Abstract
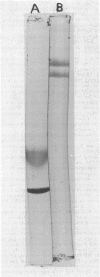
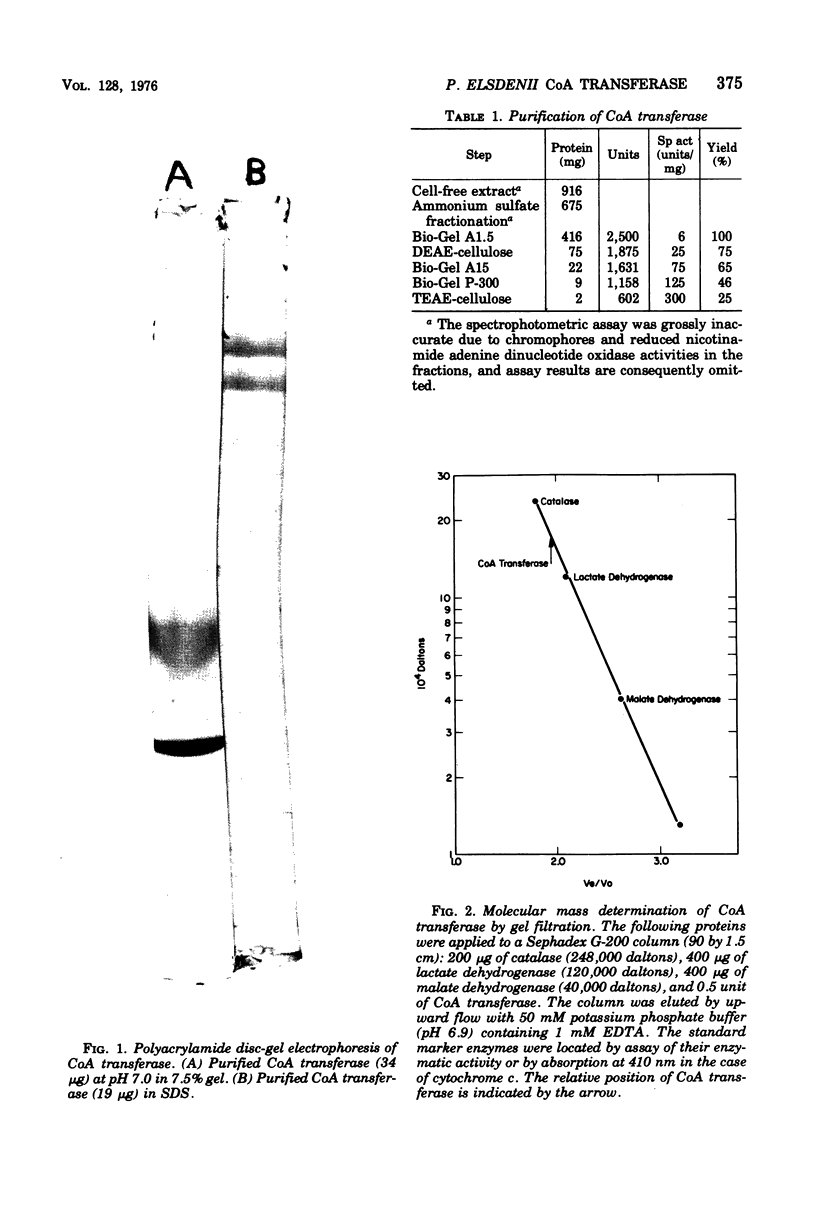
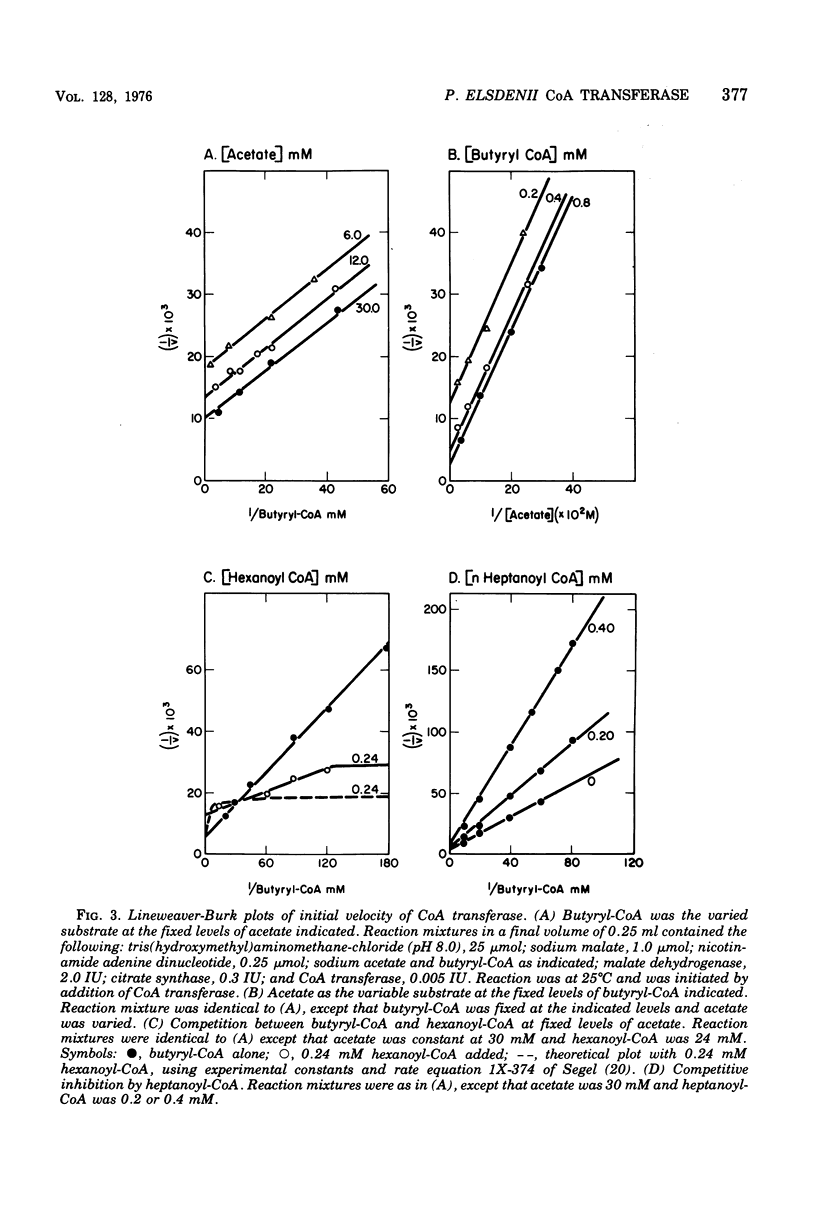
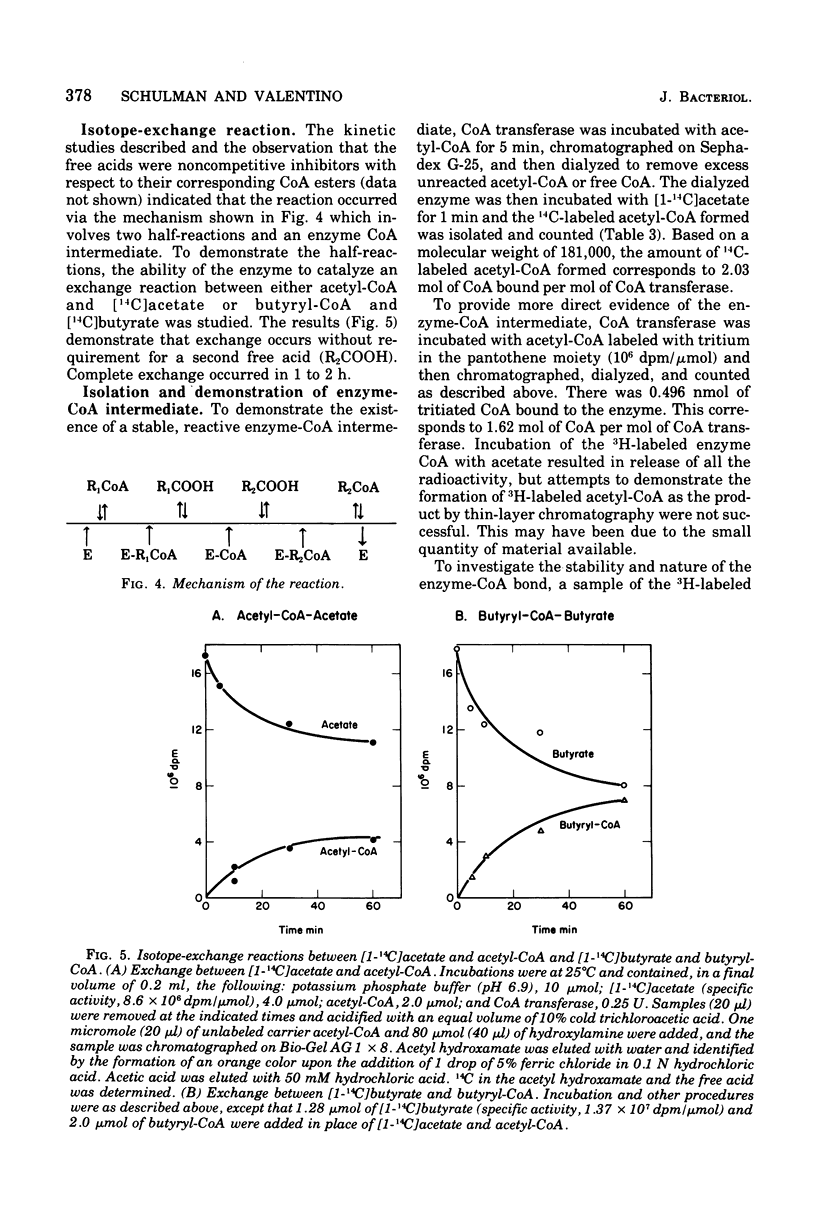
Coenzyme A (CoA) transferase from Peptostreptococcus elsdenii was purified to homogeneity, and some of its physical and catalytic properties were determined. The native enzyme has a molecular weight of 181,000 and is composed of two alpha subunits (molecular weight, 65,000) and one beta subunit (molecular weight 50,000). Heat treatment of the crude cell extract to 58 degrees C causes proteolysis of the native enzyme and yields a catalytically active enzyme with an approximate molecular weight of 120,000. The native CoA transferase is specific for CoA esters of short-chain alkyl monocarboxylic acids. With acetate as CoA acceptor the enzyme is active with propionyl-, butyryl-, isobutyryl-, valeryl-, isovaleryl,- and hexanoyl-CoA but not with heptanoyl or longer-chain CoA esters. There is no activity with acetoacetyl-CoA or the CoA esters of dicarboxylic acids. Steady-state kinetics indicated that the reaction proceeds via a classical bi-, bi-ping-pong mechanism. Maximal activity is obtained with propionyl- or butyryl-CoA, and both the Vmax and Km decrease as the alkyl chain length of the CoA ester increases. All CoA esters apompetitive inhibitor although it is not active as a substrate. Evidence for an enzyme CoA intermediate was provided by demonstration of an exchange between 14C-free acids (acetate and butyrate) and their corresponding CoA esters and by isolation of a 3H-labeled CoA enzyme after incubation of the enzyme with 3H-labeled acetyl-CoA. Approximately 2 mol of CoA was bound per mol of enzyme.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN S. H., KELLERMEYER R. W., STJERNHOLM R. L., WOOD H. G. PURIFICATION AND PROPERTIES OF ENZYMES INVOLVED IN THE PROPIONIC ACID FERMENTATION. J Bacteriol. 1964 Jan;87:171–187. doi: 10.1128/jb.87.1.171-187.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- ELSDEN S. R., GILCHRIST F. M., LEWIS D., VOLCANI B. E. Properties of a fatty acid forming organism isolated from the rumen of sheep. J Bacteriol. 1956 Nov;72(5):681–689. doi: 10.1128/jb.72.5.681-689.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fall R. R., Vagelos P. R. Acetyl coenzyme A carboxylase. Proteolytic modification of biotin carboxyl carrier protein. J Biol Chem. 1973 Mar 25;248(6):2078–2088. [PubMed] [Google Scholar]
- Fenselau A., Wallis K. Ping-pong chromatography. A novel purification of CoA-transferase. Biochem Biophys Res Commun. 1975 Jan 20;62(2):350–356. doi: 10.1016/s0006-291x(75)80145-8. [DOI] [PubMed] [Google Scholar]
- Fenselau A., Wallis K. Substrate specificity and mechanism of action of acetoacetate coenzyme A transferase from rat heart. Biochemistry. 1974 Sep 10;13(19):3884–3888. doi: 10.1021/bi00716a010. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- STERN J. R., DEL CAMPILLO A. Enzymes of fatty acid metabolism. II. Properties of crystalline crotonase. J Biol Chem. 1956 Feb;218(2):985–1002. [PubMed] [Google Scholar]
- Schulman M., Wood H. G. Determination and degradation of microquantities of acetate. Anal Biochem. 1971 Feb;39(2):505–520. doi: 10.1016/0003-2697(71)90441-6. [DOI] [PubMed] [Google Scholar]
- Schulman M., Wood H. G. Succinyl-CoA: propionate CoA-transferase from Propionibacterium shermanii. EC 2.8.3.6 succinyl-CoA:propionate CoA-transferase. Methods Enzymol. 1975;35:235–242. doi: 10.1016/0076-6879(75)35159-8. [DOI] [PubMed] [Google Scholar]
- Sramek S. J., Frerman F. E. Escherichia coli coenzyme A-transferase: kinetics, catalytic pathway and structure. Arch Biochem Biophys. 1975 Nov;171(1):27–35. doi: 10.1016/0003-9861(75)90003-x. [DOI] [PubMed] [Google Scholar]
- Sramek S. J., Frerman F. E. Purification and properties of Escherichia coli coenzyme A-transferase. Arch Biochem Biophys. 1975 Nov;171(1):14–26. doi: 10.1016/0003-9861(75)90002-8. [DOI] [PubMed] [Google Scholar]
- Swick R. W., Wood H. G. THE ROLE OF TRANSCARBOXYLATION IN PROPIONIC ACID FERMENTATION. Proc Natl Acad Sci U S A. 1960 Jan;46(1):28–41. doi: 10.1073/pnas.46.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tildon J. T., Sevdalian D. A. CoA transferase in the brain and other mammalian tissues. Arch Biochem Biophys. 1972 Feb;148(2):382–390. doi: 10.1016/0003-9861(72)90155-5. [DOI] [PubMed] [Google Scholar]
- Tung K. K., Wood W. A. Purification, new assay, and properties of coenzyme A transferase from Peptostreptococcus elsdenii. J Bacteriol. 1975 Dec;124(3):1462–1474. doi: 10.1128/jb.124.3.1462-1474.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS D. E., REISFELD R. A. DISC ELECTROPHORESIS IN POLYACRYLAMIDE GELS: EXTENSION TO NEW CONDITIONS OF PH AND BUFFER. Ann N Y Acad Sci. 1964 Dec 28;121:373–381. doi: 10.1111/j.1749-6632.1964.tb14210.x. [DOI] [PubMed] [Google Scholar]