Abstract
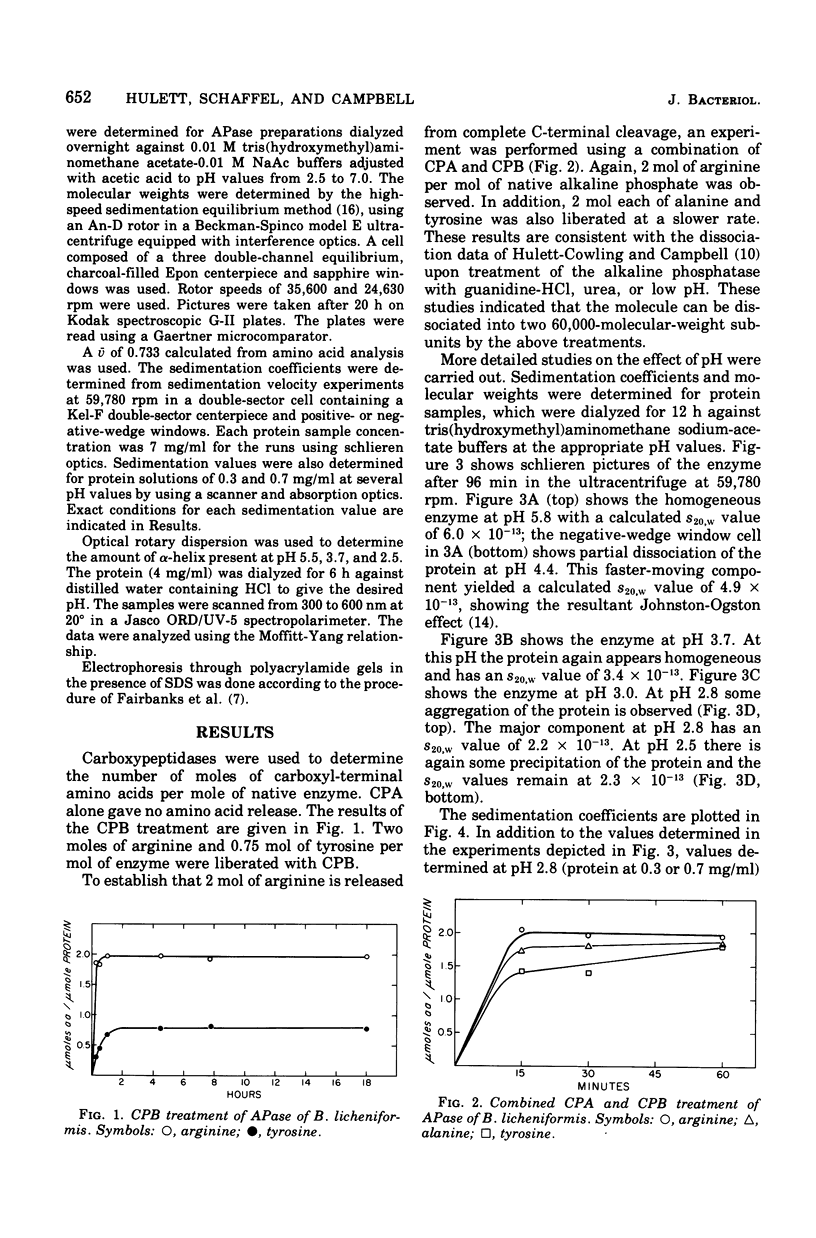
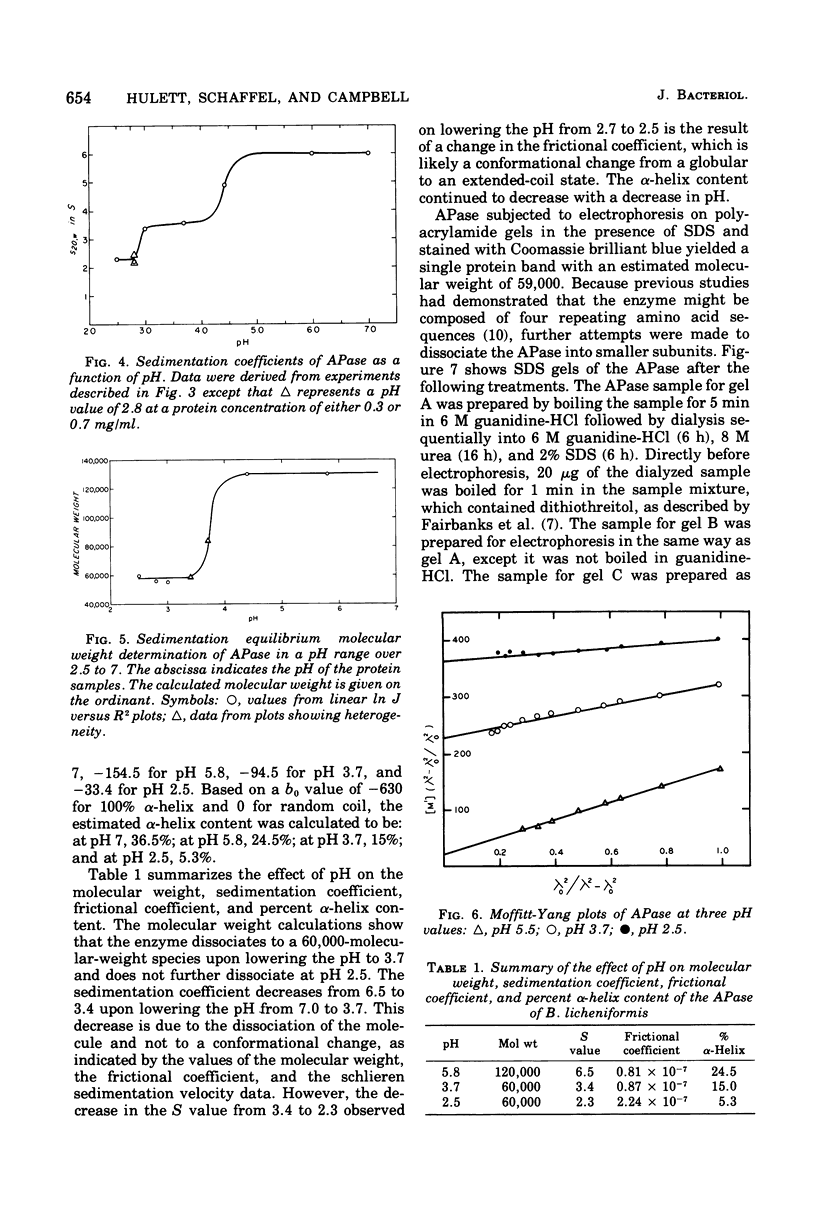
The alkaline phosphatase (orthophosphoric monoester phosphydrolase, EC 3.1.3.1) of Bacillus licheniformis MC14 was studied in an attempt to determine the number of subunits contained in the 120,000-molecular-weight native enzyme. Two moles of arginine was liberated per mole of native enzyme by carboxypeptidases A and B in the presence of sodium dodecyl sulfate. The effect on the native enzyme of progressively lowering the solvent buffer pH was monitored by determining the molecular weight by sedimentation equilibrium analysis, the sedimentation coefficient, the frictional coefficient, and the percent alpha-helix content of the enzyme. The alkaline phosphatase dissociates into two subunits around pH 4. At pH 2.8 a further decrease in S value, but no change in molecular weight, is observed, indicating a change in conformation. The frictional coefficients and percent alpha-helix content agree with this interpretation. A subunit molecular weight of 59,000 was calculated from sodium dodecyl sulfate gels.
Full text
PDF

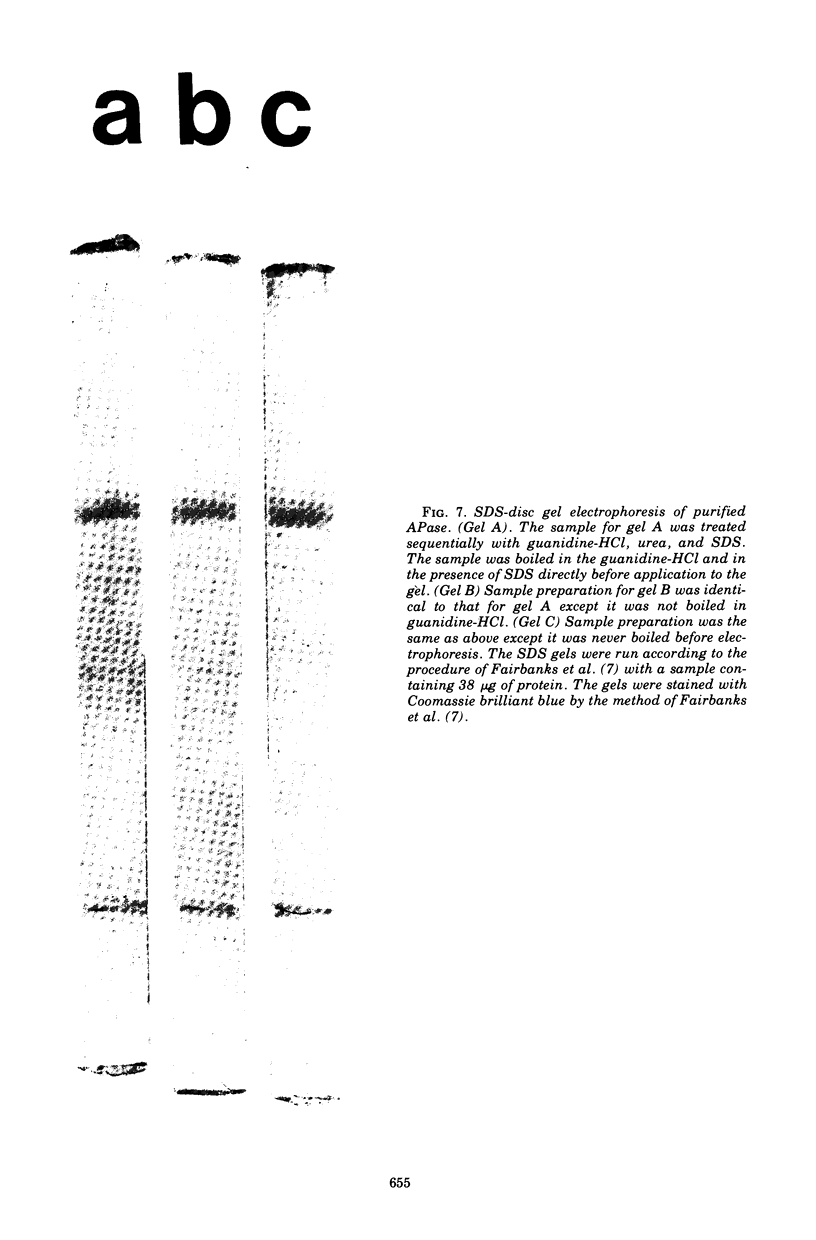
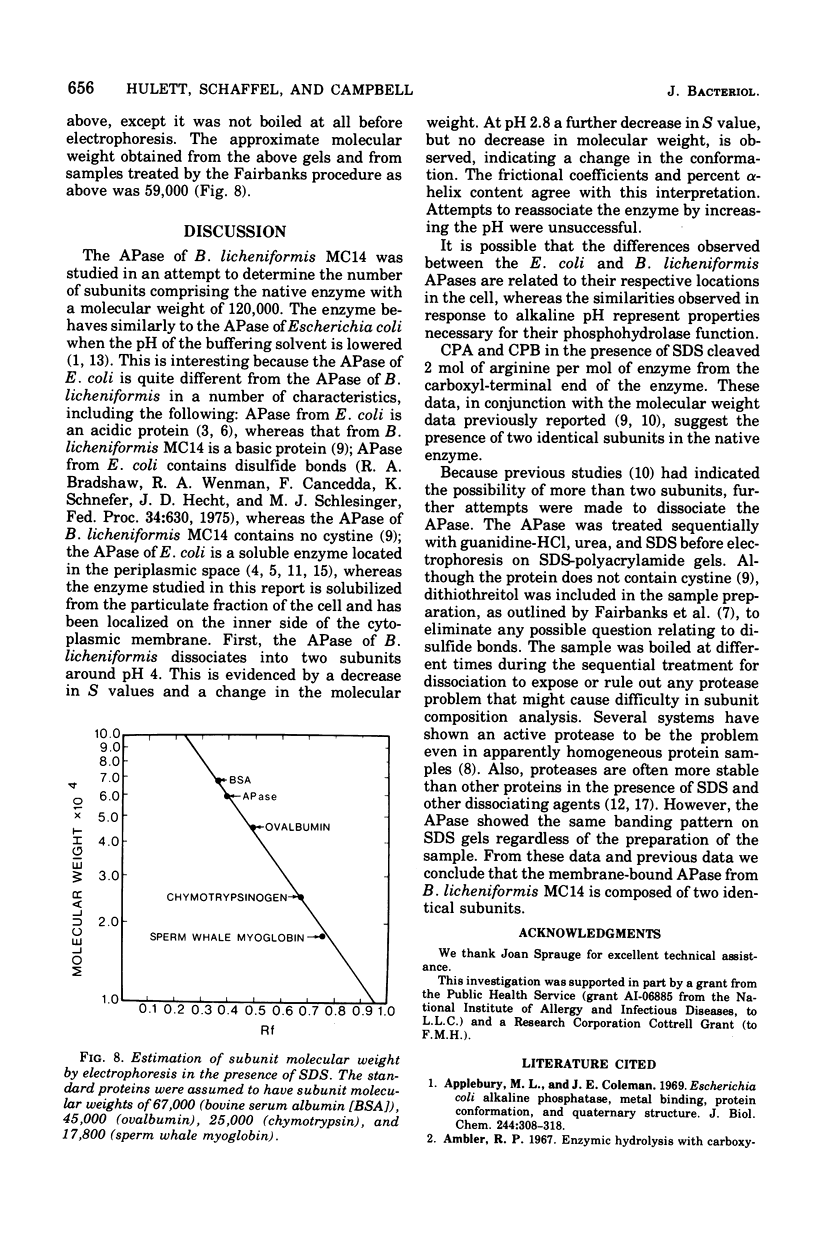

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebury M. L., Coleman J. E. Escherichia coli alkaline phosphatase. Metal binding, protein conformation, and quaternary structure. J Biol Chem. 1969 Jan 25;244(2):308–318. [PubMed] [Google Scholar]
- Bosron W. F., Vallee B. L. Effect of phosphate on multiple forms of Escherechia coli alkaline phosphatase. Biochem Biophys Res Commun. 1975 Sep 16;66(2):809–813. doi: 10.1016/0006-291x(75)90581-1. [DOI] [PubMed] [Google Scholar]
- Brockman R. W., Heppel L. A. On the localization of alkaline phosphatase and cyclic phosphodiesterase in Escherichia coli. Biochemistry. 1968 Jul;7(7):2554–2562. doi: 10.1021/bi00847a016. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Hulett-Cowling F. M., Campbell L. L. Molecular weight and subunits of the alkaline phosphatase of Bacillus licheniformis. Biochemistry. 1971 Apr 13;10(8):1371–1376. doi: 10.1021/bi00784a015. [DOI] [PubMed] [Google Scholar]
- Hulett-Cowling F. M., Campbell L. L. Purification and properties of an alkaline phosphatase of Bacillus licheniformis. Biochemistry. 1971 Apr 13;10(8):1364–1371. doi: 10.1021/bi00784a014. [DOI] [PubMed] [Google Scholar]
- Hulett F. M., DeMoss J. A. Subunit structure of anthranilate synthetase from Neurospora crassa. J Biol Chem. 1975 Sep 10;250(17):6648–6652. [PubMed] [Google Scholar]
- Kushnarev V. M., Smirnova T. A. Electron microscopy of alkaline phosphatase of Escherichia coli. Can J Microbiol. 1966 Aug;12(4):605–607. doi: 10.1139/m66-086. [DOI] [PubMed] [Google Scholar]
- Pringle J. R. The molecular weight of the undegraded polypeptide chain of yeast hexokinase. Biochem Biophys Res Commun. 1970 Apr 8;39(1):46–52. doi: 10.1016/0006-291x(70)90755-2. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Schlesinger M. J. Conformational states of the subunit of Escherichia coli alkaline phosphatase. Biochemistry. 1967 Nov;6(11):3552–3559. doi: 10.1021/bi00863a029. [DOI] [PubMed] [Google Scholar]
- Wetzel B. K., Spicer S. S., Dvorak H. F., Heppel L. A. Cytochemical localization of certain phosphatases in Escherichia coli. J Bacteriol. 1970 Oct;104(1):529–542. doi: 10.1128/jb.104.1.529-542.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Yu P. H., Kula M. R., Tsai H. Studies on the apparent instability of Neurospora tryptophan synthase. Evidence for protease. Eur J Biochem. 1973 Jan 3;32(1):129–135. doi: 10.1111/j.1432-1033.1973.tb02588.x. [DOI] [PubMed] [Google Scholar]