Abstract
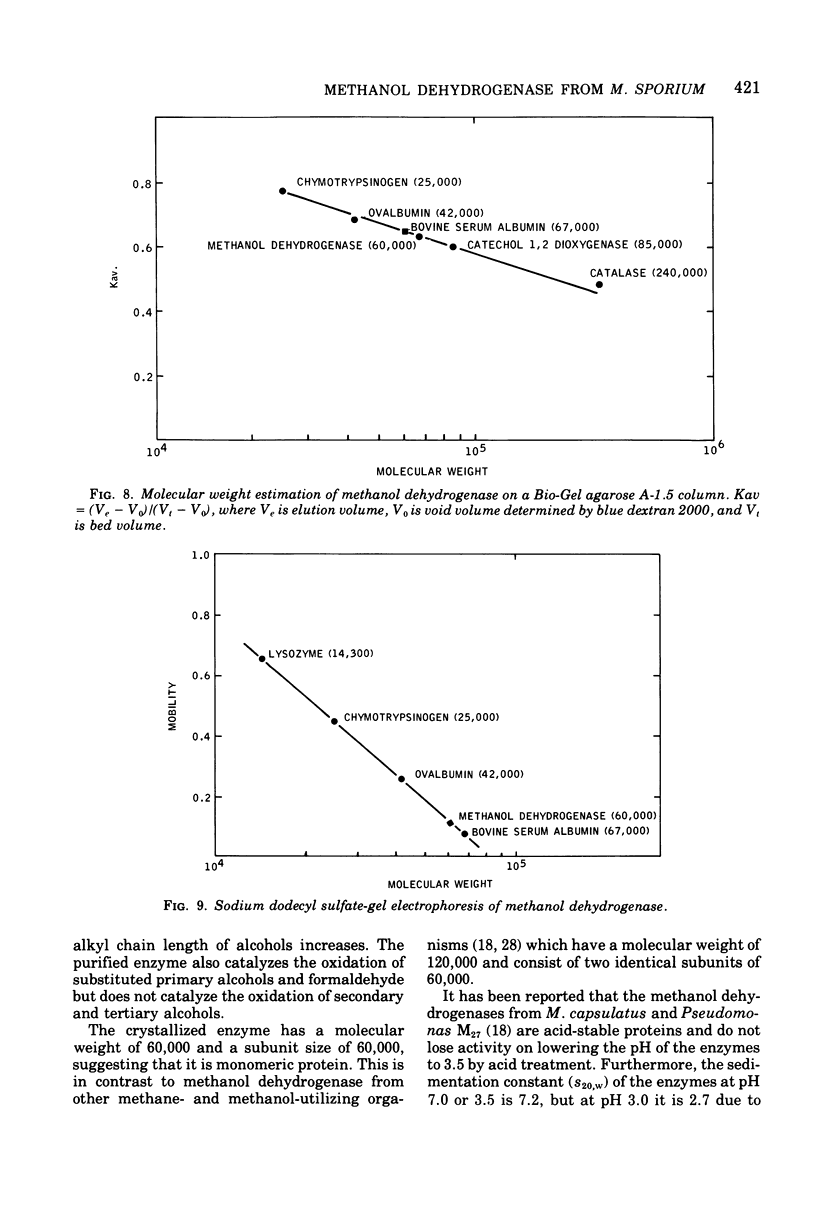
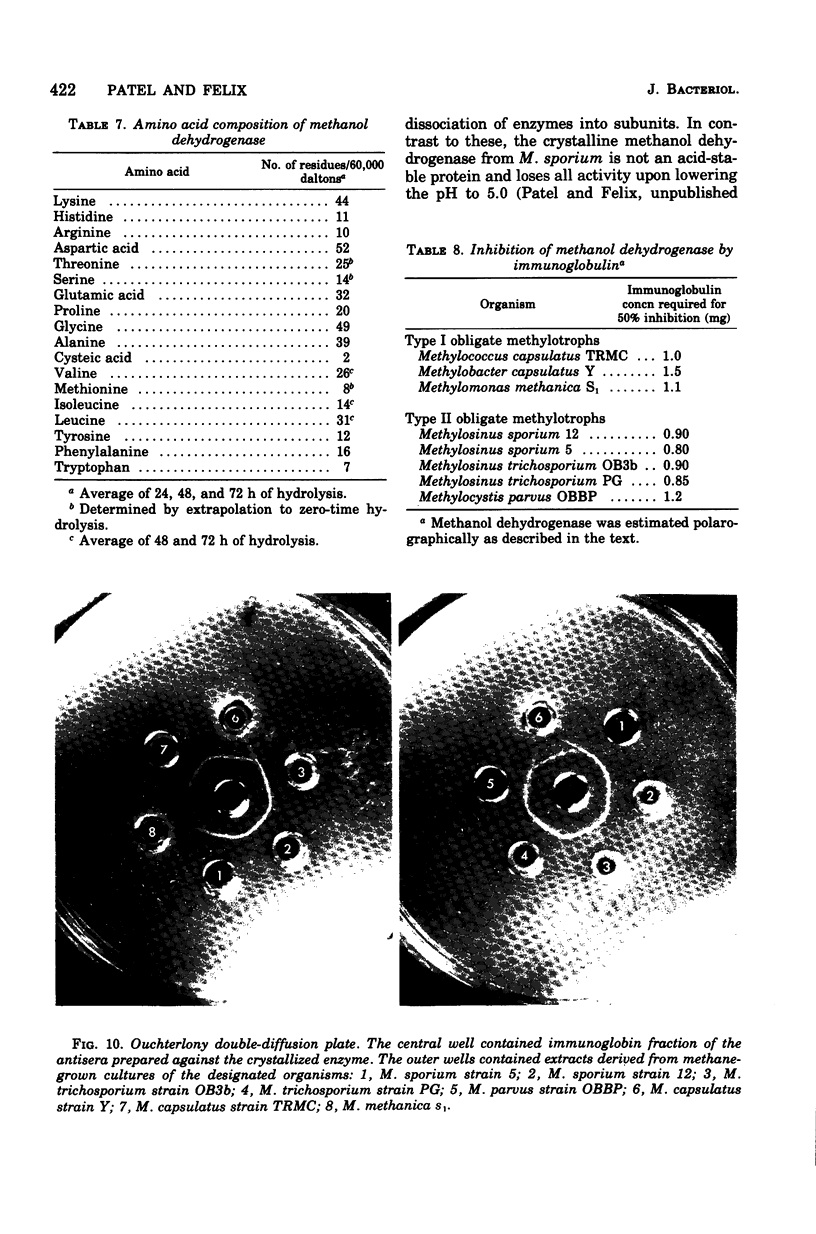
Obligate methylotrophs are divisible into two types on the basis of ultrastructural biochemical characteristics. Both groups possess a soluble phenazine methosulfate (PMS)-dependent methanol dehydrogenase. In addition, particulate PMS-dependent methanol dehydrogenase and PMS-independent methanol oxidase have been found in the type I membrane group. A procedure was developed for the crystallization of methanol dehydrogenase from the soluble fraction of the type II obligate methylotroph Methylosinus sporium. This is the first report of a crystalline methanol dehydrogenase from a methylotrophic bacterium. The crystallized enzyme is homogeneous as judged by ultracentrifugation and by acrylamide gel electrophoresis. In the presence of an electron acceptor (phenazine or phenazinium compound) and an activator (ammonium compound), the crystallized enzyme catalyzed the oxidation of primary alcohols and formaldehyde. Secondary, tertiary, and aromatic alcohols were not oxidized. The molecular weight of the enzyme as estimated by gel filtration is approximately 60,000, and as estimated by sedimentation equilibrium analysis it is 62,000. The sedimentation constant (S20,W) is 2.9. The subunit size determined by sodium dodecyl sulfate-gel electrophoresis is approximately 60,000. The amino acid composition and spectral properties of the enzyme are also presented. Antisera prepared against the crystalline enzyme are nonspecific, they cross-reacted and inhibited isofunctional enzymes from other obligate methylotrophic bacteria.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony C., Zatman L. J. The microbial oxidation of methanol. Purification and properties of the alcohol dehydrogenase of Pseudomonas sp. M27. Biochem J. 1967 Sep;104(3):953–959. doi: 10.1042/bj1040953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony C., Zatman L. J. The microbial oxidation of methanol. The prosthetic group of the alcohol dehydrogenase of Pseudomonas sp. M27: a new oxidoreductase prosthetic group. Biochem J. 1967 Sep;104(3):960–969. doi: 10.1042/bj1040960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J. F., Whittenbury R., Wilkinson J. F. The distribution in the methylobacteria of some key enzymes concerned with intermediary metabolism. Arch Mikrobiol. 1972;87(4):359–366. doi: 10.1007/BF00409135. [DOI] [PubMed] [Google Scholar]
- Davies S. L., Whittenbury R. Fine structure of methane and other hydrocarbon-utilizing bacteria. J Gen Microbiol. 1970 May;61(2):227–232. doi: 10.1099/00221287-61-2-227. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Strom T., Quayle J. R. Oxidation of carbon monoxide and methane by Pseudomonas methanica. J Gen Microbiol. 1975 Nov;91(1):79–91. doi: 10.1099/00221287-91-1-79. [DOI] [PubMed] [Google Scholar]
- Foster J. W., Davis R. H. A methane-dependent coccus, with notes on classification and nomenclature of obligate, methane-utilizing bacteria. J Bacteriol. 1966 May;91(5):1924–1931. doi: 10.1128/jb.91.5.1924-1931.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman E. P., Chichester C. O., Simpson K. L. Mechanism of the isomerization of isopentenyl pyrophosphate in Rhodotorual rubra-1. J Bacteriol. 1975 Jul;123(1):385–386. doi: 10.1128/jb.123.1.385-386.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. A., Quayle J. R. Microbial growth on C-1 compounds. 6. Oxidation of methanol, formaldehyde and formate by methanol-grown Pseudomonas AM-1. Biochem J. 1964 Nov;93(2):281–290. doi: 10.1042/bj0930281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekwick R. A. The serum proteins in multiple myelomatosis. Biochem J. 1940 Sep;34(8-9):1248–1257. doi: 10.1042/bj0341248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsubara H., Sasaki R. M. High recovery of tryptophan from acid hydrolysates of proteins. Biochem Biophys Res Commun. 1969 Apr 29;35(2):175–181. doi: 10.1016/0006-291x(69)90263-0. [DOI] [PubMed] [Google Scholar]
- Meagher R. B., Ornston L. N. Relationships among enzymes of the beta-ketoadipate pathway. I. Properties of cis,cis-muconate-lactonizing enzyme and muconolactone isomerase from Pseudomonas putida. Biochemistry. 1973 Aug 28;12(18):3523–3530. doi: 10.1021/bi00742a027. [DOI] [PubMed] [Google Scholar]
- Patel R. N., Bose H. R., Mandy W. J., Hoare D. S. Physiological studies of methane- and methanol-oxidizing bacteria: comparison of a primary alcohol dehydrogenase from Methylococcus capsulatus (Texas strain) and Pseudomonas species M27. J Bacteriol. 1972 May;110(2):570–577. doi: 10.1128/jb.110.2.570-577.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Hoare D. S. Physiological studies of methane and methanol-oxidizing bacteria: oxidation of C-1 compounds by Methylococcus capsulatus. J Bacteriol. 1971 Jul;107(1):187–192. doi: 10.1128/jb.107.1.187-192.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Mandy W. J., Hoare D. S. Physiological studies of methane- and methanol-oxidizing bacteria: immunological comparison of a primary alcohol dehydrogenase from Methylococcus capsulatus and Pseudomonas sp. M27. J Bacteriol. 1973 Feb;113(2):937–945. doi: 10.1128/jb.113.2.937-945.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R., Hoare L., Hoare D. S., Taylor B. F. Incomplete tricarboxylic acid cycle in a type I methylotroph, Methylococcus capsulatus. J Bacteriol. 1975 Jul;123(1):382–384. doi: 10.1128/jb.123.1.382-384.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patt T. E., Cole G. C., Bland J., Hanson R. S. Isolation and characterization of bacteria that grow on methane and organic compounds as sole sources of carbon and energy. J Bacteriol. 1974 Nov;120(2):955–964. doi: 10.1128/jb.120.2.955-964.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAYMOND S. A convenient apparatus for vertical gel electrophoresis. Clin Chem. 1962 Sep-Oct;8:455–470. [PubMed] [Google Scholar]
- Ribbons D. W., Harrison J. E., Wadzinski A. M. Metabolism of single carbon compounds. Annu Rev Microbiol. 1970;24:135–158. doi: 10.1146/annurev.mi.24.100170.001031. [DOI] [PubMed] [Google Scholar]
- Sahm H., Wagner F. Microbial assimilation of methanol. The ethanol- and methanol-oxidizing enzymes of the yeast Candida boidinii. Eur J Biochem. 1973 Jul 2;36(1):250–256. doi: 10.1111/j.1432-1033.1973.tb02907.x. [DOI] [PubMed] [Google Scholar]
- Sheehan B. T., Johnson M. J. Production of bacterial cells from methane. Appl Microbiol. 1971 Mar;21(3):511–515. doi: 10.1128/am.21.3.511-515.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer R. L., Wold F. A new convenient method for estimation of total cystine-cysteine in proteins. Anal Biochem. 1969 Oct 15;32(1):185–190. doi: 10.1016/0003-2697(69)90123-7. [DOI] [PubMed] [Google Scholar]
- Sperl G. T., Forrest H. S., Gibson D. T. Substrate specificity of the purified primary alcohol dehydrogenases from methanol-oxidizing bacteria. J Bacteriol. 1974 May;118(2):541–550. doi: 10.1128/jb.118.2.541-550.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Wachter D., Gasser C., Wilson A. C. Comparative immunological studies of two Pseudomonas enzymes. J Bacteriol. 1970 May;102(2):351–362. doi: 10.1128/jb.102.2.351-362.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadzinski A. M., Ribbons D. W. Oxidation of C1 compounds by particulate fractions from Methylococcus capsulatus: properties of methanol oxidase and methanol dehydrogenase. J Bacteriol. 1975 Jun;122(3):1364–1374. doi: 10.1128/jb.122.3.1364-1374.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Whittenbury R., Phillips K. C., Wilkinson J. F. Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol. 1970 May;61(2):205–218. doi: 10.1099/00221287-61-2-205. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]







