Abstract
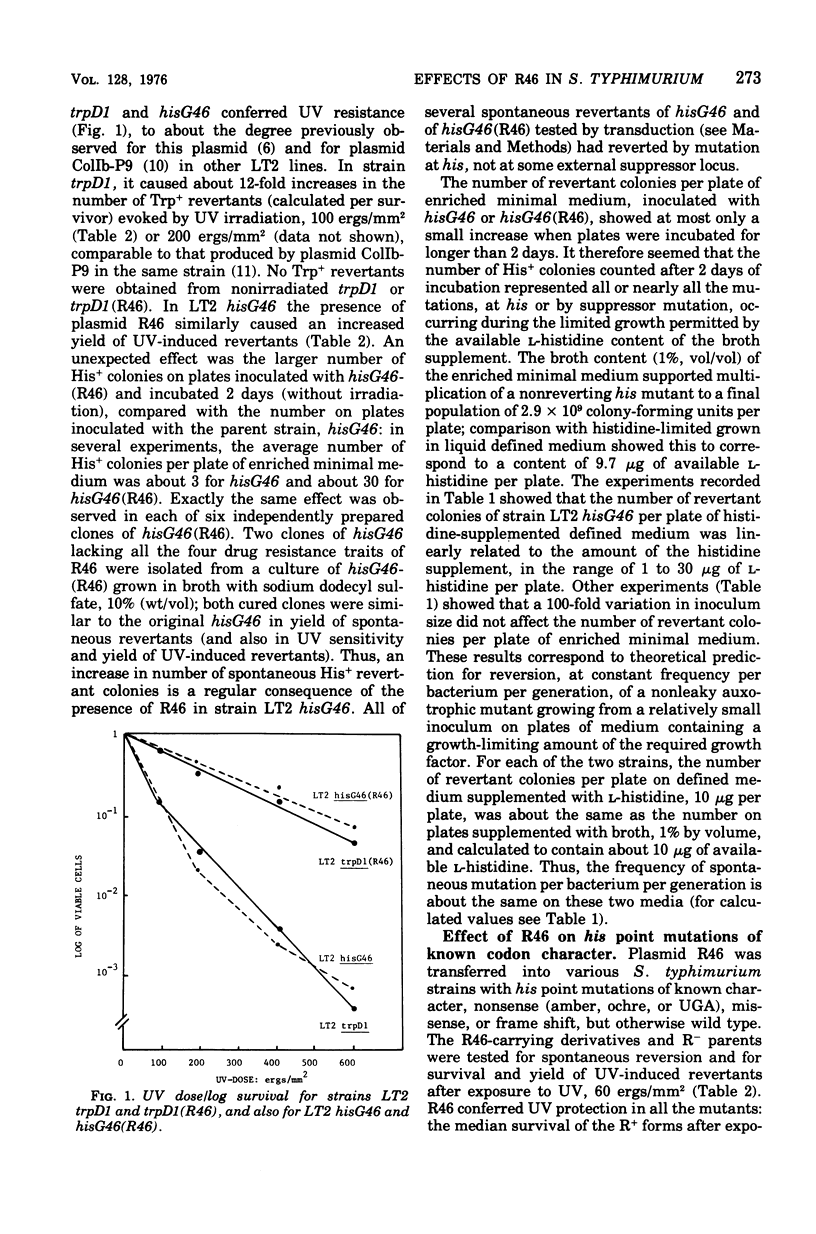
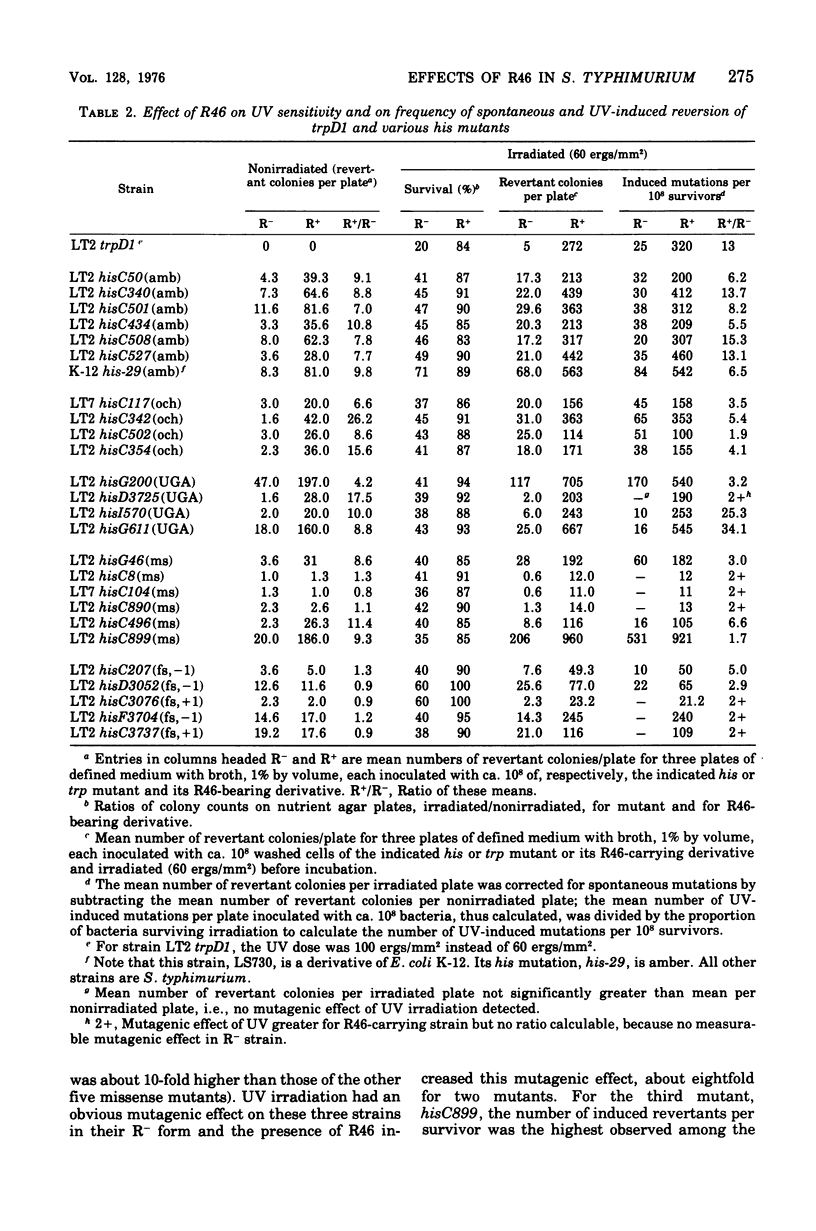
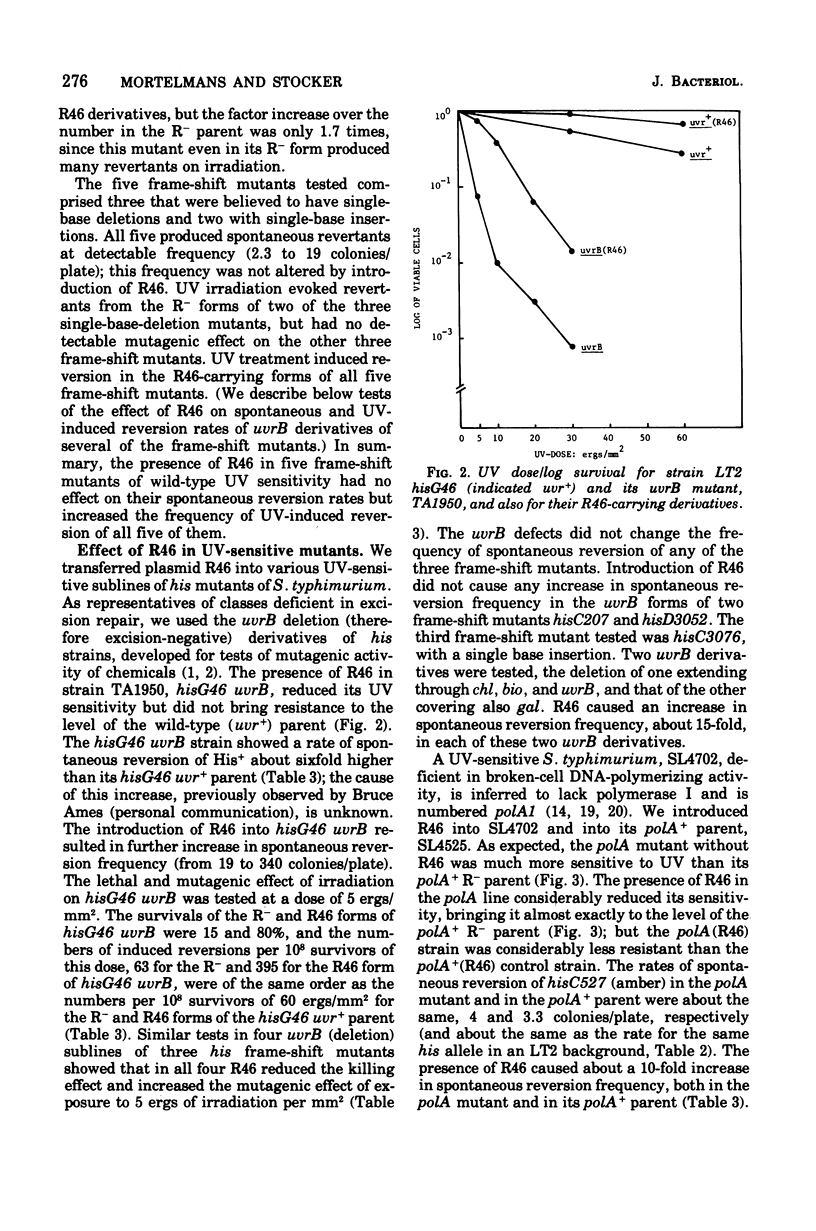
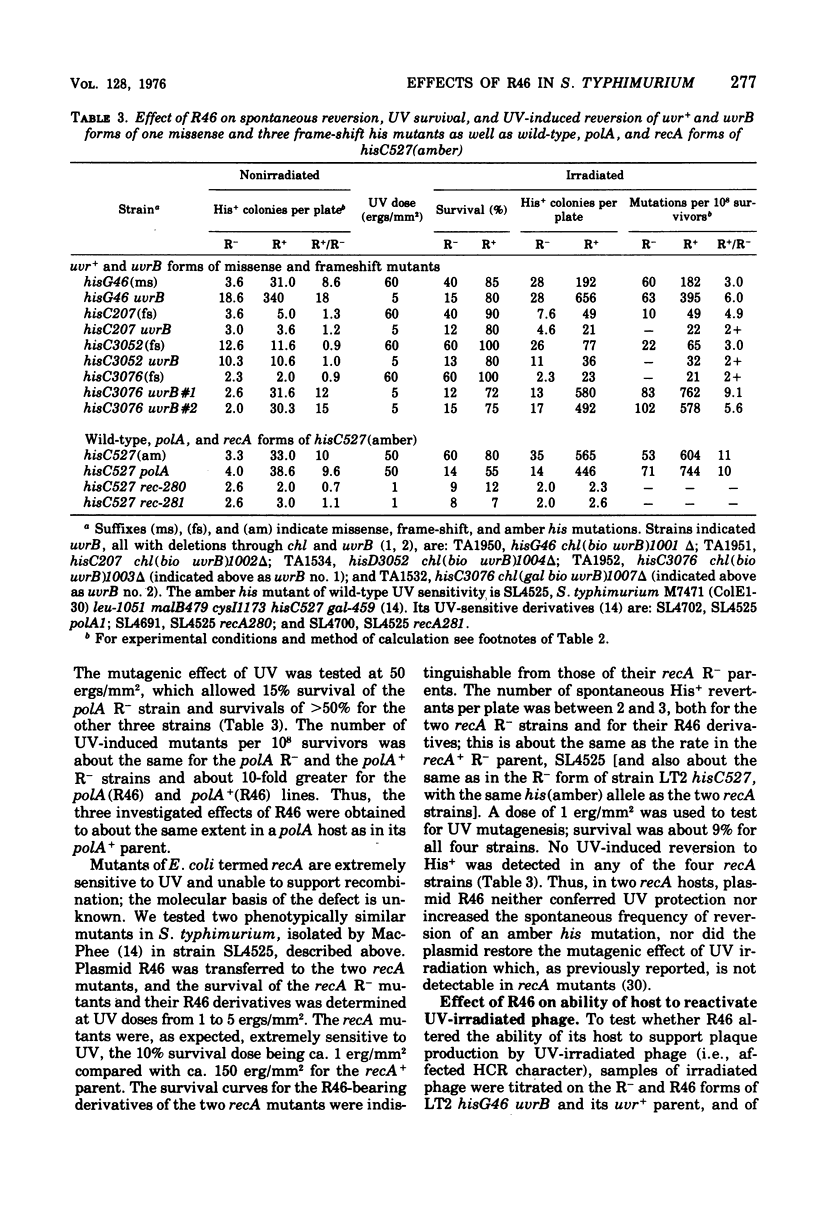
Plasmid R46 partially protected Salmonella typhimurium, wild type or uvrB or polA, against the lethal effect of ultraviolet (UV) irradiation, but did not protect recA mutants. The plasmid also increased frequency of UV-induced reversion to His+ in all tested his point mutants (wild type for UV sensitivity), including amber, ochre, UGA, missense, and frame-shift mutants. Plasmid R46 also increased UV-induced reversion to His+ in uvrB and polA strains, but no UV mutagenic effect was detected in R- or R46-carrying recA derivatives of a his (amber) mutant. The spontaneous reversion frequency of his nonsense mutants of all classes, and of some his missense mutants, was increased about 10-fold when the strains carried R46, but the plasmid had no effect on the spontaneous reversion frequency of some other his missense mutations or of reversion rate of his frame-shift mutants (except for two uvrB derivatives of one single-base insertion mutant). The plasmid increased the ability of wild-type, polA, and uvrB hosts to support plaque production by UV-irradiated phage, and made strain LT2 hisG46 less sensitive to methyl methane sulfonate and to X rays and more responsive to the mutagenic effect of visible-light irradiation. R46 increased spontaneous reversion frequency of a his (amber) rec+ strain, but had no such effect in its recA sublines. Since the plasmid in the absence of host recA function fails to produce its mutator effect, or to confer UV protection or to enhance UV mutagenesis, these three effects may be produced via some mechanism involved in recA-dependent deoxyribonucleic acid repair, perhaps by an increase in activity of the "error prone" component of the inducible repair pathway.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON E. S., DATTA N. RESISTANCE TO PENICILLINS AND ITS TRANSFER IN ENTEROBACTERIACEAE. Lancet. 1965 Feb 20;1(7382):407–409. doi: 10.1016/s0140-6736(65)90004-8. [DOI] [PubMed] [Google Scholar]
- Alper M. D., Ames B. N. Positive selection of mutants with deletions of the gal-chl region of the Salmonella chromosome as a screening procedure for mutagens that cause deletions. J Bacteriol. 1975 Jan;121(1):259–266. doi: 10.1128/jb.121.1.259-266.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleichrodt J. F., Verheij W. S. Mutagenesis by ultraviolet radiation in bacteriophage phiX174: on the mutation stimulating processes induced by ultraviolet radiation in the host bacterium. Mol Gen Genet. 1974;135(1):19–27. doi: 10.1007/BF00433897. [DOI] [PubMed] [Google Scholar]
- Datta N., Kontomichalou P. Penicillinase synthesis controlled by infectious R factors in Enterobacteriaceae. Nature. 1965 Oct 16;208(5007):239–241. doi: 10.1038/208239a0. [DOI] [PubMed] [Google Scholar]
- Drabble W. T., Stocker B. A. R (transmissible drug-resistance) factors in Salmonella typhimurium: pattern of transduction by phage P22 and ultraviolet-protection effect. J Gen Microbiol. 1968 Aug;53(1):109–123. doi: 10.1099/00221287-53-1-109. [DOI] [PubMed] [Google Scholar]
- Grindley N. D., Humphreys G. O., Anderson E. S. Molecular studies of R factor compatibility groups. J Bacteriol. 1973 Jul;115(1):387–398. doi: 10.1128/jb.115.1.387-398.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman P. E., Hartman Z., Stahl R. C. Classification and mapping of spontaneous and induced mutations in the histidine operon of Salmonella. Adv Genet. 1971;16:1–34. doi: 10.1016/s0065-2660(08)60352-1. [DOI] [PubMed] [Google Scholar]
- Howarth S. Increase in frequency of ultraviolet-induced mutation brought about by the colicine factor, col-I in Salmonella typhimurium. Mutat Res. 1966 Apr;3(2):129–134. doi: 10.1016/0027-5107(66)90026-1. [DOI] [PubMed] [Google Scholar]
- Howarth S. Resistance to the bactericidal effect of ultraviolet radiation conferred on Enterobacteria by the colicine factor coli. J Gen Microbiol. 1965 Jul;40(1):43–55. doi: 10.1099/00221287-40-1-43. [DOI] [PubMed] [Google Scholar]
- JAGGER J. A small and inexpensive ultraviolet dose-rate meter useful in biological experiements. Radiat Res. 1961 Apr;14:394–403. [PubMed] [Google Scholar]
- Kuo T. T., Stocker B. A. ES18, a general transducing phage for smooth and nonsmooth Salmonella typhimurium. Virology. 1970 Nov;42(3):621–632. doi: 10.1016/0042-6822(70)90308-9. [DOI] [PubMed] [Google Scholar]
- MacPhee D. G., Beazer M. R. Mutants of Salmonella typhimurium deficient in DNA polymerase. I. Detection by their failure to produce colicin E1. Mol Gen Genet. 1973 Dec 31;127(3):229–240. doi: 10.1007/BF00333762. [DOI] [PubMed] [Google Scholar]
- MacPhee D. G. DNA polymerase activity determined by the ultraviolet-protecting plasmid, R-Utrecht. Nature. 1974 Oct 4;251(5474):432–434. doi: 10.1038/251432a0. [DOI] [PubMed] [Google Scholar]
- MacPhee D. G. Effect of an R factor on resistance of Salmonella typhimurium to radiation and chemical treatment. Mutat Res. 1972 Apr;14(4):450–453. doi: 10.1016/0027-5107(72)90146-7. [DOI] [PubMed] [Google Scholar]
- MacPhee D. G. Effect of rec mutations on the ultraviolet protecting and mutation-enhancing properties of the plasmid R-Utrecht in Salmonella typhimurium. Mutat Res. 1973 Sep;19(3):357–359. doi: 10.1016/0027-5107(73)90237-6. [DOI] [PubMed] [Google Scholar]
- MacPhee D. G. Effects of an R factor and caffeine on ultraviolet mutability in Salmonella typhimurium. Mutat Res. 1973 Jun;18(3):367–370. doi: 10.1016/0027-5107(73)90221-2. [DOI] [PubMed] [Google Scholar]
- MacPhee D. G. Recombination-deficient mutants of colicinogenic Salmonella typhimurium detected by their failure to produce colicin. J Bacteriol. 1970 Oct;104(1):345–350. doi: 10.1128/jb.104.1.345-350.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhee D. G. Salmonella typhimurium hisG46 (R-Utrecht): possible use in screening mutagens and carcinogens. Appl Microbiol. 1973 Dec;26(6):1004–1005. doi: 10.1128/am.26.6.1004-1005.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh E. B., Jr, Smith D. H. R factors improving survival of Escherichia coli K-12 after ultraviolet irradiation. J Bacteriol. 1969 Oct;100(1):128–139. doi: 10.1128/jb.100.1.128-139.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J., Spingarn N. E., Kobori J., Ames B. N. Detection of carcinogens as mutagens: bacterial tester strains with R factor plasmids. Proc Natl Acad Sci U S A. 1975 Mar;72(3):979–983. doi: 10.1073/pnas.72.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZEKI H., STOCKER B. A., SMITH S. M. Transmission of colicinogeny between strains of Salmonella typhimurium grown together. J Gen Microbiol. 1962 Sep;28:671–687. doi: 10.1099/00221287-28-4-671. [DOI] [PubMed] [Google Scholar]
- Radman M. SOS repair hypothesis: phenomenology of an inducible DNA repair which is accompanied by mutagenesis. Basic Life Sci. 1975;5A:355–367. doi: 10.1007/978-1-4684-2895-7_48. [DOI] [PubMed] [Google Scholar]
- Schmieger H. Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet. 1972;119(1):75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- Siccardi A. G. Effect of R factors and other plasmids on ultraviolet susceptibility and host cell reactivation property of Escherichia coli. J Bacteriol. 1969 Oct;100(1):337–346. doi: 10.1128/jb.100.1.337-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck W. T., Rosenkranz H. S. Base substitution mutations induced in Salmonella strains by visible light (450 nm). Photochem Photobiol. 1975 May;21(5):369–371. doi: 10.1111/j.1751-1097.1975.tb06687.x. [DOI] [PubMed] [Google Scholar]
- Tweats D. J., Thompson M. J., Pinney R. J., Smith J. T. R factor-mediated resistance to ultraviolet light in strains of Escherichia coli deficient in known repair functions. J Gen Microbiol. 1976 Mar;93(1):103–110. doi: 10.1099/00221287-93-1-103. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Thermal enhancement of ultraviolet mutability in a tif-1 uvrA derivative of Escherichia coli B-r: evidence that ultraviolet mutagenesis depends upon an inducible function. Proc Natl Acad Sci U S A. 1974 May;71(5):1930–1934. doi: 10.1073/pnas.71.5.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet-induced mutation and DNA repair. Annu Rev Microbiol. 1969;23:487–514. doi: 10.1146/annurev.mi.23.100169.002415. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Ito J., Horn V. Amino acid replacements and the genetic code. Cold Spring Harb Symp Quant Biol. 1966;31:151–162. doi: 10.1101/sqb.1966.031.01.023. [DOI] [PubMed] [Google Scholar]



