Abstract
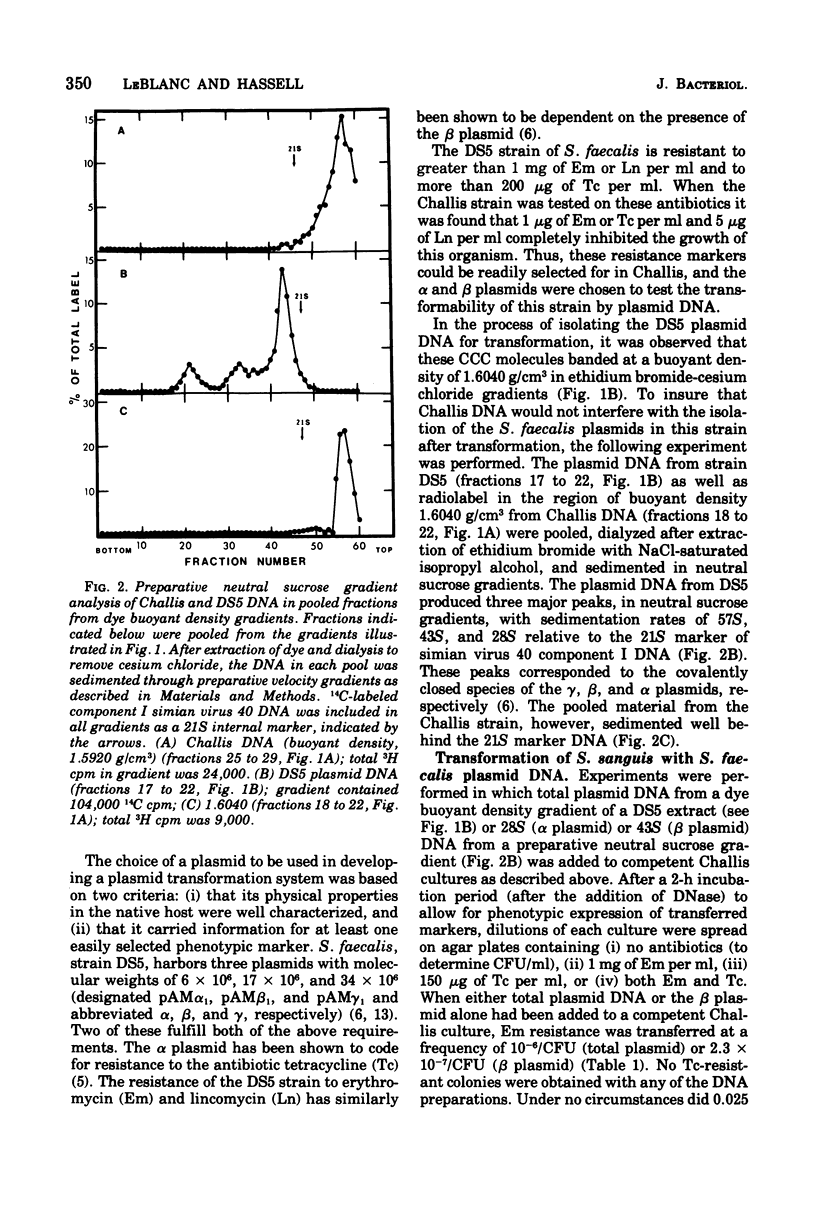
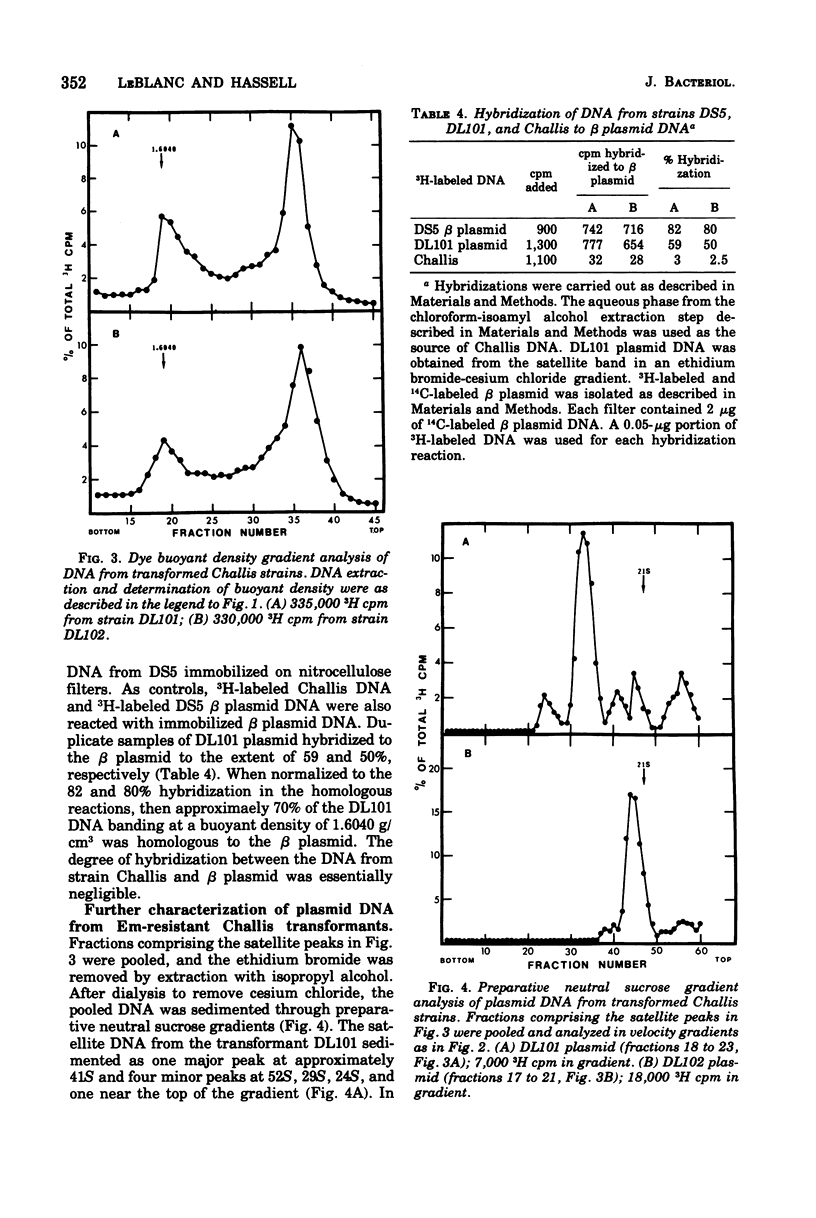
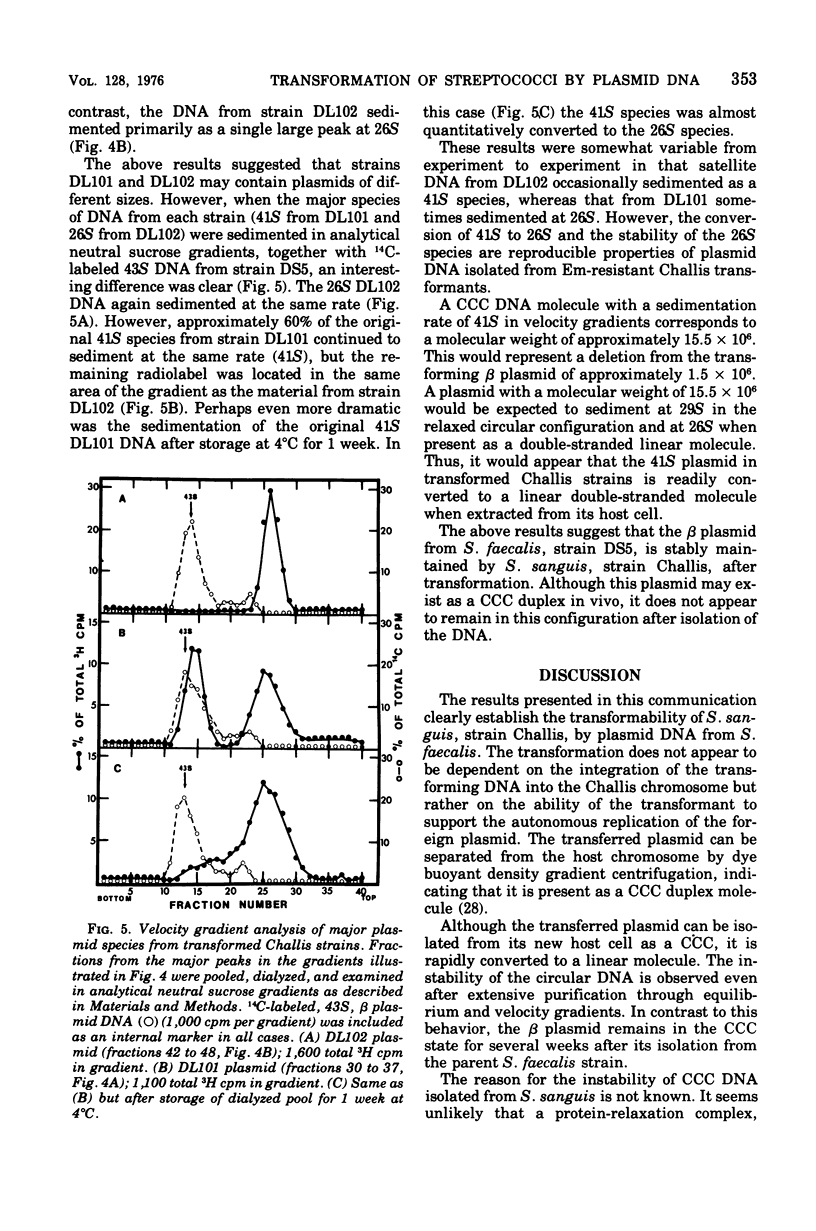
Plasmid deoxyribonucleic acid (DNA) from Streptococcus faecalis, strain DS5, was transferred to the Challis strain of Streptococcus sanguis by transformation. Two antibiotic resistance markers carried by the beta plasmid from strain DS5, erythromycin and lincomycin, were transferred to S. sanguis at a maximum frequency of 1.8 x 10-5/colony-forming unit. Approximately 70% of the covalently closed circular DNA isolated from transformant cultures by dye buoyant density gradients was shown to be hybridizable to beta plasmid DNA. Two major differences were observed between the beta plasmid from S. faecalis and the plasmid isolated from transformed S. sanguis: (i) the beta plasmid from strain DS5 sedimented in velocity gradients at 43S, whereas the covalently closed circular DNA from transformed Challis sedimented at 41S, suggesting a 1.5-Mdal deletion from the beta plasmid occurred; (ii) although the 43S beta plasmid remained in the supercoiled configuration for several weeks after isolation, the 41S plasmid was rapidly converted to a linear double-stranded molecule. Attempts to transform S. sanguis with the alpha plasmid from S. faecalis, strain DS5, were unsuccessful.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chassy B. M. A gentle method for the lysis of oral streptococci. Biochem Biophys Res Commun. 1976 Jan 26;68(2):603–608. doi: 10.1016/0006-291x(76)91188-8. [DOI] [PubMed] [Google Scholar]
- Cleary P. P., Johnson Z., Wannamaker L. Genetic instability of M protein and serum opacity factor of group A streptocci: evidence suggesting extrachromosomal control. Infect Immun. 1975 Jul;12(1):109–118. doi: 10.1128/iai.12.1.109-118.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Franke A. E. Characterization of a plasmid determining resistance to erythromycin, lincomycin, and vernamycin Balpha in a strain Streptococcus pyogenes. Antimicrob Agents Chemother. 1974 May;5(5):534–537. doi: 10.1128/aac.5.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Bauer B. Plasmid-determined tetracycline resistance in Streptococcus faecalis: evidence for gene amplification during growth in presence of tetracycline. Proc Natl Acad Sci U S A. 1975 May;72(5):1720–1724. doi: 10.1073/pnas.72.5.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Dunny G. M., Schultz S. K. Characterization of three plasmid deoxyribonucleic acid molecules in a strain of Streptococcus faecalis: identification of a plasmid determining erythromycin resistance. J Bacteriol. 1974 Jan;117(1):283–289. doi: 10.1128/jb.117.1.283-289.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cords B. R., McKay L. L., Guerry P. Extrachromosomal elements in group N streptococci. J Bacteriol. 1974 Mar;117(3):1149–1152. doi: 10.1128/jb.117.3.1149-1152.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P. M., Carlier C., Chabbert Y. A. Plasmid-linked tetracycline and erythromycin resistance in group D "streptococcus". Ann Inst Pasteur (Paris) 1972 Dec;123(6):755–759. [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Evidence for diverse types of large plasmids in tumor-inducing strains of Agrobacterium. J Bacteriol. 1976 Apr;126(1):157–165. doi: 10.1128/jb.126.1.157-165.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson J. R., Jr, Blevins W. T., Feary T. W. Interspecies transformation of streptomycin resistance in oral streptococci. Antimicrob Agents Chemother. 1976 Jan;9(1):145–150. doi: 10.1128/aac.9.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Birch N., Hascall G., Clewell D. B. Isolation and characterization of plasmid deoxyribonucleic acid from Streptococcus mutans. J Bacteriol. 1973 Jun;114(3):1362–1364. doi: 10.1128/jb.114.3.1362-1364.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Clewell D. B. Transmissible toxin (hemolysin) plasmid in Streptococcus faecalis and its mobilization of a noninfectious drug resistance plasmid. J Bacteriol. 1975 Nov;124(2):784–790. doi: 10.1128/jb.124.2.784-790.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furano A. V. A very rapid method for washing large numbers of precipitates of proteins and nucleic acids. Anal Biochem. 1971 Oct;43(2):639–640. doi: 10.1016/0003-2697(71)90300-9. [DOI] [PubMed] [Google Scholar]
- Higuchi M., Araya S., Higuchi M. Plasmid DNA satellite bands seen in lysates of Streptococcus mutans that form insoluble extracellular polysaccharides. J Dent Res. 1976 Mar-Apr;55(2):266–271. doi: 10.1177/00220345760550021801. [DOI] [PubMed] [Google Scholar]
- Jacob A. E., Douglas G. J., Hobbs S. J. Self-transferable plasmids determining the hemolysin and bacteriocin of Streptococcus faecalis var. zymogenes. J Bacteriol. 1975 Mar;121(3):863–872. doi: 10.1128/jb.121.3.863-872.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi S., Rozenblatt S., Singer M. F., Winocour E. Acquisition of sequences homologous to host DNA by closed circular simian virus 40 DNA. II. Further studies on the serial passage of virus clones. J Virol. 1973 Sep;12(3):492–500. doi: 10.1128/jvi.12.3.492-500.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi S., Winocour E. Acquisition of sequences homologous to host deoxyribonucleic acid by closed circular simian virus 40 deoxyribonucleic acid. J Virol. 1972 Feb;9(2):309–316. doi: 10.1128/jvi.9.2.309-316.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Singer M. F. Localization of replicating DNA of simian virus 40 in monkey kidney cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2236–2240. doi: 10.1073/pnas.71.6.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A. Plasmid distribution and evidence for a proteinase plasmid in Streptococcus lactis C2-1. Appl Microbiol. 1975 Apr;29(4):546–548. doi: 10.1128/am.29.4.546-548.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A. Simultaneous loss of proteinase- and lactose-utilizing enzyme activities in Streptococcus lactis and reversal of loss by transduction. Appl Microbiol. 1974 Sep;28(3):342–346. doi: 10.1128/am.28.3.342-346.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molskness T. A., Sandine W. E., Brown L. R. Characterization of lac+ transductants of Streptococcus lactis. Appl Microbiol. 1974 Nov;28(5):753–758. doi: 10.1128/am.28.5.753-758.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae M., Inoue M., Mitsuhashi S. Artificial elimination of drug resistance from group A beta-hemolytic streptococci. Antimicrob Agents Chemother. 1975 May;7(5):719–720. doi: 10.1128/aac.7.5.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY D., SLADE H. D. INTRASPECIFIC AND INTERSPECIFIC TRANSFORMATION IN STREPTOCOCCI. J Bacteriol. 1964 Sep;88:595–601. doi: 10.1128/jb.88.3.595-601.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY D., SLADE H. D. Transformation of streptococci to streptomycin resistance. J Bacteriol. 1962 Mar;83:443–449. doi: 10.1128/jb.83.3.443-449.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yag Y., Franke A. E., Clewell D. B. Plasmid-determined resistance to erythromycin: comparison of strains of streptococcus faecalis and streptococcus pyogenes with regard to plasmid hmology and resistance inducibility. Antimicrob Agents Chemother. 1975 Jun;7(6):871–873. doi: 10.1128/aac.7.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]


