Abstract
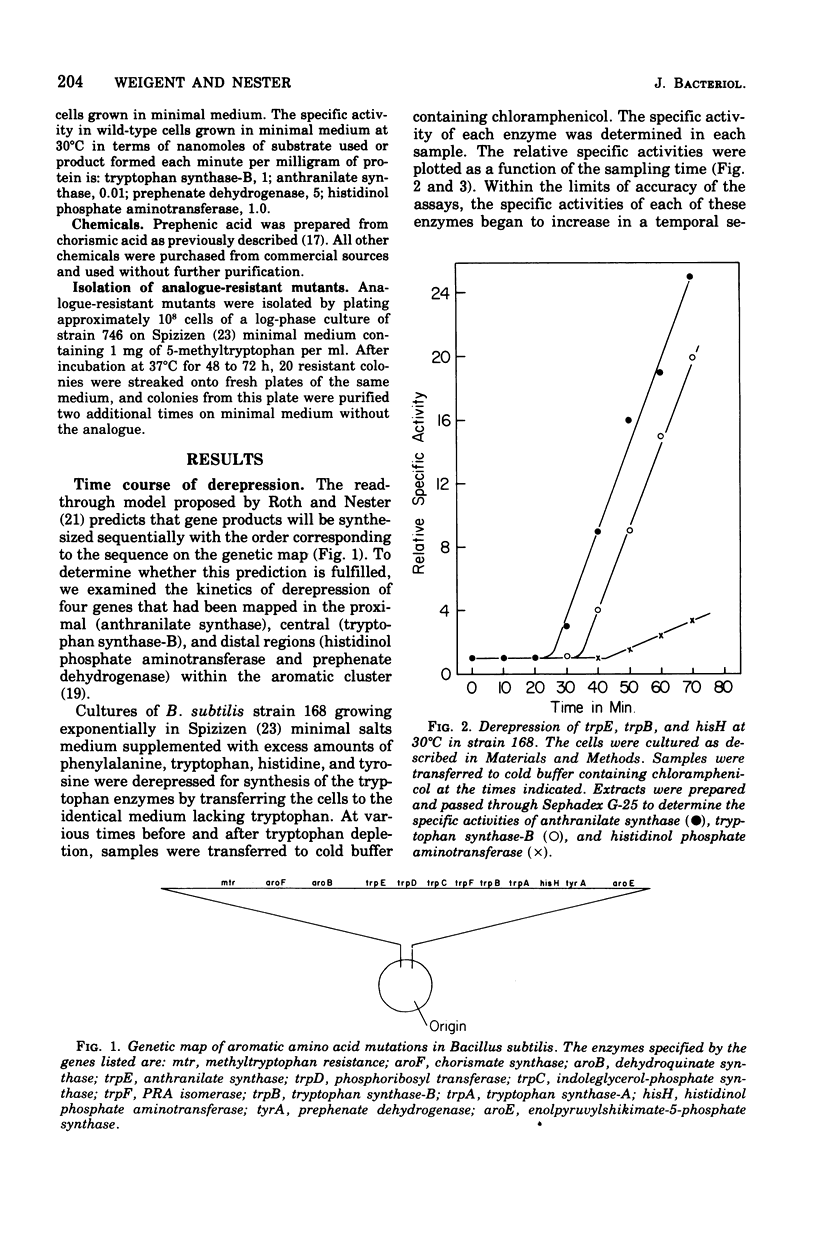
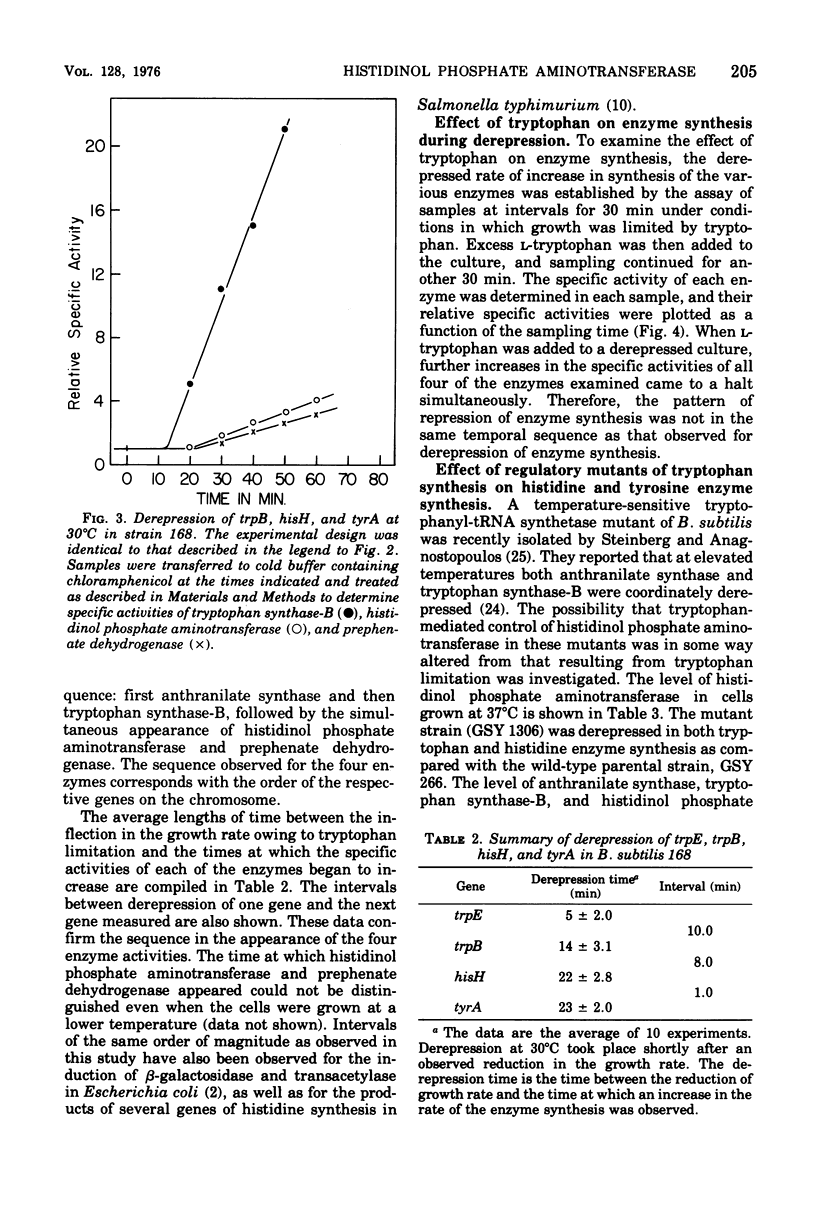
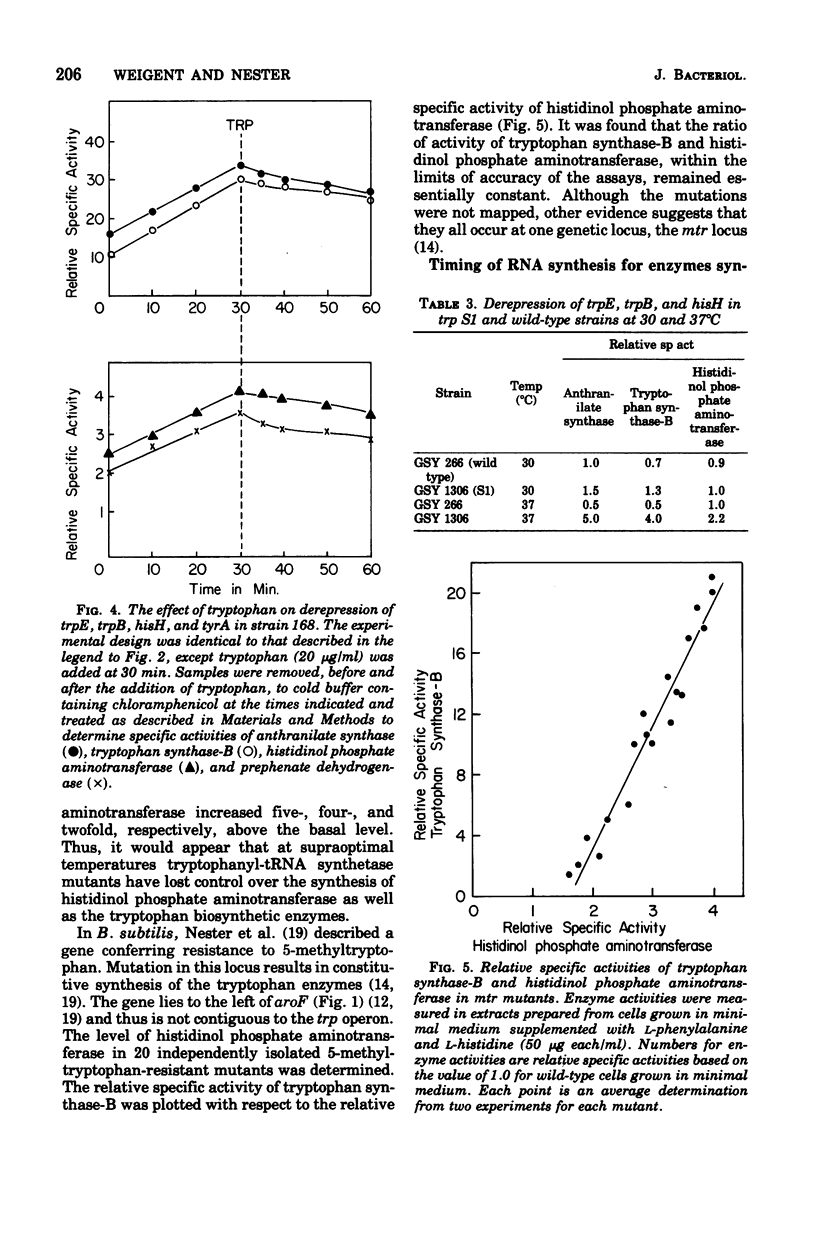
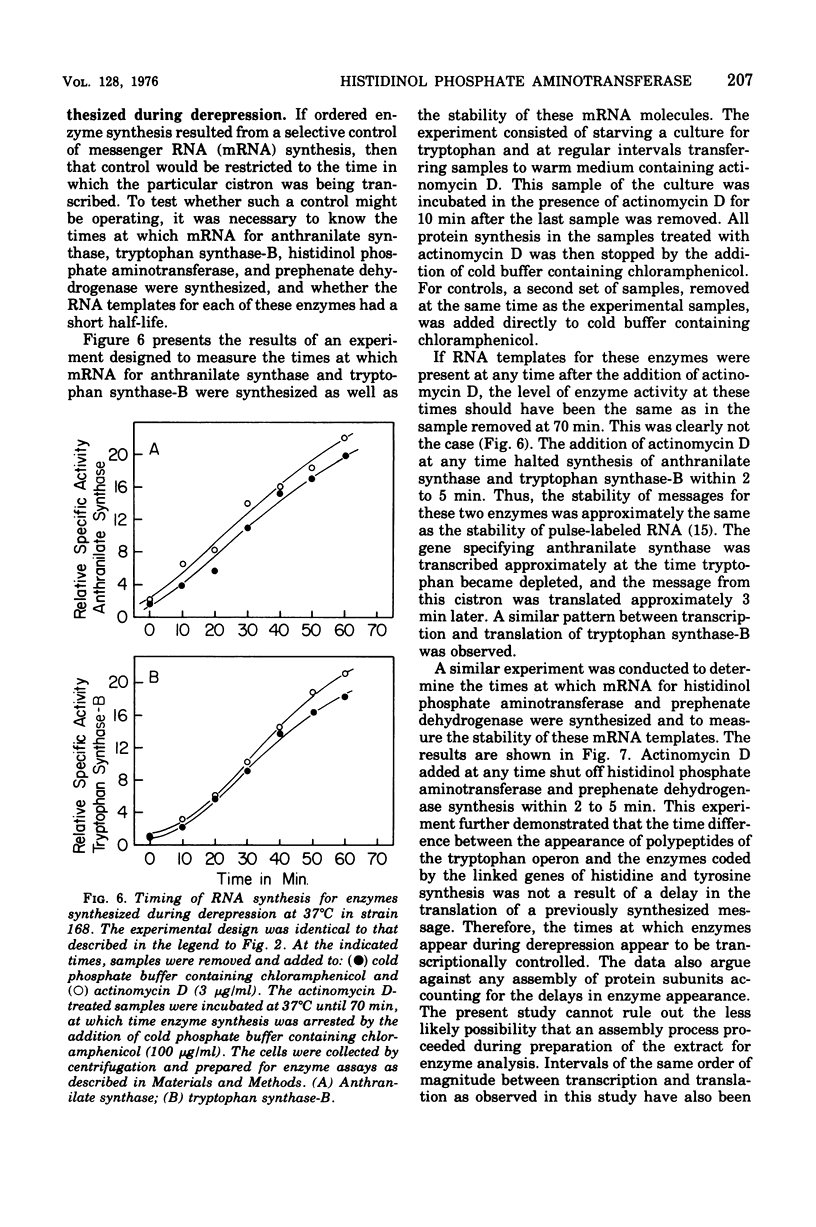
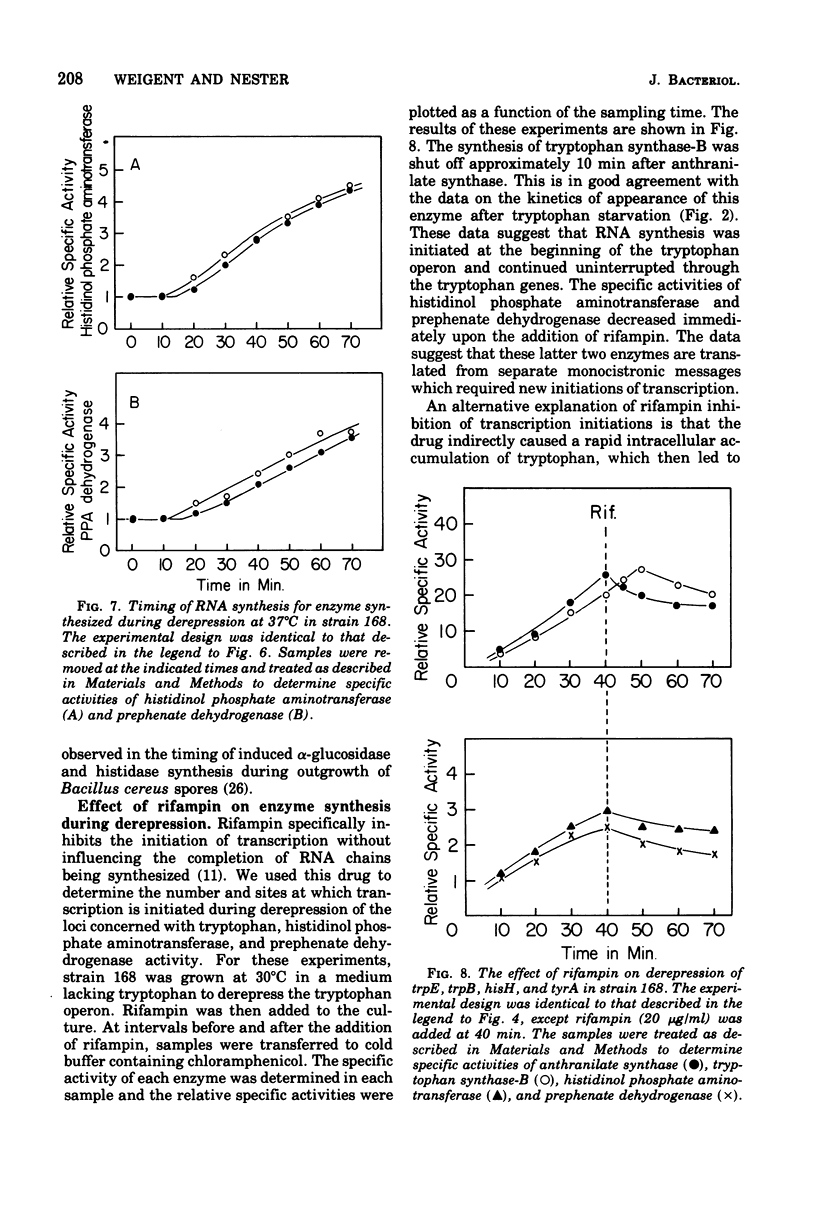
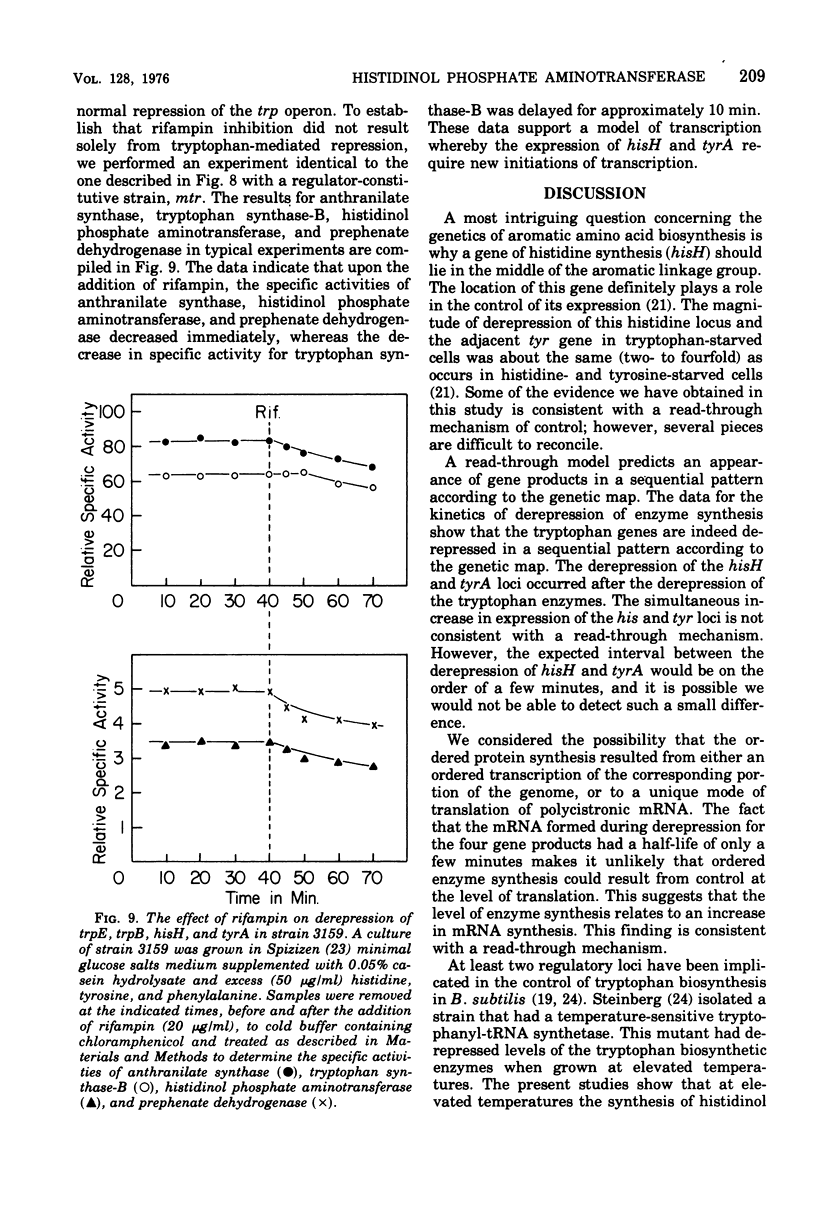
The effect of tryptophan on the synthesis of histidinol phosphate aminotransferase and prephenate dehydrogenase has been examined. The genes specifying two enzymes for tryptophan biosynthesis (anthranilate synthase and tryptophan synthase-B) were found to be derepressed in a temporal sequence according to their chromosomal location. The genes for histidinol phosphate aminotransferase and prephenate dehydrogenase were derepressed simultaneously approximately 8 min after tryptophan synthase-B. When excess tryptophan was added to a derepressed culture, the pattern of repression of trpE (anthranilate synthase), trpB (tryptophan synthase-B), hisH (histidinol phosphate aminotransferase), and tyrA (prephenate dehydrogenase) was found to be simultaneous. Methyl tryptophan-resistant mutants, which synthesize elevated levels of the tryptophan enzymes, also synthesized elevated levels of histidinol phosphate aminotransferase. Qualitatively similar data were obtained in a temperature-sensitive tryptophanyl-transferase ribonucleic acid synthetase mutant grown at elevated temperatures. The time at which messenger ribonucleic acid was synthesized for anthranilate synthase, tryptophan synthase-B, histidinol phosphate aminotransferase, and prephenate dehydrogenase in the presence of actinomycin D indicated that ordered enzyme synthesis was a result of ordered transcription of the corresponding portion of the genome. The effect of the drug rifampin on enzyme synthesis was also examined. The addition of this drug halted the transcription of anthranilate synthase very rapidly, but later regions of the tryptophan region continued to be transcribed. The transcription of the hisH and tyrA genes was also shut off rapidly after rifampin was added. The significance of these observations to the control of transcription of the hisH gene by tryptophan is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALPERS D. H., TOMKINS G. M. THE ORDER OF INDUCTION AND DEINDUCTION OF THE ENZYMES OF THE LACTOSE OPERON IN E. COLI. Proc Natl Acad Sci U S A. 1965 Apr;53:797–802. doi: 10.1073/pnas.53.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhya S., Gottesman M., De Crombrugghe B. Release of polarity in Escherichia coli by gene N of phage lambda: termination and antitermination of transcription. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2534–2538. doi: 10.1073/pnas.71.6.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames B. N., Garry B. COORDINATE REPRESSION OF THE SYNTHESIS OF FOUR HISTIDINE BIOSYNTHETIC ENZYMES BY HISTIDINE. Proc Natl Acad Sci U S A. 1959 Oct;45(10):1453–1461. doi: 10.1073/pnas.45.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostopoulos C., Crawford I. P. Le groupe des gènes régissant la biosynthèse du tryptophane chez Bacillus subtilis. C R Acad Sci Hebd Seances Acad Sci D. 1967 Jul 3;265(1):93–96. [PubMed] [Google Scholar]
- Carsiotis M., Jones R. F. Cross-pathway regulation: tryptophan-mediated control of histidine and arginine biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1974 Sep;119(3):889–892. doi: 10.1128/jb.119.3.889-892.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsiotis M., Jones R. F., Lacy A. M., Cleary T. J., Fankhauser D. B. Histidine-mediated control of tryptophan biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1970 Oct;104(1):98–106. doi: 10.1128/jb.104.1.98-106.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsiotis M., Jones R. F., Wesseling A. C. Cross-pathway regulation: histidine-mediated control of histidine, tryptophan, and arginine biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1974 Sep;119(3):893–898. doi: 10.1128/jb.119.3.893-898.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman L. F., Nester E. W. Common element in the repression control of enzymes of histidine and aromatic amino acid biosynthesis in Bacillus subtilus. J Bacteriol. 1968 Nov;96(5):1658–1663. doi: 10.1128/jb.96.5.1658-1663.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. ROLE OF VALYL-SRNA SYNTHETASE IN ENZYME REPRESSION. Proc Natl Acad Sci U S A. 1965 Mar;53:539–543. doi: 10.1073/pnas.53.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger R. F., Berberich M. A. Sequential repression and derepression of the enzymes for histidine biosynthesis in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1965 Jul;54(1):279–286. doi: 10.1073/pnas.54.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle D. C., Mangel W. F., Chamberlin M. J. Studies of the binding of Escherichia coli RNA polymerase to DNA. IV. The effect of rifampicin on binding and on RNA chain initiation. J Mol Biol. 1972 Sep 28;70(2):209–220. doi: 10.1016/0022-2836(72)90534-7. [DOI] [PubMed] [Google Scholar]
- Hoch S. O., Anagnostopoulos C., Crawford I. P. Enzymes of the tryptophan operon of Bacillus subtilis. Biochem Biophys Res Commun. 1969 Jun 27;35(6):838–844. doi: 10.1016/0006-291x(69)90700-1. [DOI] [PubMed] [Google Scholar]
- Hoch S. O. Mapping of the 5-methyltryptophan resistance locus in Bacillus subtilis. J Bacteriol. 1974 Jan;117(1):315–317. doi: 10.1128/jb.117.1.315-317.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch S. O., Roth C. W., Crawford I. P., Nester E. W. Control of tryptophan biosynthesis by the methyltryptophan resistance gene in Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):38–45. doi: 10.1128/jb.105.1.38-45.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E W, Schafer M, Lederberg J. Gene Linkage in DNA Transfer: A Cluster of Genes Concerned with Aromatic Biosynthesis in Bacillus Subtilis. Genetics. 1963 Apr;48(4):529–551. doi: 10.1093/genetics/48.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E. W., Dale B., Montoya A., Vold B. Cross pathway regulation of tyrosine and histidine synthesis in Bacillus subtilis. Biochemical, genetic, and transfer RNA studies. Biochim Biophys Acta. 1974 Aug 15;361(1):59–72. doi: 10.1016/0005-2787(74)90209-3. [DOI] [PubMed] [Google Scholar]
- Nester E. W., Jensen R. A. Control of aromatic acid biosynthesis in Bacillus subtilis: sequenial feedback inhibition. J Bacteriol. 1966 Apr;91(4):1594–1598. doi: 10.1128/jb.91.4.1594-1598.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E. W., Montoya A. L. An enzyme common to histidine and aromatic amino acid biosynthesis in Bacillus subtilis. J Bacteriol. 1976 May;126(2):699–705. doi: 10.1128/jb.126.2.699-705.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth C. W., Nester E. W. Co-ordinate control of tryptophan, histidine and tyrosine enzyme synthesis in Bacillus subtilis. J Mol Biol. 1971 Dec 28;62(3):577–589. doi: 10.1016/0022-2836(71)90157-4. [DOI] [PubMed] [Google Scholar]
- Roth J. R., Ames B. N. Histidine regulatory mutants in Salmonella typhimurium II. Histidine regulatory mutants having altered histidyl-tRNA synthetase. J Mol Biol. 1966 Dec 28;22(2):325–333. doi: 10.1016/0022-2836(66)90135-5. [DOI] [PubMed] [Google Scholar]
- Schürch A., Miozzari J., Hütter R. Regulation of tryptophan biosynthesis in Saccharomyces cerevisiae: mode of action of 5-methyl-tryptophan and 5-methyl-tryptophan-sensitive mutants. J Bacteriol. 1974 Mar;117(3):1131–1140. doi: 10.1128/jb.117.3.1131-1140.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg W., Anagnostopoulos C. Biochemical and genetic characterization of a temperature-sensitive, tryptophanyl-transfer ribonucleic acid synthetase mutant of Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):6–19. doi: 10.1128/jb.105.1.6-19.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg W., Halvorson H. O. Timing of enzyme synthesis during outgrowth of spores of Bacillus cereus. I. Ordered enzyme synthesis. J Bacteriol. 1968 Feb;95(2):469–478. doi: 10.1128/jb.95.2.469-478.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg W. Temperature-induced derepression of tryptophan biosynthesis in a tryptophanyl-transfer ribonucleic acid synthetase mutant of Bacillus subtilis. J Bacteriol. 1974 Mar;117(3):1023–1034. doi: 10.1128/jb.117.3.1023-1034.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfner M., Yep D., Messenguy F., Fink G. R. Integration of amino acid biosynthesis into the cell cycle of Saccharomyces cerevisiae. J Mol Biol. 1975 Aug 5;96(2):273–290. doi: 10.1016/0022-2836(75)90348-4. [DOI] [PubMed] [Google Scholar]



