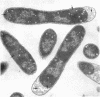
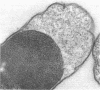
Abstract
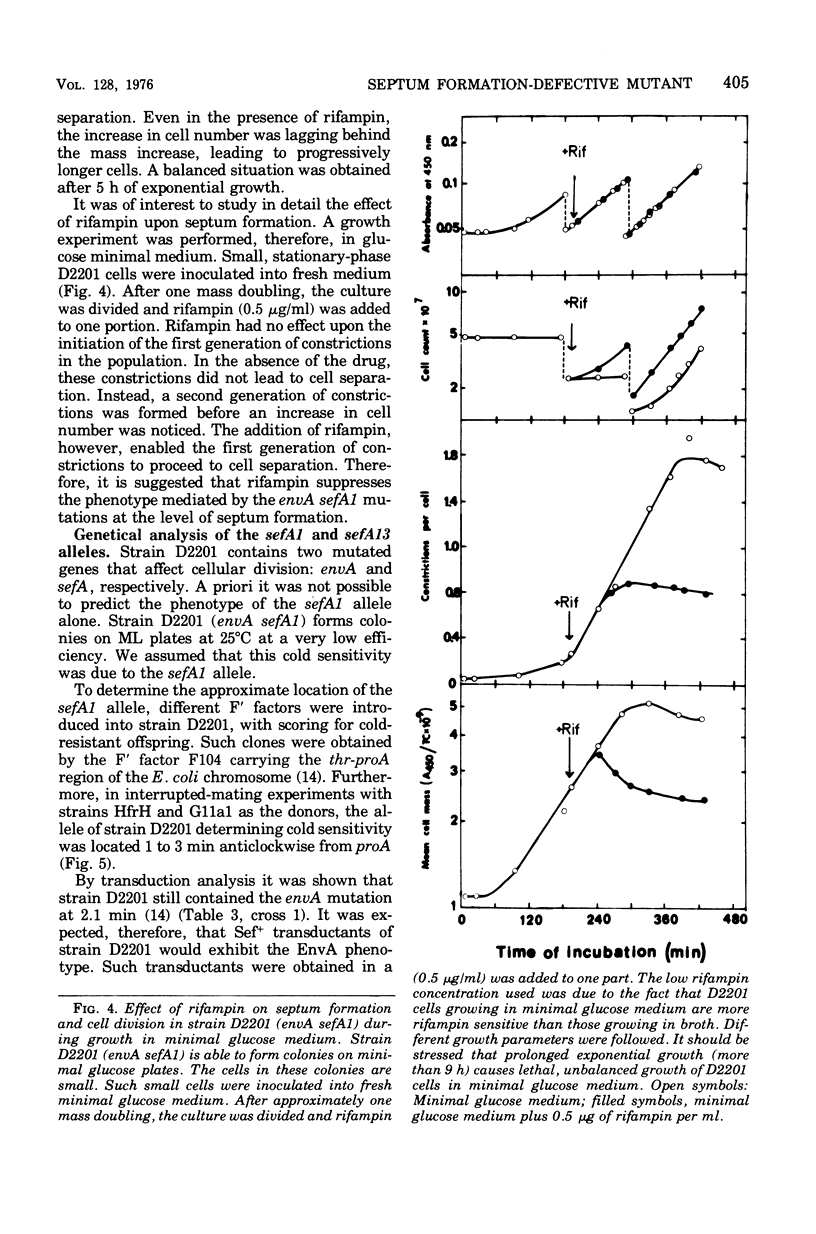
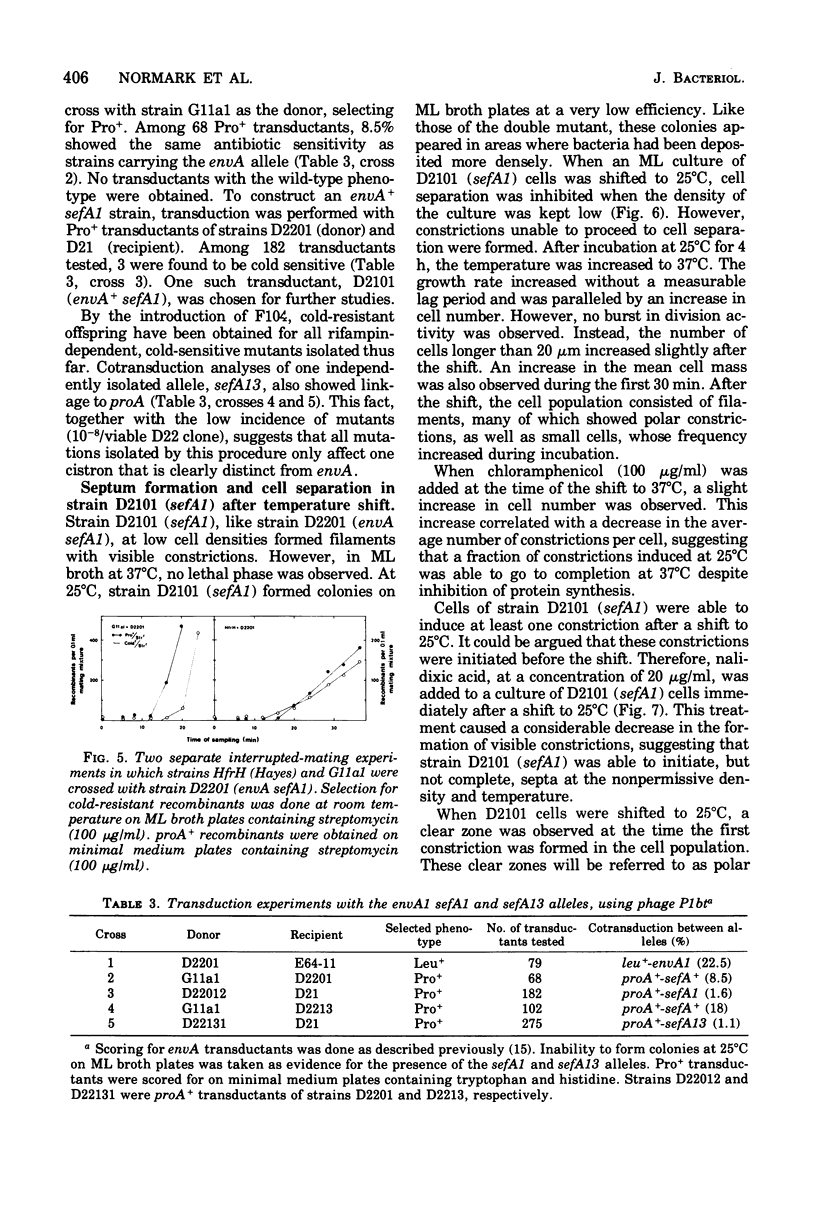
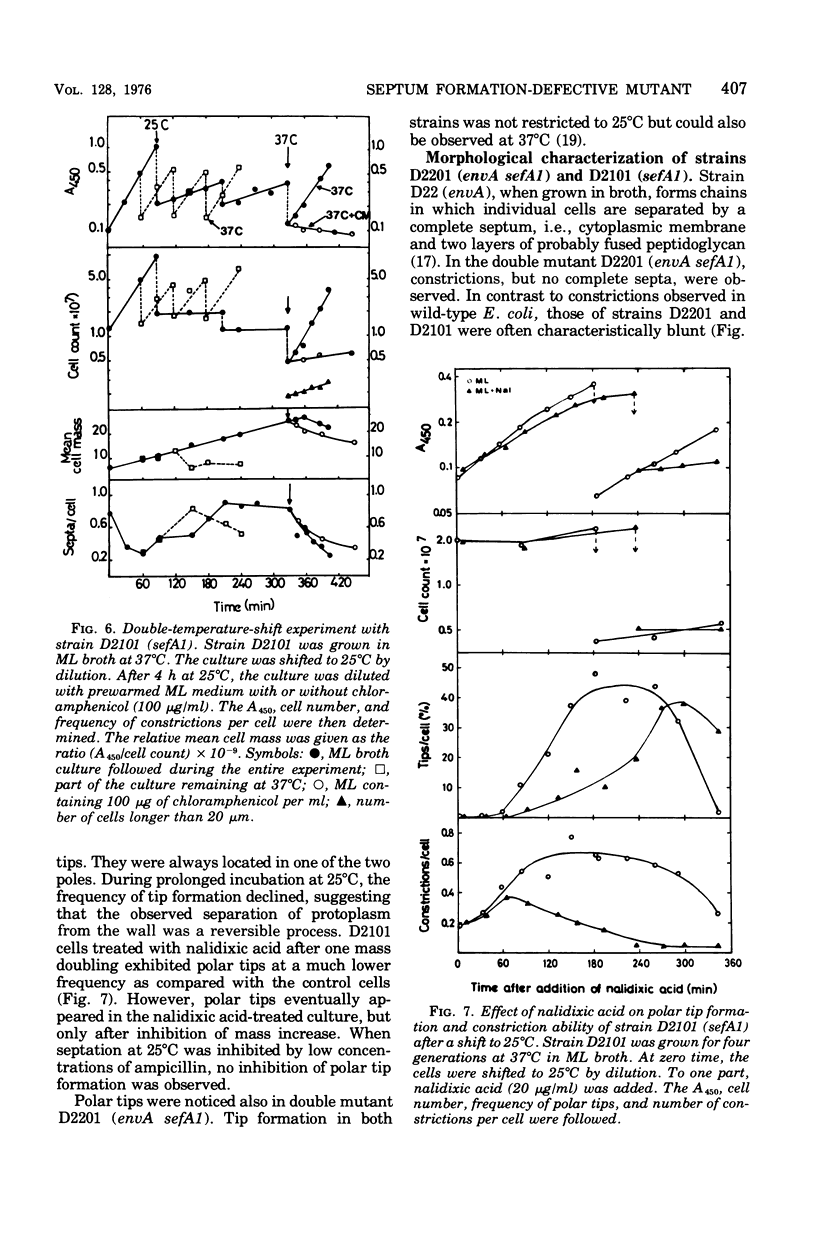
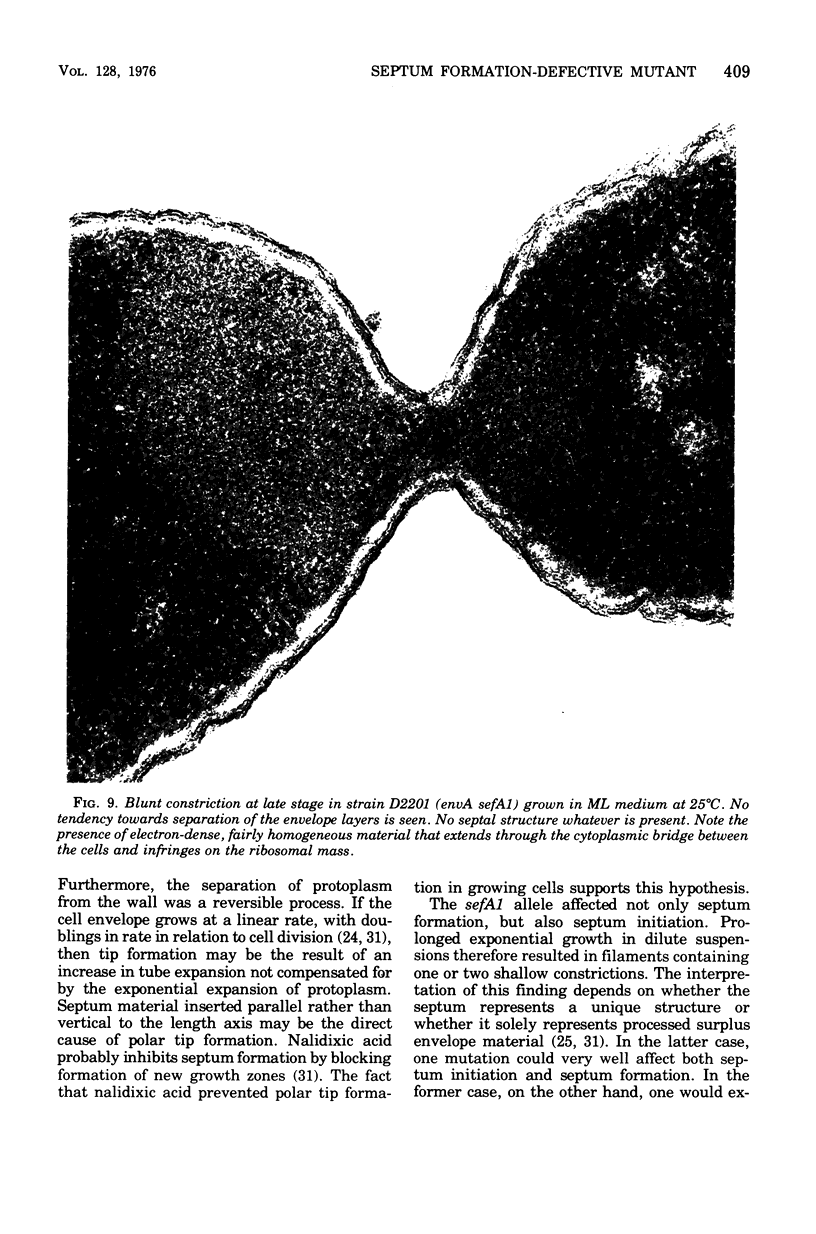
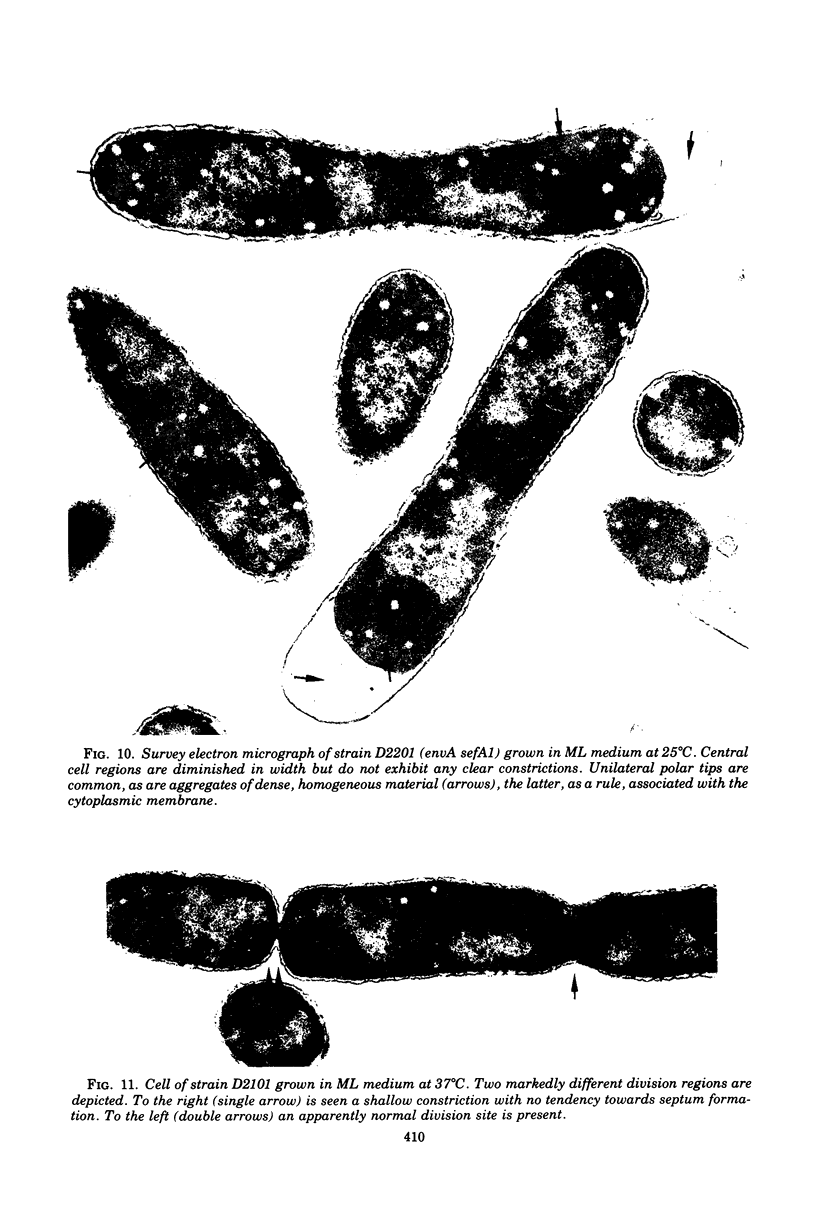
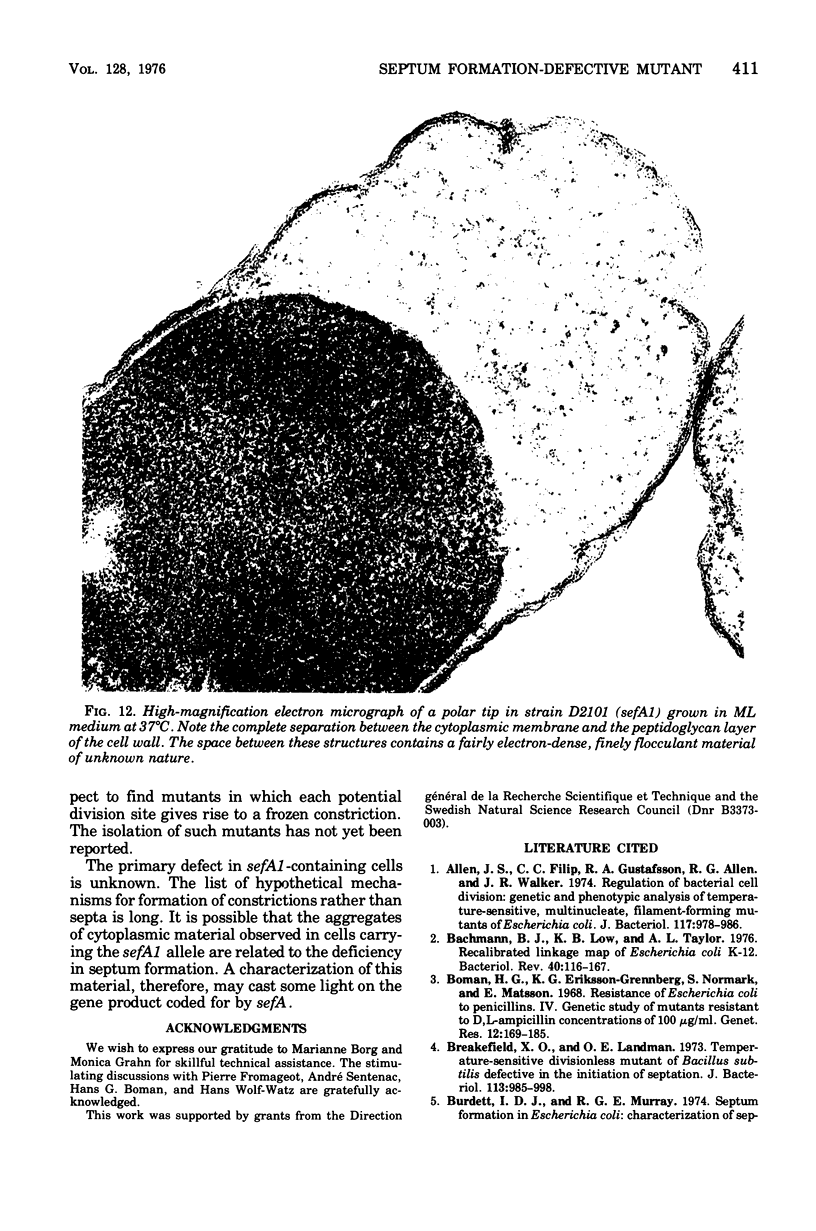
Mutants of Escherichia coli defective in septum initiation, as well as in septum formation were obtained spontaneously, without mutagenic treatment, by selection of rifampin-tolerant mutants of an antibiotic-permeable strain carrying the envA mutation. The disturbed phenotype was in all mutants aggrevated the low incubation temperatures. One allele, sefA1, was studied in detail. Septum initiation, as well as septum formation, was promoted by high cell densities or by the addition of low concentrations of certain antibiotics, e.g., rifampin and chloramphenicol, to low-density cultures. The observed rifampicin depencence was studied in detail. These experiments indicated that a very modest shift-down situation suppressed the phenotype and enabled constrictions to proceed to cell separation. The rifampicin sensitivity of the partially purified deoxyribonucleic acid polymerase was not affected by the sefA1 allele, which is located close to proA and is thus distinct from envA. Growth parameters during the shift to 25 degrees C were followed in a transductant carrying HE SEFA1 allele. This constriction was characteristically blunt and did not lead to cell separation. At the time of formation of these frozen constrictions, clear zones representing a separation of wall from cytoplasmic membrane appeared. These polar tips did not inhibit expansion of the cell envelope. The phenotype of cells carrying the sefA1 allele suggests a disturbed relationship among protoplasm expansion, envelope growth, and septum formation. It is thought that the blunt constrictions observed are caused by an inability of the two septal peptidoglycan layers to fuse during an early stage of septation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. S., Filip C. C., Gustafson R. A., Allen R. G., Walker J. R. Regulation of bacterial cell division: genetic and phenotypic analysis of temperature-sensitive, multinucleate, filament-forming mutants of Escherichia. J Bacteriol. 1974 Mar;117(3):978–986. doi: 10.1128/jb.117.3.978-986.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman H. G., Eriksson-Grennberg K. G., Normark S., Matsson E. Resistance of Escherichia coli to penicillins. IV. Genetic study of mutants resistant to D,L-ampicillin concentrations o 100 mu-g-ml. Genet Res. 1968 Oct;12(2):169–185. doi: 10.1017/s0016672300011782. [DOI] [PubMed] [Google Scholar]
- Breakefield X. O., Landman O. E. Temperature-sensitive divisionless mutant of Bacillus subtilis defective in the initiation of septation. J Bacteriol. 1973 Feb;113(2):985–998. doi: 10.1128/jb.113.2.985-998.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett I. D., Murray R. G. Electron microscope study of septum formation in Escherichia coli strains B and B-r during synchronous growth. J Bacteriol. 1974 Sep;119(3):1039–1056. doi: 10.1128/jb.119.3.1039-1056.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Burgess R. R. RNA polymerase. Annu Rev Biochem. 1971;40:711–740. doi: 10.1146/annurev.bi.40.070171.003431. [DOI] [PubMed] [Google Scholar]
- Cieśla Z., Bagdasarian M., Szczurkiewicz W., Przygońska M., Klopotowski T. Defective cell division in thermosensitive mutants of Salmonella typhimurium. Mol Gen Genet. 1972;116(2):107–125. doi: 10.1007/BF00582221. [DOI] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. G. Resistance of Escherichia coli to penicillins. II. An improved mapping of the ampA gene. Genet Res. 1968 Oct;12(2):147–156. doi: 10.1017/s0016672300011769. [DOI] [PubMed] [Google Scholar]
- Gustafsson P., Nordström K., Normark S. Outer penetration barrier of Escherichia coli K-12: kinetics of the uptake of gentian violet by wild type and envelope mutants. J Bacteriol. 1973 Nov;116(2):893–900. doi: 10.1128/jb.116.2.893-900.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Boman H. G., Bloom G. D. Cell division in a chain-forming envA mutant of Escherichia coli K12. Fine structure of division sites and effects of EDTA, lysozyme and ampicillin. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(5):651–664. doi: 10.1111/j.1699-0463.1971.tb00093.x. [DOI] [PubMed] [Google Scholar]
- Normark S., Boman H. G., Matsson E. Mutant of Escherichia coli with anomalous cell division and ability to decrease episomally and chromosomally mediated resistance to ampicillin and several other antibiotics. J Bacteriol. 1969 Mar;97(3):1334–1342. doi: 10.1128/jb.97.3.1334-1342.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S. Genetics of a chain-forming mutant of Escherichia coli. Transduction and dominance of the envA gene mediating increased penetration to some antibacterial agents. Genet Res. 1970 Aug;16(1):63–78. doi: 10.1017/s0016672300002287. [DOI] [PubMed] [Google Scholar]
- Normark S. Phenethyl alcohol as a suppressor of the envA phenotype associated with the envA gene in Escherichia coli K-12. J Bacteriol. 1971 Oct;108(1):51–58. doi: 10.1128/jb.108.1.51-58.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Wolf-Watz H. Cell division and permeability of unbalanced envelope mutants of Escherichia coli K12. Ann Microbiol (Paris) 1974 Sep;125 B(2):211–226. [PubMed] [Google Scholar]
- Reeve J. N., Groves D. J., Clark D. J. Regulation of Cell Division in Escherichia coli: Characterization of Temperature-Sensitive Division Mutants. J Bacteriol. 1970 Dec;104(3):1052–1064. doi: 10.1128/jb.104.3.1052-1064.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyn A., Birch-Andersen A., Berger U. Fine structure and taxonomic position of Neisseria haemolysans (Thjotta and Boe 1938) or Gemella haemolysans (Berger 1960). Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(3):375–389. doi: 10.1111/j.1699-0463.1970.tb04317.x. [DOI] [PubMed] [Google Scholar]
- Ricard M., Hirota Y. Process of cellular division in Escherichia coli: physiological study on thermosensitive mutants defective in cell division. J Bacteriol. 1973 Oct;116(1):314–322. doi: 10.1128/jb.116.1.314-322.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodolakis A., Thomas P., Starka J. Morphological mutants of Escherichia coli. Isolation and ultrastructure of a chain-forming envC mutant. J Gen Microbiol. 1973 Apr;75(2):409–416. doi: 10.1099/00221287-75-2-409. [DOI] [PubMed] [Google Scholar]
- Sargent M. G. Control of cell length in Bacillus subtilis. J Bacteriol. 1975 Jul;123(1):7–19. doi: 10.1128/jb.123.1.7-19.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater M., Schaechter M. Control of cell division in bacteria. Bacteriol Rev. 1974 Jun;38(2):199–221. doi: 10.1128/br.38.2.199-221.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone A. B. Regulation of cell division in a temperature-sensitive division mutant of Escherichia coli. J Bacteriol. 1973 Nov;116(2):741–750. doi: 10.1128/jb.116.2.741-750.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DE PUTTE P., VAN DILLEWIJN, ROERSCH A. THE SELECTION OF MUTANTS OF ESCHERICHIA COLI WITH IMPAIRED CELL DIVISION AT ELEVATED TEMPERATURE. Mutat Res. 1964 Jul;106:121–128. doi: 10.1016/0027-5107(64)90014-4. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Van Alstyne D., Simon M. I. Division mutants of Bacillus subtilis: isolation and PBS1 transduction of division-specific markers. J Bacteriol. 1971 Dec;108(3):1366–1379. doi: 10.1128/jb.108.3.1366-1379.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. R., Kovarik A., Allen J. S., Gustafson R. A. Regulation of bacterial cell division: temperature-sensitive mutants of Escherichia coli that are defective in septum formation. J Bacteriol. 1975 Aug;123(2):693–703. doi: 10.1128/jb.123.2.693-703.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky A., Pritchard R. H. Changes in cell size and shape associated with changes in the replication time of the chromosome of Escherichia coli. J Bacteriol. 1973 May;114(2):824–837. doi: 10.1128/jb.114.2.824-837.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]