Abstract
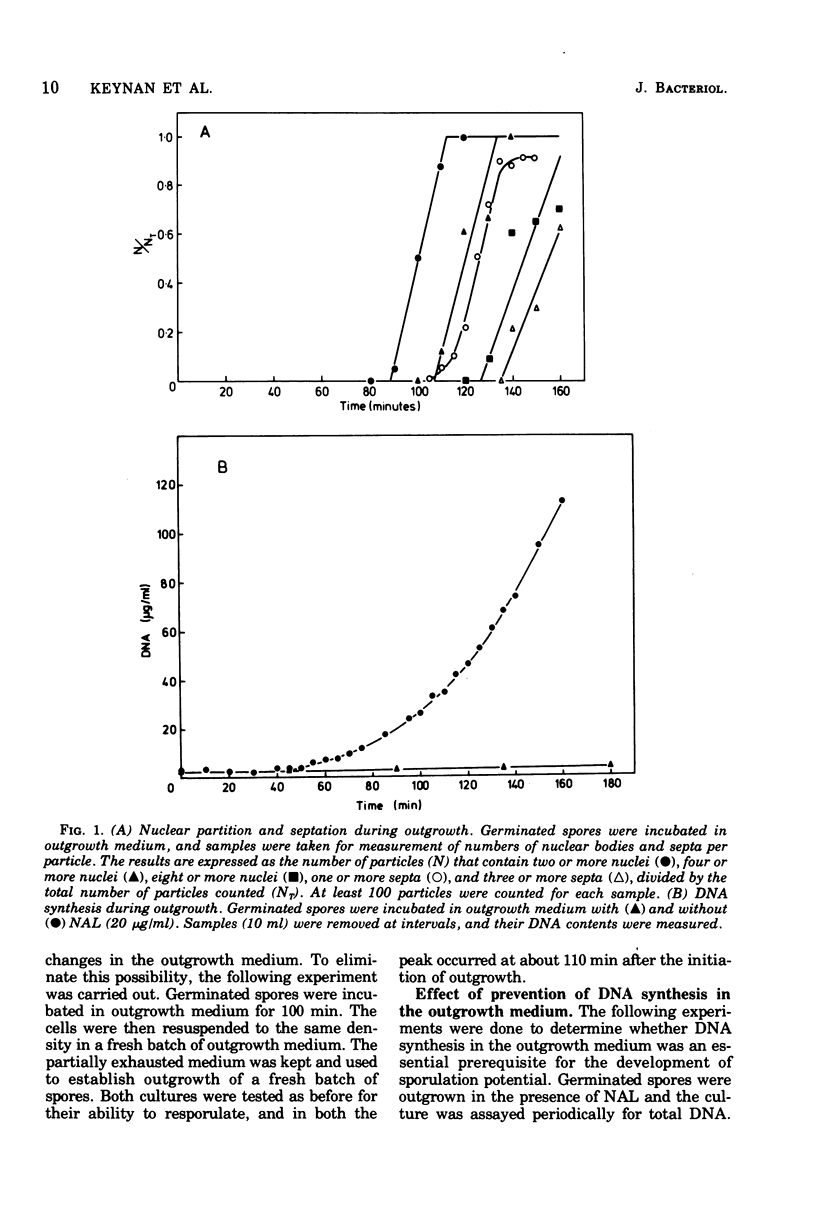
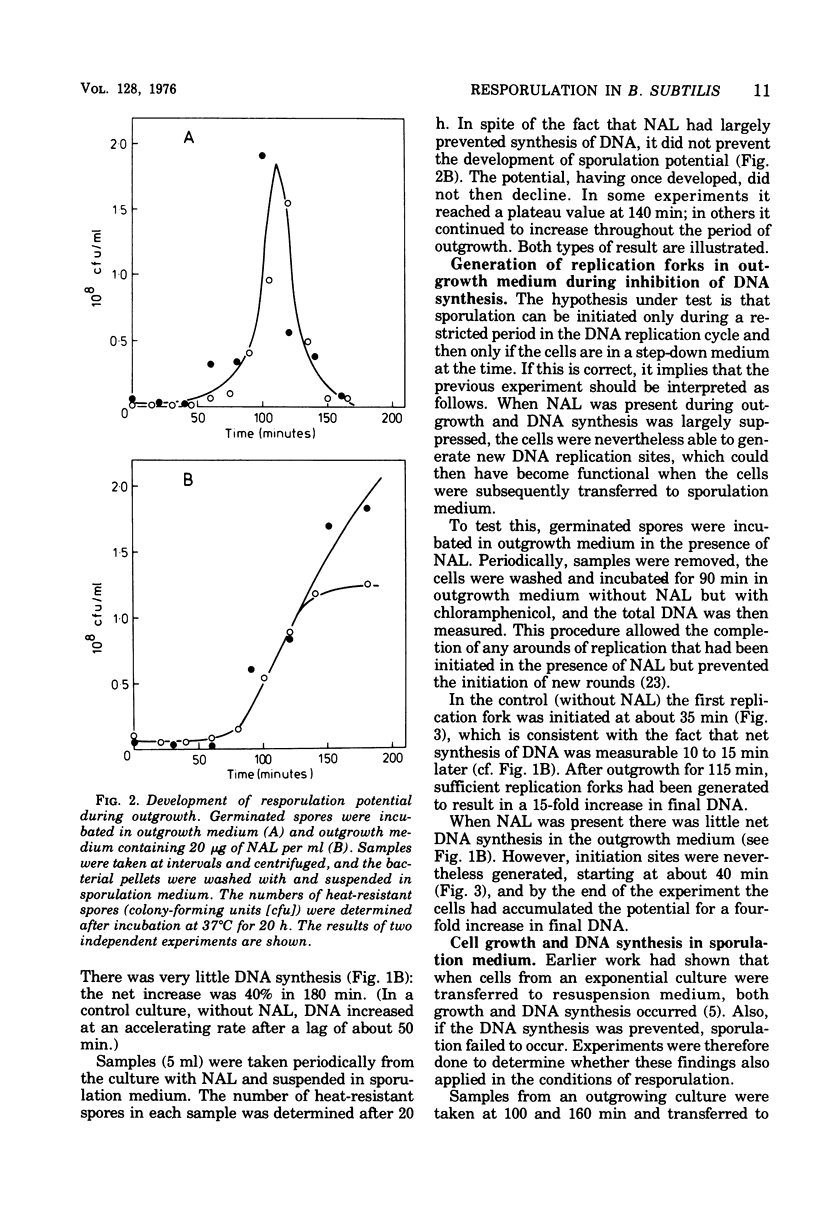
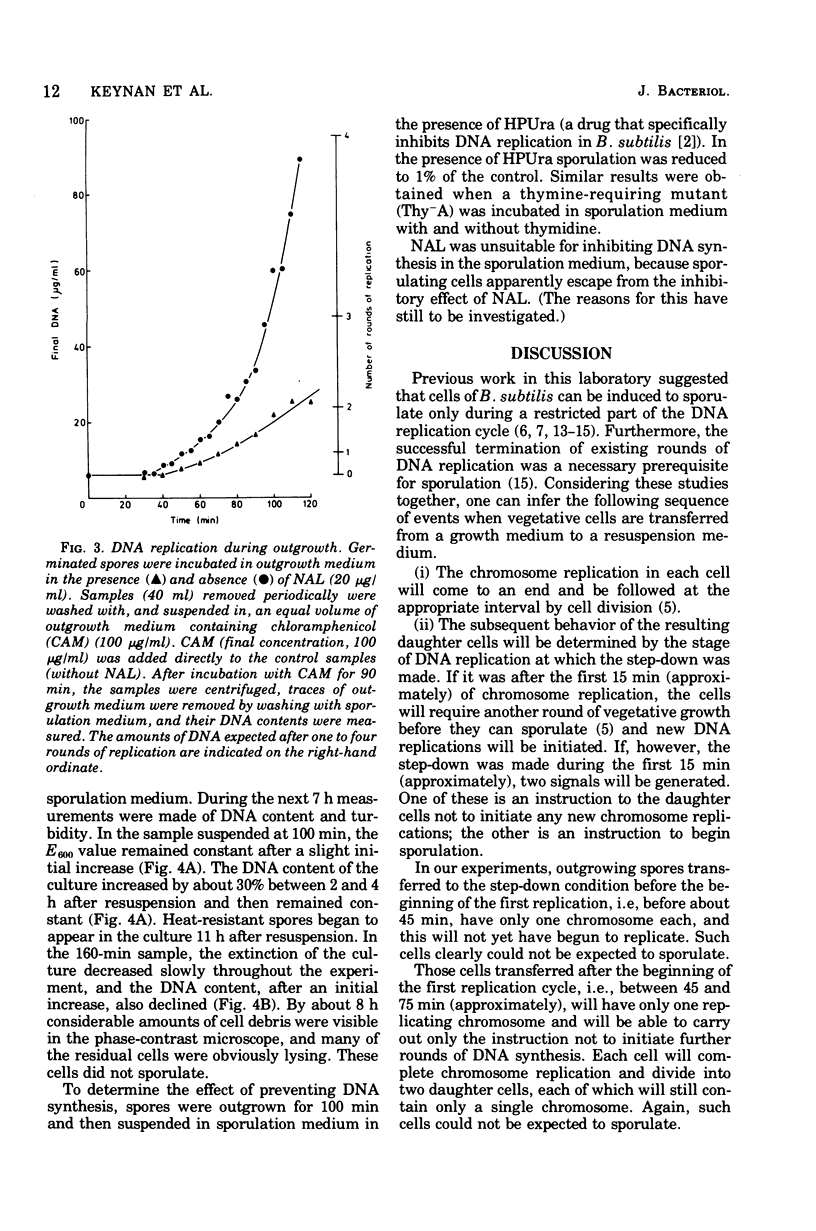
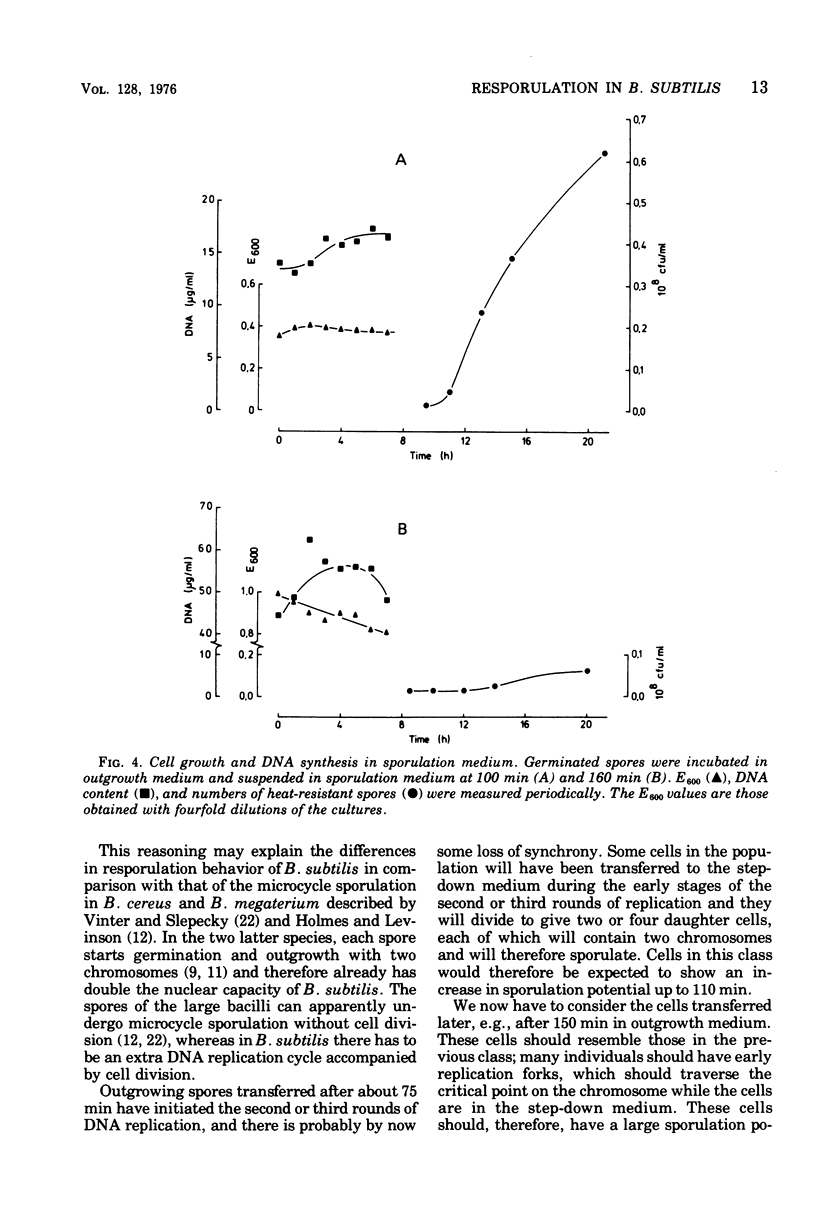
Germinated spores of Bacillus subtilis were incubated in outgrowth medium and tested periodically for capacity to sporulate when suspended in sporulation medium. Concurrent measurements were made of deoxyribonucleic acid (DNA) content and numbers of cell division septa and nucleoids. Sporulation potential is shown to reach a peak at about 110 min at which time the chromosomes are probably well into the second round of replication. Experiments with nalidixic acid show that sporulation potential can be generated in the outgrowth medium even when DNA synthesis is largely prevented. Further experiments show that nalidixic acid apparently does not prevent the formation of DNA initiation complexes, which can subsequently function after resuspension in the sporulation medium. The results support those previously obtained with a temperature-sensitive DNA mutant which indicated that sporulation could only be induced at a specific stage of chromosome replication, and then only if the cells are in a state of nutritional "step-down".
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsen P. P., Spudich J. A., Nelson D. L., Kornberg A. Biochemical studies of bacterial sporulation and germination. XII. A sulfonic acid as a major sulfur compound of Bacillus subtilis spores. J Bacteriol. 1969 Apr;98(1):62–68. doi: 10.1128/jb.98.1.62-68.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. C. 6-(p-hydroxyphenylazo)-uracil: a selective inhibitor of host DNA replication in phage-infected Bacillus subtilis. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1454–1461. doi: 10.1073/pnas.67.3.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callister H., Wake R. G. Completed chromosomes in thymine-requiring Bacillus subtilis spores. J Bacteriol. 1974 Nov;120(2):579–582. doi: 10.1128/jb.120.2.579-582.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes I. W., Kay D., Mandelstam J. Determining effect of growth medium on the shape and position of daughter chromosomes and on sporulation in Bacillus subtilis. Nature. 1971 Apr 30;230(5296):567–569. doi: 10.1038/230567a0. [DOI] [PubMed] [Google Scholar]
- Dawes I. W., Mandelstam J. Sporulation of Bacillus subtilis in continuous culture. J Bacteriol. 1970 Sep;103(3):529–535. doi: 10.1128/jb.103.3.529-535.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrati-Elizur E., Borenstein S. Velocity of chromosome replication in thymine-requiring and independent strains of Bacillus subtilis. J Bacteriol. 1971 Apr;106(1):58–64. doi: 10.1128/jb.106.1.58-64.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. C., YOUNG I. E. Comparison of species and yarieties of the genus Bacillus. Structure and nucleic acid content of spores. J Bacteriol. 1959 Dec;78:743–754. doi: 10.1128/jb.78.6.743-754.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillin F. D., Ganesan A. T. Control of chromosome replication in thymine-requiring strains of Bacillus subtilis 168. J Bacteriol. 1975 Sep;123(3):1055–1067. doi: 10.1128/jb.123.3.1055-1067.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring E. S., Wake R. G. A comparison of the segregation of chromosomes within microcolonies developing from single Bacillus subtilis and Bacillus megaterium spores. J Mol Biol. 1968 Aug 14;35(3):647–650. doi: 10.1016/s0022-2836(68)80022-1. [DOI] [PubMed] [Google Scholar]
- Holmes P. K., Levinson H. S. Metabolic requirements for microcycle sporogenesis of Bacillus megaterium. J Bacteriol. 1967 Aug;94(2):434–440. doi: 10.1128/jb.94.2.434-440.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelstam J., Higgs S. A. Induction of sporulation during synchronized chromosome replication in Bacillus subtilis. J Bacteriol. 1974 Oct;120(1):38–42. doi: 10.1128/jb.120.1.38-42.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelstam J., Sterlini J. M., Kay D. Sporulation in Bacillus subtilis. Effect of medium on the form of chromosome replication and on initiation to sporulation in Bacillus subtilis. Biochem J. 1971 Nov;125(2):635–641. doi: 10.1042/bj1250635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelstam J. The Leeuwenhoek lecture, 1975: bacterial sporulation: a problem in the biochemistry and genetics of a primitive developmental system. Proc R Soc Lond B Biol Sci. 1976 Apr 13;193(1111):89–106. doi: 10.1098/rspb.1976.0033. [DOI] [PubMed] [Google Scholar]
- Mychajlonka M., Slepecky R. A. Requirement of deoxyribonucleic acid synthesis for microcycle sporulation in Bacillus megaterium. J Bacteriol. 1974 Dec;120(3):1331–1338. doi: 10.1128/jb.120.3.1331-1338.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OISHI M., YOSHIKAWA H., SUEOKA N. SYNCHRONOUS AND DICHOTOMOUS REPLICATIONS OF THE BACILLUS SUBTILIS CHROMOSOME DURING SPORE GERMINATION. Nature. 1964 Dec 12;204:1069–1073. doi: 10.1038/2041069a0. [DOI] [PubMed] [Google Scholar]
- Sargent M. G. Nuclear segregation in Bacillus subtilis. Nature. 1974 Jul 19;250(463):252–254. doi: 10.1038/250252a0. [DOI] [PubMed] [Google Scholar]
- Siccardi A. G., Galizzi A., Mazza G., Clivio A., Albertini A. M. Synchronous germination and outgrowth of fractionated Bacillus subtilis spores: tool for the analysis of differentiation and division of bacterial cells. J Bacteriol. 1975 Jan;121(1):13–19. doi: 10.1128/jb.121.1.13-19.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterlini J. M., Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969 Jun;113(1):29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinter V., Slepecky R. A. Direct Transition of Outgrowing Bacterial Spores to New Sporangia Without Intermediate Cell Division. J Bacteriol. 1965 Sep;90(3):803–807. doi: 10.1128/jb.90.3.803-807.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H. DNA synthesis during germination of Bacillus subtilis spores. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1476–1483. doi: 10.1073/pnas.53.6.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]



