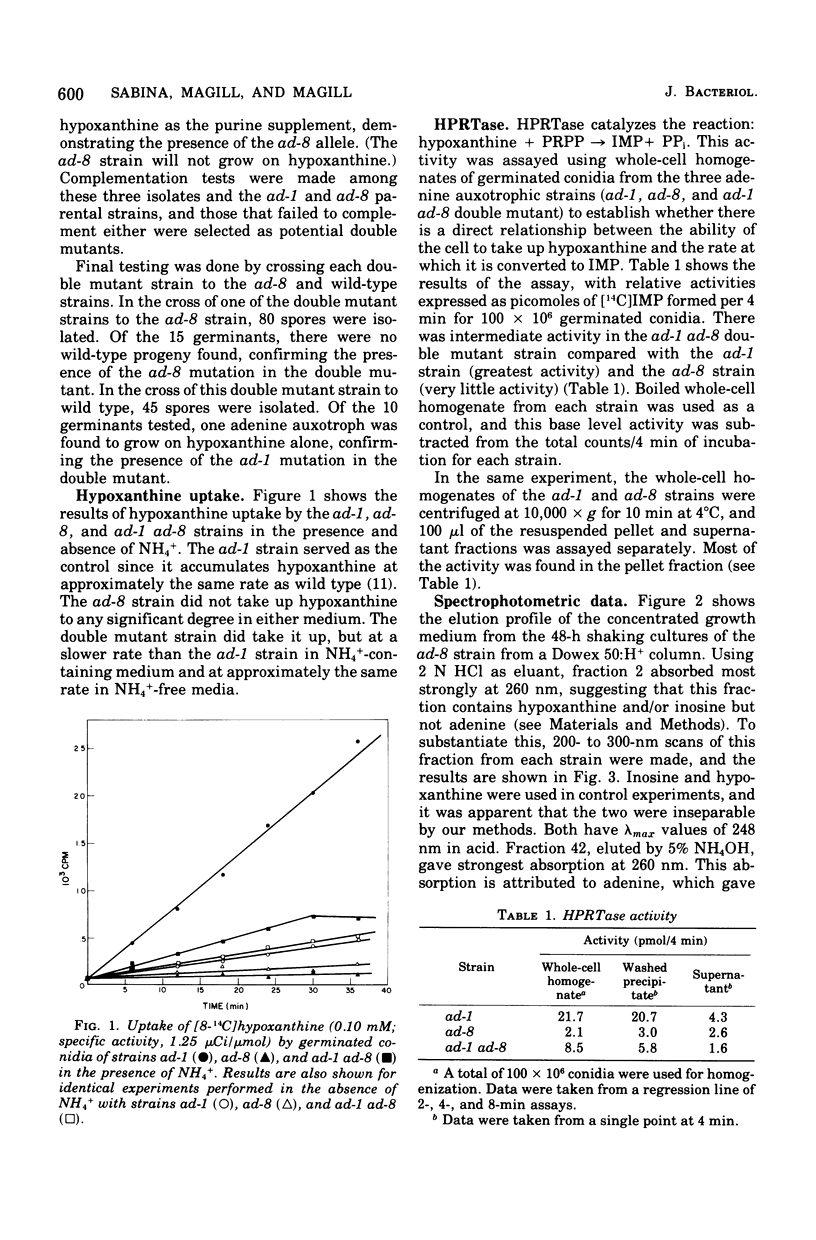
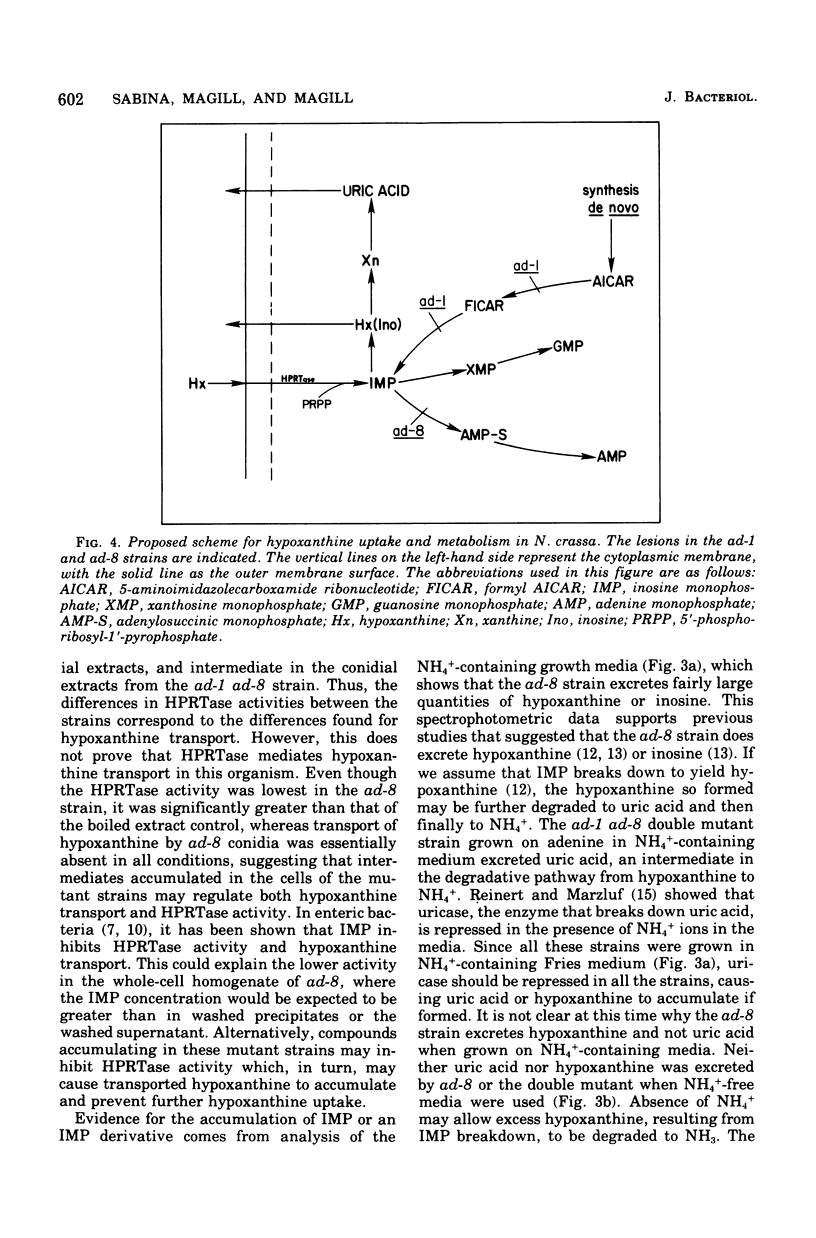
Hypoxanthine uptake and hypoxanthine phosphoribosyltransferase activity (EC 2.4.2.8) were determined in germinated conidia from the adenine auxotrophic strains ad-1 and ad-8 and the double mutant strain ad-1 ad-8. The mutant strain ad-1 appears to lack aminoimidazolecarboximide ribonucleotide formyltransferase (EC 2.1.2.3) or inosine 5'monophosphate cyclohydrolase (EC 3.5.1.10) activities, or both, whereas the ad-8 strain lacks adenylosuccinate synthase activity (EC 6.3.4.4). Normal (or wild-type) hypoxanthine transport capacity was found to the ad-1 conidia, whereas the ad-8 strains failed to take up any hypoxanthine. The double mutant strains showed intermediate transport capacities. Similar results were obtained for hypoxanthine phosphoribosyl-transferase activity assayed in germinated conidia. The ad-1 strain showed greatest activity, the ad-8 strain showed the least activity, and the double mutant strain showed intermediate activity levels. Ion-exchange chromatography of the growth media revealed that in the presence of NH+/4, the ad-8 strain excreted hypoxanthine or inosine, the ad-1 strain did not excrete any purines, and the ad-1 ad-8 double mutant strain excreted uric acid. In the absence of NH+/4, none of the strains excreted any detectable purine compounds.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benke P. J., Herrick N., Herbert A. Transport of hypoxanthine in fibroblasts with normal and mutant hypoxanthine-guanine phosphoribosyltransferase. Biochem Med. 1973 Oct;8(2):309–323. doi: 10.1016/0006-2944(73)90035-5. [DOI] [PubMed] [Google Scholar]
- Berlin R. D., Stadtman E. R. A possible role of purine nucleotide pyrophosphorylases in the regulation of purine uptake by Bacillus subtilis. J Biol Chem. 1966 Jun 10;241(11):2679–2686. [PubMed] [Google Scholar]
- Cohn W. E. The Separation of Purine and Pyrimidine Bases and of Nucleotides by Ion Exchange. Science. 1949 Apr 15;109(2833):377–378. doi: 10.1126/science.109.2833.377. [DOI] [PubMed] [Google Scholar]
- Dybing E. Cycloheximide inhibition of hypoxanthine transport cultured cells. Biochim Biophys Acta. 1974 Nov 27;373(1):100–105. doi: 10.1016/0005-2736(74)90109-6. [DOI] [PubMed] [Google Scholar]
- Giles N. H., Partridge C. W., Nelson N. J. THE GENETIC CONTROL OF ADENYLOSUCCINASE IN Neurospora Crassa. Proc Natl Acad Sci U S A. 1957 Apr 15;43(4):305–317. doi: 10.1073/pnas.43.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Stadtman E. R. The regulation of purine utilization in bacteria. II. Adenine phosphoribosyltransferase in isolated membrane preparations and its role in transport of adenine across the membrane. J Biol Chem. 1971 Sep 10;246(17):5304–5311. [PubMed] [Google Scholar]
- Hochstadt-Ozer J. The regulation of purine utilization in bacteria. IV. Roles of membrane-localized and pericytoplasmic enzymes in the mechanism of purine nucleoside transport across isolated Escherichia coli membranes. J Biol Chem. 1972 Apr 25;247(8):2419–2426. [PubMed] [Google Scholar]
- ISHIKAWA T. Genetic studies of ad-8 mutants in Neurospora crassa. I. Genetic fine structure of the ad-8 locus. Genetics. 1962 Sep;47:1147–1161. doi: 10.1093/genetics/47.9.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman L. E., Hochstadt J. Regulation of purine utilization in bacteria. VI. Characterization of hypoxanthine and guanine uptake into isolated membrane vesicles from Salmonella typhimurium. J Bacteriol. 1976 Apr;126(1):312–326. doi: 10.1128/jb.126.1.312-326.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill J. M., Magill C. W. Purine base transport in Neurospora crassa. J Bacteriol. 1975 Oct;124(1):149–154. doi: 10.1128/jb.124.1.149-154.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARTRIDGE C. W., GILES N. H. Identification of major accumulation products of adenine-specific mutants of Neurospora. Arch Biochem Biophys. 1957 Mar;67(1):237–238. doi: 10.1016/0003-9861(57)90261-8. [DOI] [PubMed] [Google Scholar]
- Pendyala L., Wellman A. M. Effect of histidine on purine nucleotide synthesis and utilization in Neurospora crassa. J Bacteriol. 1975 Oct;124(1):78–85. doi: 10.1128/jb.124.1.78-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao E. Y., Rao T. K., Debusk A. G. Isolation and characterization of a mutant of Neurospora crassa deficient in general amino acid permease activity. Biochim Biophys Acta. 1975 Nov 17;413(1):45–51. doi: 10.1016/0005-2736(75)90057-7. [DOI] [PubMed] [Google Scholar]
- Reinert W. R., Marzluf G. A. Regulation of the purine catabolic enzymes in Neurospora crassa. Arch Biochem Biophys. 1975 Feb;166(2):565–574. doi: 10.1016/0003-9861(75)90421-x. [DOI] [PubMed] [Google Scholar]
- Rosenbloom F. M., Henderson J. F., Caldwell I. C., Kelley W. N., Seegmiller J. E. Biochemical bases of accelerated purine biosynthesis de novo in human fibroblasts lacking hypoxanthine-guanine phosphoribosyltransferase. J Biol Chem. 1968 Mar 25;243(6):1166–1173. [PubMed] [Google Scholar]
- Roy-Burman S., Visser D. W. Transport of purines and deoxyadenosine in Escherichia coli. J Biol Chem. 1975 Dec 25;250(24):9270–9275. [PubMed] [Google Scholar]
- Rubin C. S., Dancis J., Yip L. C., Nowinski R. C., Balis M. E. Purification of IMP:pyrophosphate phosphoribosyltransferases, catalytically incompetent enzymes in Lesch-Nyhan disease. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1461–1464. doi: 10.1073/pnas.68.7.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]


