Abstract
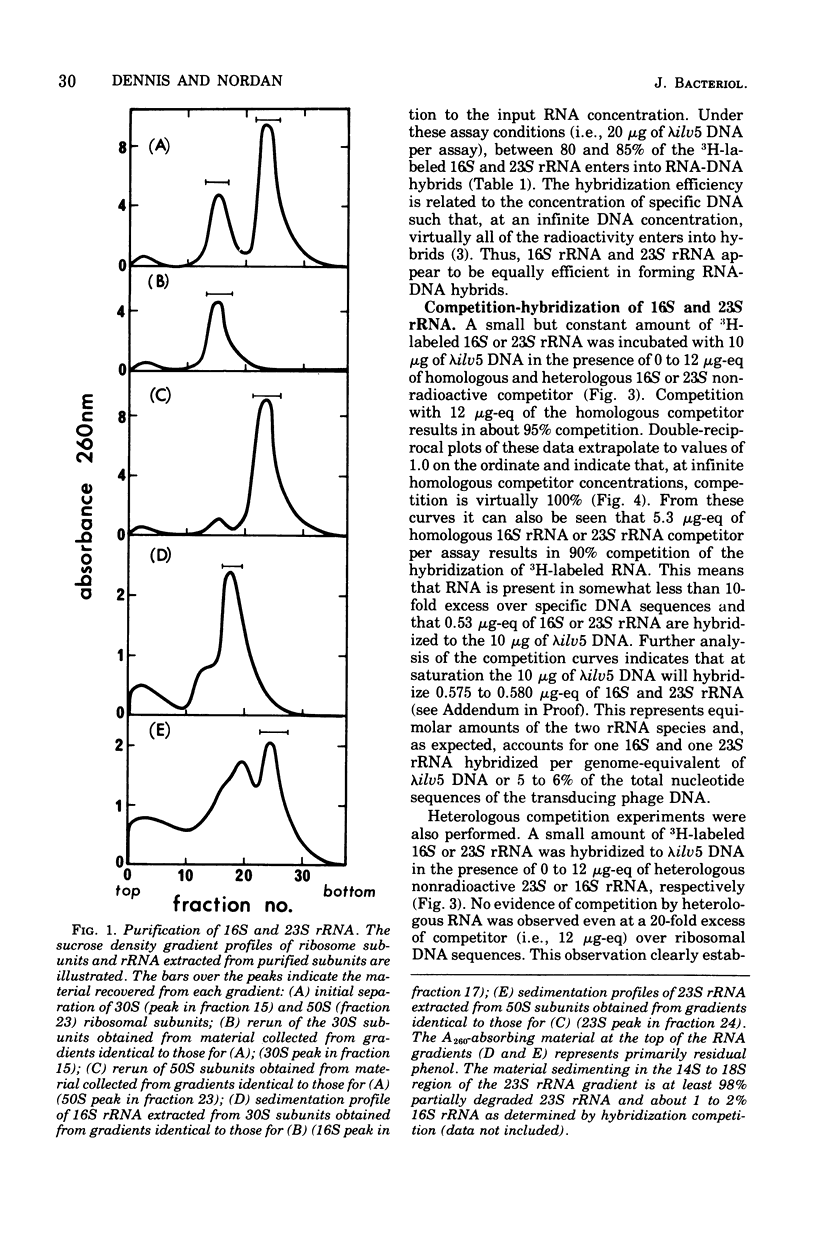
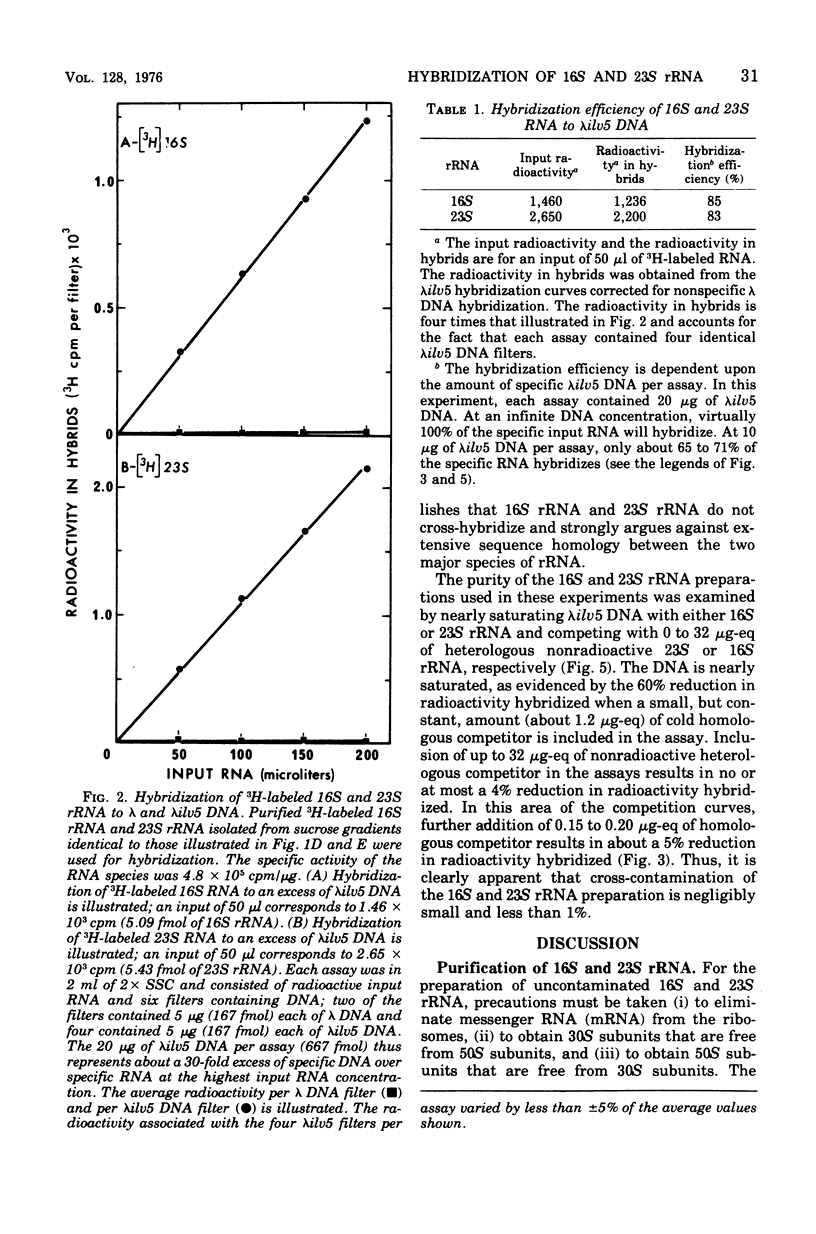
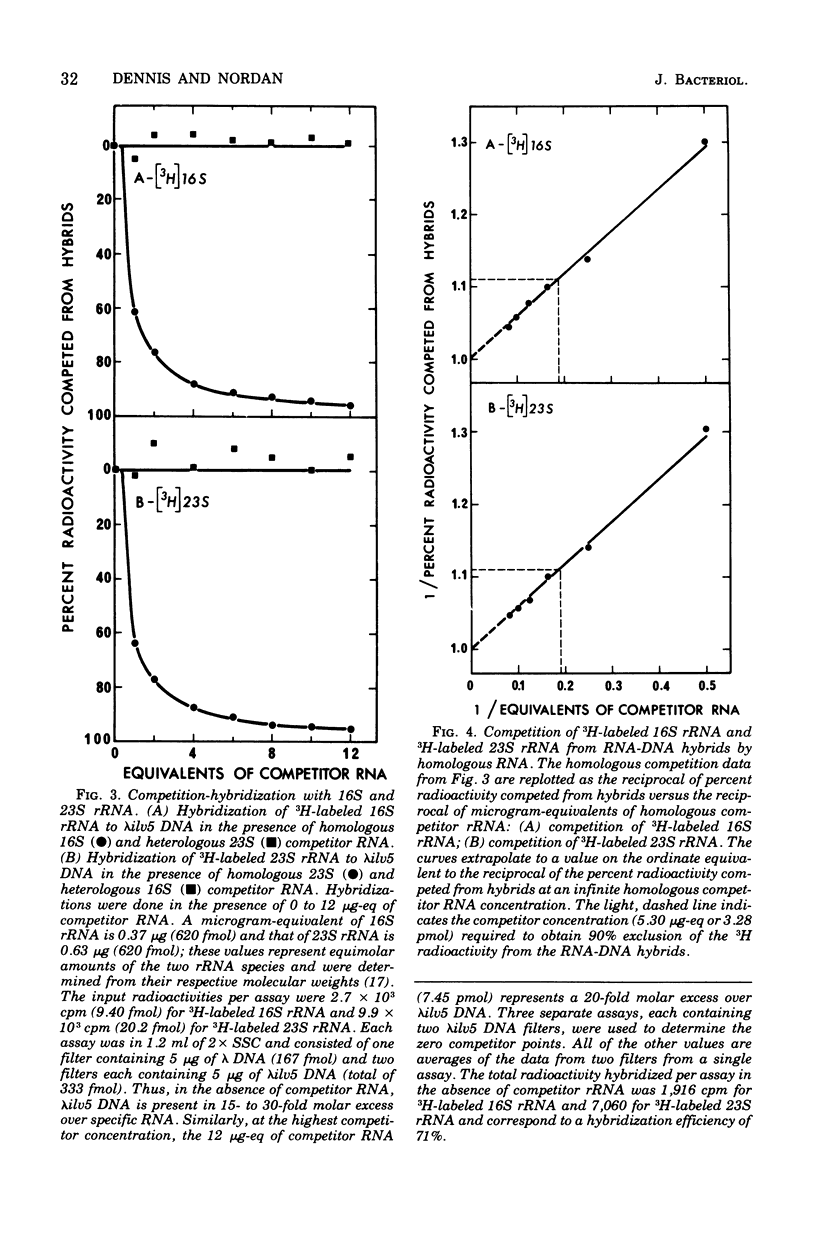
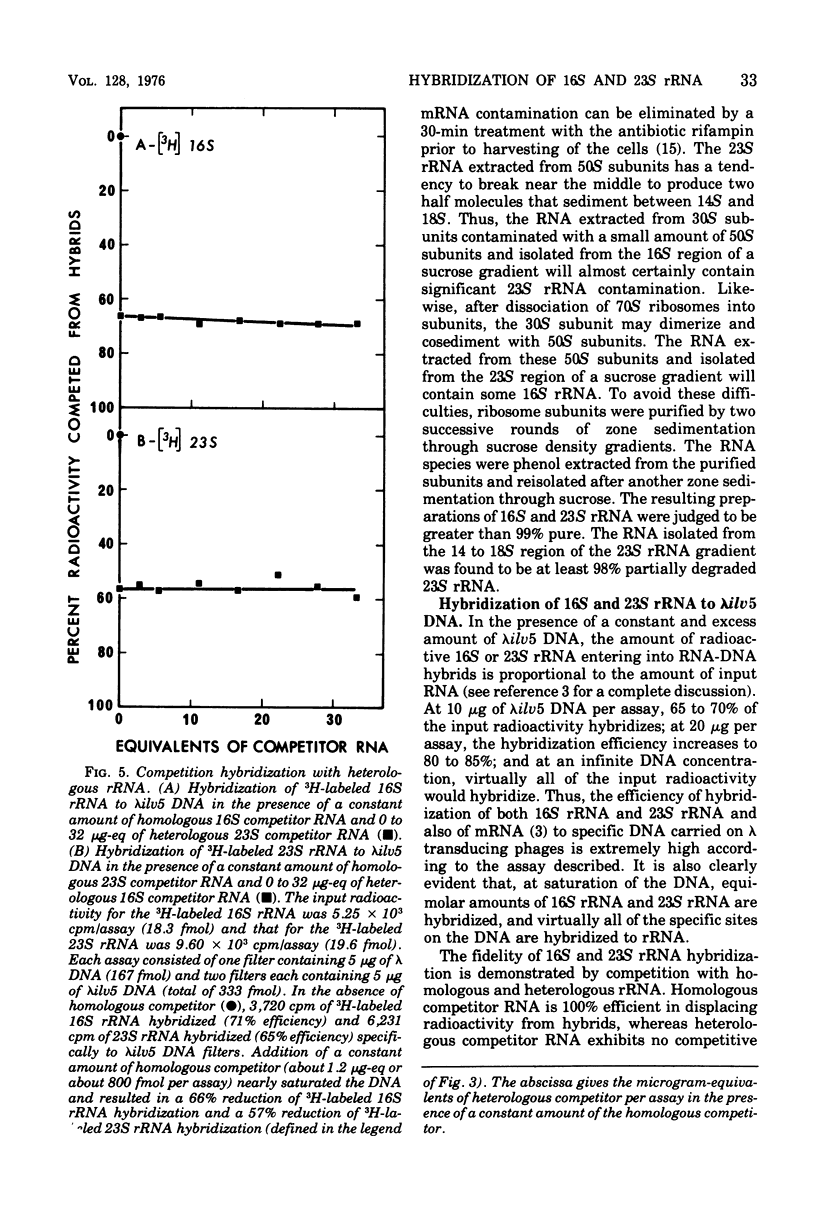
An assay to distinguish specifically between 16S and 23S ribosomal ribonucleic acid (rRNA) has been developed. The assay involves hybridization of radioactive rRNA to deoxyribonucleic acid (DNA) from lambdailv5 transducing phage, which carries an rRNA transcription unit. Radioactive 16S or 23S rRNA can be specifically and completely competed from hybrids by using highly purified nonradioactive 16S or 23S competitor RNA, respectively. The preparation and purification of 16S rRNA and 23S rRNA are described in detail. The hybridization assay is extremely sensitive and efficient; 65 to 70% or more of the input radioactivity hybridizes to specific DNA in the absence of homologous competitor RNA, and at saturation virtually all of the specific DNA sequences are hybridized to rRNA. The results indicate that: (i) the 16S rRNA and 23S rRNA prepared as described are greater than 99% pure, (ii) 16S RRNA and 23S rRNA hybridize with equal efficiency and in equal molar amounts of lambdailv5 DNA; (iii) at saturation, about one molecule of 16S and one molecule of 23S rRNA are hybridized per genome equivalent of lambda ilv 5 DNA; (iv) essentially no cross-hybridization occurs between 16S and 23S rRNA; and (v) the sequence homology between 16S and 23S rRNA is negligible.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi G., Huang P. C., Kabat S. Recognition of ribosomal RNA sites in DNA. I. Analysis of the E. coli system. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1490–1498. doi: 10.1073/pnas.53.6.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P., Nomura M. Regulation of the expression of ribosomal protein genes in Escherichia coli. J Mol Biol. 1975 Sep 5;97(1):61–76. doi: 10.1016/s0022-2836(75)80022-2. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs and Escherichia coli ribosomal RNAs are cut from large precursor RNAs in vivo by ribonuclease 3. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3296–3300. doi: 10.1073/pnas.70.12.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner P., Ebel J. P. Observations on the primary structure of the 23S ribosomal RNA from E. coli. Nature. 1970 Mar 21;225(5238):1131–1132. doi: 10.1038/2251131a0. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Ginsburg D., Steitz J. A. The 30 S ribosomal precursor RNA from Escherichia coli. A primary transcript containing 23 S, 16 S, and 5 S sequences. J Biol Chem. 1975 Jul 25;250(14):5647–5654. [PubMed] [Google Scholar]
- Hayes F., Vasseur M., Nikolaev N., Schlessinger D., Sri Widada J., Krol A., Branlant C. Structure of a 30 S pre-ribosomal RNA of E. coli. FEBS Lett. 1975 Aug 1;56(1):85–91. doi: 10.1016/0014-5793(75)80117-7. [DOI] [PubMed] [Google Scholar]
- Jaskunas S. R., Lindahl L., Nomura M. Specialized transducing phages for ribosomal protein genes of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jan;72(1):6–10. doi: 10.1073/pnas.72.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E., Lindahl L., Jaskunas S. R., Dennis P. P., Nomura M. Transfer RNA genes between 16S and 23S rRNA genes in rRNA transcription units of E. coli. Cell. 1976 Feb;7(2):165–177. doi: 10.1016/0092-8674(76)90016-7. [DOI] [PubMed] [Google Scholar]
- Mangiarotti G., Apirion D., Schlessinger D., Silengo L. Biosynthetic precursors of 30S and 50S ribosomal particles in Escherichia coli. Biochemistry. 1968 Jan;7(1):456–472. doi: 10.1021/bi00841a058. [DOI] [PubMed] [Google Scholar]
- Nikolaev N., Silengo L., Schlessinger D. Synthesis of a large precursor to ribosomal RNA in a mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3361–3365. doi: 10.1073/pnas.70.12.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Engbaek F. Expression of ribosomal protein genes as analyzed by bacteriophage Mu-induced mutations. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1526–1530. doi: 10.1073/pnas.69.6.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace N. R. Structure and synthesis of the ribosomal ribonucleic acid of prokaryotes. Bacteriol Rev. 1973 Dec;37(4):562–603. doi: 10.1128/br.37.4.562-603.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I., Dubnau D., Morrell P., Marmur J. Chromosomal location of DNA base sequences complementary to transfer RNA and to 5 s, 16 s and 23 s ribosomal RNA in Bacillus subtilis. J Mol Biol. 1968 Apr 14;33(1):123–140. doi: 10.1016/0022-2836(68)90285-4. [DOI] [PubMed] [Google Scholar]
- Wu M., Davidson N. Use of gene 32 protein staining of single-strand polynucleotides for gene mapping by electron microscopy: application to the phi80d3ilvsu+7 system. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4506–4510. doi: 10.1073/pnas.72.11.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]



