Abstract
5-HT-moduline is an endogenous tetrapeptide [Leu-Ser-Ala-Leu (LSAL)] that was first isolated from bovine brain tissue. To understand the physiological role of this tetrapeptide, we studied the localization of 5-HT-moduline binding sites in rat and mouse brains. Quantitative data obtained with a gaseous detector of β-particles (β-imager) indicated that [3H]-5-HT-moduline bound specifically to rat brain sections with high affinity (Kd = 0.77 nM and Bmax = 0.26 dpm/mm2). Using film autoradiography in parallel, we found that 5-HT-moduline binding sites were expressed in a variety of rat and mouse brain structures. In 5-HT1B receptor knock-out mice, the specific binding of [3H]-5-HT-moduline was not different from background labeling, indicating that 5-HT-moduline targets are exclusively located on the 5-HT1B receptors. Although the distribution of 5-HT-moduline binding sites was similar to that of 5-HT1B receptors, they did not overlap totally. Differences in distribution patterns were found in regions containing either high levels of 5-HT1B receptors such as globus pallidus and subiculum that were poorly labeled or in other regions such as dentate gyrus of hippocampus and cortex where the relative density of 5-HT-moduline binding sites was higher than that of 5-HT1B receptors. In conclusion, our data, based on autoradiographic localization, indicate that 5-HT-moduline targets are located on 5-HT1B receptors present both on 5-HT afferents and postsynaptic neurons. By interacting specifically with 5-HT1B receptors, this tetrapeptide may play a pivotal role in pathological states such as stress that involves the dysfunction of 5-HT neurotransmission.
Keywords: 5-HT-moduline, β-imager, serotonergic system, 5-HT1B receptor, 5-HT1B receptor knock-out mice
In the central nervous system, serotonin [5-hydroxytryptamine (5-HT)] is implicated in a wide range of physiological and behavioral functions. The 5-HT neurotransmission is mediated by several serotonergic receptors that are present either on serotonergic neurons or on postsynaptic targets (1). Recent studies indicate that there are at least 14 distinct serotonergic receptors that have been classified into subfamilies based on their structure, sequence similarities, and transduction systems (2, 3). Among them and in agreement with the recent nomenclature of the serotonin club nomenclature Committee (4), the r5-HT1B and h5-HT1B receptor subtypes play a key role in controlling the level of 5-HT in the synaptic cleft by regulating its release from rat or human nerve endings, respectively. The r5-HT1B and h5-HT1B subtypes function as autoreceptors on serotonergic terminals (5, 6). In addition, these receptor subtypes have been suggested to function as terminal heteroreceptors by regulating the release of the neurotransmitter present in corresponding nerve ending (7–10). Although r5-HT1B and h5-HT1B receptors have distinct pharmacological properties, they are distributed with similar patterns with an enrichment in basal ganglia and associated structures in the brain (11).
By controlling 5-HT levels in the synaptic cleft, r5-HT1B and h5-HT1B receptors may play an important role in neurological disorders where imbalances in serotonin neurotransmission have been reported to occur. Indeed, excessive 5-HT has been associated with anxiety, whereas 5-HT deficits have been observed in depression (12–14). Stress has been considered as a closely related factor of such disorders. We have previously shown that both auto- and hetero-presynaptic r5-HT1B receptors were desensitized immediately under stress conditions (15). Moreover, an increase in synthesis, release, and turnover of 5-HT has been observed in the brain of animals subjected to stress (16–19).
5-HT-moduline is a cerebral tetrapeptide that was recently isolated and characterized in our laboratory (20, 21). We have shown by in vivo and in vitro studies that functional activities of 5-HT1B receptor subtypes were significantly decreased by this tetrapeptide. 5-HT-moduline may interact with r5-HT1B and h5-HT1B receptors at the molecular level by noncompetitive mechanisms and at the functional level by interacting with 5-HT release (20, 21). This tetrapeptide may therefore play a fundamental role in various pathological conditions such as depression and anxiety.
Knowledge of the distribution of 5-HT-moduline binding sites in rodent brain may represent an essential step in understanding the mechanisms by which 5-HT-moduline behaves under normal physiological conditions. We undertook the present study to determine the distribution of 5-HT-moduline binding sites and to compare it with that of 5-HT1B receptors. For these purposes, we used both digital autoradiography with a newly developed high resolution β-imager, a gaseous detector of β particles (22) as described (23, 24), and film autoradiography.
MATERIALS AND METHODS
Animals and Tissue Preparation.
Adult male Wistar rats were obtained from Iffa Credo and housed in groups of five per cage. Animals were kept under controlled conditions, 20 ± 1°C temperature, 12-h light/12-h dark cycle (lights on at 6 a.m.) with water and food available ad libitum for at least 1 week before experiments. For autoradiographic studies, rats were decapitated, and their brains were removed and immediately frozen in dry ice. Ten micrometer-thick coronal sections were cut using a Reichert–Jung Cryostat at −20°C, thaw-mounted onto gelatin-coated glass slides, dried under cold air-stream, and processed for autoradiography.
Constitutive 5-HT1B receptor knock-out mice were generated by homologous recombination (25). Mutant brains were a generous gift of R. Hen (Columbia University, New York). These brains were dissected, frozen, and processed as described above.
Autoradiography.
5-HT-moduline binding sites were detected by using [Leu1,4-3H]-5-HT-moduline (8.95 Tbq/mmol) synthesized by Amersham as the radioactive ligand. Experimental binding conditions were adapted from those reported previously for brain membranes (21) with slight modifications as described below. Brain sections were successively pre-incubated in buffer A containing 0.1 mM GTP and in buffer B at room temperature for 20 min. Buffer A consisted of 50 mM Tris⋅HCl (pH 7.4) and buffer B was a 50 mM Tris⋅HCl (pH 7.4) containing 0.3 mg/ml of bacitracin, 0.1% BSA, 2 mM EDTA, 0.1 mM phenylmethylsulfonyl fluoride, and 5 units/liter aprotinin. Sections were then rinsed in buffer A and air-dried at room temperature. Dried sections were incubated overnight in buffer A in the presence of [3H]-5-HT-moduline (0.1 to 1.5 nM) at 4°C, and then rinsed twice in ice-cold (4°C) buffer A for 10 min. Finally, the sections were dipped quickly in distilled water (4°C) to remove excess salts and air-dried. Nonspecific binding was assessed on adjacent sections by adding 1 μM of nonradioactive 5-HT-moduline tetrapeptide to buffer A. For 5-HT1B receptor labeling, sections were preincubated twice for 15 min in 170 mM Tris⋅HCl buffer (pH 7.4) containing 150 mM of NaCl. This was followed by an incubation for 2 hr at room temperature in the same buffer supplemented with 20 pM [125I]iodocyanopindolol (Amersham) containing both 20 μM isoproterenol, which masks β-adrenoreceptors, and 0.2 μM (±)8-hydroxy-2-(di-npropylamino)tetralin (8-OHDPAT) to mask the 5-HT1A receptors. Then, the sections were washed twice in 170 mM Tris⋅HCl buffer (4°C) for 15 min, rinsed in cold distilled water, and air-dried. Nonspecific binding was determined by adding 1 μM of 5-methoxy-3-(1,2,3,6-tetrahydro-4-pyridinyl)-1H-indole (RU 24969). Dried sections were exposed to 3H-Hyperfilm in x-ray cassettes (Amersham) either for 50 days and 2 days with [3H]5-HT-moduline and [125I]iodocyanopindolol, respectively. Films were developed in Kodak D19 for 3 min and the sections were stained with toluidine blue. Brain structures were identified by referring to atlases of rat (26, 27) and mouse brains (28).
Quantitative Analysis.
Specific binding was determined in each region by the difference between total and nonspecific binding. Optical densities of autoradiograms were measured using a microcomputer-based image analysis system (RAG 200 Biocom, Les Ulis, France). Absolute quantification of [3H]-5-HT binding sites was performed on a high resolution β-imager (Biospace, France), which provides an accurate linear detection of dpm (24). Counting of β particles emitted was assessed using a delimited window placed over different brain areas. Consequently, results have been expressed in dpm/mm2.
RESULTS
Characteristics of [3H]-5-HT-Moduline Binding.
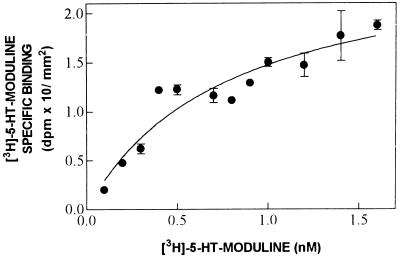
Saturation experiments were performed by incubating rat brain sections with increasing concentrations of [3H]-5-HT-moduline. The specific binding was saturable with an estimated Kd = 0.77 nM and a corresponding Bmax = 0.26 dpm/mm2 (Fig. 1). This is equivalent to 0.48 attomole/mm2.
Figure 1.
Representative saturation analysis of [3H]-5-HT-moduline specific binding. Slide-mounted sections of rat brain were incubated with increasing concentrations of [3H]-5-HT-moduline. Binding was measured using β-imager by quantitative autoradiography in whole slices (midbrain level). Each point represents the mean ± SE of triplicate determinations. Transformation of the data into Scatchard coordinates indicated a single site with a Kd = 0.77 nM and Bmax = 0.26 dpm/mm2 as binding parameters. These experiments were performed twice using two sets of animals, with similar results.
Distribution of [3H]-5-HT-Moduline Binding Sites in Rat Brain.
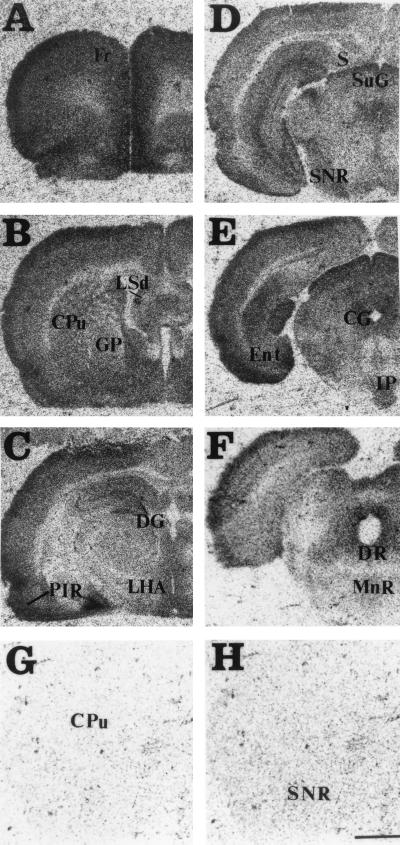
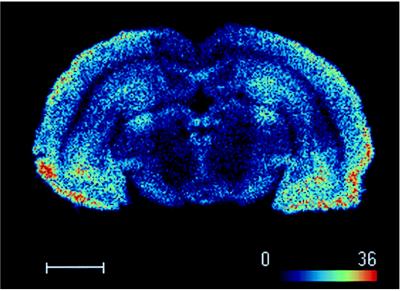
[3H]-5-HT-moduline binding sites were mapped in consecutive coronal sections of rat brain by film autoradiography. The autoradiograms revealed that the binding sites were distributed heterogeneously in the brain (Fig. 2 A–F). Incubation with 1 μM unlabeled 5-HT-moduline resulted in a complete displacement of [3H]-5-HT-moduline binding from rat brain sections (Fig. 2 G and H). Results of the semiquantitative analysis obtained from film autoradiograms are summarized in Table 1. The estimated maximal and minimal densities of binding sites in different brain regions were obtained by quantification with the β-imager (Table 1, legend). The highest binding densities were found in septum, olfactory system, frontoparietal, and cingulate cortices. In septum, the strongest signals were observed in lateral septal nuclei, horizontal limb diagonal band, whereas in olfactory system, the primary olfactory cortex and the anterior olfactory nucleus were the predominant labeled structures. Other areas with significant [3H]-5-HT-moduline binding sites included the amygdala, hypothalamus, frontal, entorhinal and cingulate cortices, accumbens nucleus, ventral pallidum, dentate gyrus of hippocampus, superficial gray layer of the superior colliculus. Table 1 contains the complete list of the regions with significant binding sites. Moderate labeling was observed in globus pallidus, caudate putamen, CA1-CA3 fields of Ammon’s horn in hippocampus, some thalamic nuclei, substantia nigra, central gray, interpeduncular nucleus, and dorsal raphe nucleus. The dorsal subiculum, entopeduncular nucleus, subthalamic nucleus, and ventral thalamic nuclei revealed a low density of binding sites (Table 1). [3H]-5-HT-moduline binding sites were also observed in fiber tracts such as olfactory tract and anterior commissure. Fig. 3 shows a digital autoradiogram of [3H]-5-HT-moduline binding sites in rat midbrain section using the β-imager.
Figure 2.
(A–F) Distribution of [3H]-5-HT-moduline binding in antero-posterior coronal sections of rat brain. Sections (10 μM) were incubated with 1.5 nM of [3H]-5-HT-moduline as described in text and apposed to 3H-Hyperfilm for 50 days. (G and H) Nonspecific binding was defined in the presence of 1 μM unlabeled 5-HT-moduline. CG, central gray; CPu, caudate putamen; DG, dentate gyrus of hippocampus; DR: dorsal raphe; Ent, Entorhinal cortex; Fr, frontal cortex; GP, globus pallidus; IP, interpeduncular nucleus; LHA, lateral hypothalamus area; LSd, lateral septum, dorsal part; MnR, median raphe nucleus; PIR, pyriform cortex; S, subiculum; SNR, substantia nigra, reticular part; SuG, superficial gray layer of the superior colliculus. (Bar = 2 mm.)
Table 1.
Comparative regional distribution of 5-HT-moduline and 5-HT1B binding sites in rat brain
| Area | Relative grain density
|
|
|---|---|---|
| 5-HT-moduline | 5-HT1B | |
| Olfactory system | ||
| Primary olfactory cortex | ++++ | ++(+) |
| Anterior olfactory nucleus | ++++ | ++ |
| Lateral olfactory tract | ++ | ND |
| Olfactory tubercle | +++ | +++ |
| Amygdala | ||
| Central amygdaloid nucleus | +++ | +++ |
| Medial amygdaloid nucleus | +++ | ++(+) |
| Basolateral amygdaloid nucleus | +++ | +++ |
| Septal region | ||
| Lateral septal nucleus | ++++ | +++ |
| Septohippocampal nucleus | +++ | ++++ |
| Bed nucleus of the stria terminalis | +++ | ++ |
| Vertical limb diagonal band | +++ | ND |
| Horizontal limb diagonal band | ++++ | ND |
| Anterior commissure | ++ | ND |
| Basal ganglia | ||
| Accumbens nucleus | +++ | ++++ |
| Globus pallidus | ++ | ++++ |
| Ventral pallidum | +++ | ++++ |
| Caudate putamen | ++ | +++ |
| Subthalamic nucleus | −(+) | +++ |
| Entopeduncular nucleus | −(+) | +++ |
| Hippocampus | ||
| Dentate gyrus | +++ | ++ |
| CA1 field, oriens layer | ++ | +* |
| CA1 field, radiatum layer | ++ | +(+)* |
| CA3 field, oriens layer | ++ | ++ |
| CA3 field, radiatum layer | ++ | ND |
| Dorsal subiculum | + | ++++ |
| Hypothalamus | ||
| Lateral hypothalamic area | ++ | +++(+) |
| Ventromedial hypothalamic area | +++ | +++ |
| Anterior hypothalamic nucleus | +++ | +++(+) |
| Paraventricular hypothalamic nucleus | +++ | ND |
| Thalamus | ||
| Paraventricular thalamic nucleus, anterior part | ++ | ++ |
| Mediodorsal thalamic nucleus | ++ | +++(+) |
| Laterodorsal thalamic nucleus | ++ | ++ |
| Central medial thalamic nucleus | ++ | +++ |
| Ventral anterior lateral complex thalamus | + | ND |
| Ventral posteromedial thalamic nucleus | + | + |
| Paraventricular thalamic nucleus | + | +++(+) |
| Lateral post thalamic nucleus | ++ | +++ |
| Visual system | ||
| Superficial gray layer of the superior colliculus | +++ | ++++ |
| Optic nerve layer of the superior colliculus | +++ | ++(+) |
| Midbrain | ||
| Central gray | ++ | ++++ |
| Substantia nigra | ++ | ++++ |
| Interpeduncular nucleus | ++ | ++++ |
| Ventral tegmental area | + | +++ |
| Dorsal raphe nucleus | ++ | +++ |
| Median raphe nucleus | +(+) | +* |
| Anterior pretectal nucleus | + | ND |
| Deep mesencephalic nucleus | +(+) | ND |
| Claustrum, neo, cingulate, and entorhinal cortex | ||
| Area | Relative grain density | |
| 5-HT-moduline | 5-HT1B | |
| Claustrum | ++ | +++ |
| Frontal cortex | +++ | +++ |
| Frontal parietal cortex | +++(+) | ++ |
| Striate cortex, areas 17 and 18 | ++ | + |
| Retrospenial cortex | + | +++ |
| Entorhinal cortex | +++ | +++ |
| Cingulate cortex | +++(+) | ND |
Brain sections were labeled as described in the Materials and Methods with 1.5 nM of [3H]-5-HT-moduline and apposed to 3H-Hyperfilm for 50 days. Nonspecific binding was defined in the presence of 1 μM unlabeled 5-HT-moduline. Gray levels were analyzed on autoradiograms and converted to a four-point relative scale. The corresponding binding intensities were quantified using the images obtained with the β-imager. In dpm/mm2: −, 0 to 0.12; +, 0.13 to 0.24; ++, 0.25 to 0.36; +++, 0.37 to 0.48; ++++, 0.49 to 0.6. Comparison with 5-HT1B binding sites was obtained by converting quantitative data of Bruinvels et al. (11) and Pazos et al. (29)* to a four-point relative scale. ND, not determined.
Figure 3.
Digital autoradiogram of [3H]-5-HT-moduline binding sites on rat midbrain section obtained with the β-imager. Brain sections were labeled as described in text with 1.5 nM of [3H]-5-HT-moduline and β-particles emitted were detected by β-imager. Note that binding is concentrated in regions such as cortex, central gray, hippocampus and substantia nigra. (Bar = 2.6 mm.)
5-HT-Moduline Binding Sites in Wild-Type and in 5-HT1B Receptor Knock-Out Mice.
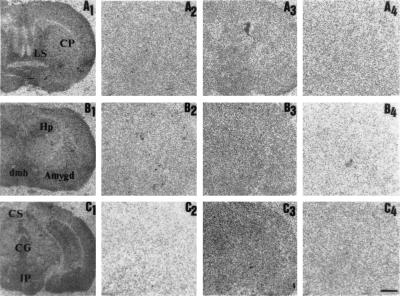
To determine whether the binding of 5-HT-moduline was restricted to the 5-HT1B receptor subtype as shown previously (20), we studied the distribution pattern of [3H]-5-HT-moduline in wild-type and mutant mice lacking the 5-HT1B gene by film autoradiography. Similar to our observations with rat brains, total [3H]-5-HT-moduline binding was completely displaced when wild-type mouse brain sections were incubated in the presence of 1 μM unlabeled 5-HT-moduline (Fig. 4 A2–C2). Moreover, the distribution pattern of labeling in mice brain regions as shown on Fig. 4 A1–C1 was identical to that observed in rat brain (Fig. 2). Indeed, a high density of binding sites was detected in regions such as septum, hippocampus, cortex, amygdala, and hypothalamus. In brain sections of 5-HT1B knock-out mice, total binding (Fig. 4 A3–C3) was not significantly distinct from nonspecific binding (Fig. 4 A4–C4).
Figure 4.
[3H]-5-HT-moduline binding sites in coronal sections of wild-type and 5-HT1B receptor knock-out 5-HT1B mice. The levels of forebrain (A), basal ganglia (B), and midbrain (C). Autoradiograms showing total [3H]-5-HT-moduline binding [3H]-5-HT-moduline binding (A1–C1 and A3–C3) or nonspecific binding (A2–C2 and A4–C4) in control and mutant mice, respectively. Brain sections were labeled as described in text with 1.5 nM of [3H]-5-HT-moduline and apposed to 3H-Hyperfilm for 50 days. Nonspecific binding was defined in the presence of 1 μM unlabeled 5-HT-moduline. Note the absence of specific binding in mice lacking the 5-HT1B receptor. Amygd, amygdala; CG, central gray; CP, caudate putamen; CS, colliculus superior; dmh, nucleus dorsomedialis hypothalami; GP, globus pallidus; Hp, hippocampus; IP, interpeduncular nucleus. (Bar = 1 mm.)
Comparative Distribution of 5-HT-Moduline Binding Sites and 5-HT1B Receptors.
Since 5-HT1B receptors represent the specific target for 5-HT-moduline binding on brain membranes, we compared the distribution of 5-HT-moduline binding sites with that of 5-HT1B receptors (Fig. 5). In agreement with previous data (11) reported in Table 1, the highest densities of 5-HT1B receptors were found in globus pallidus, substantia nigra, dorsal subiculum, superficial gray layer of the superior colliculus, and stratum lacunosum moleculare of the CA1 field of hippocampus (Fig. 5 A′ and B′). The distributions of 5-HT-moduline binding sites and 5-HT1B receptors were quite similar, particularly at the midbrain level as illustrated in Fig. 5 B–B′. However, in proportion to the total amount, much less 5-HT-moduline binding was observed in regions such as globus pallidus and dorsal subiculum. These regions, however, contain high densities of 5-HT1B receptors as shown by 125I-iodocyanopindolol labeling. Whereas 5-HT1B receptors were dense and distributed homogeneously within globus pallidus (Fig. 5A′), [3H]-5-HT-moduline binding did not represent a homogeneous distribution. The binding density was lower in the medial than in the lateral section of this structure (Figs. 2B and 5A).
Figure 5.
Comparative distribution of [3H]-5-HT-moduline binding sites and 5-HT1B receptors on coronal sections of rat brain. Autoradiograms showing total [3H]-5-HT-moduline and [125I]cyanopindolol binding at the level of basal ganglia (A and A′) and midbrain (B and B′), respectively. Brain sections were labeled as described in text with either 1.5 nM of [3H]-5-HT-moduline or 20 pM [125I]cyanopindolol and apposed to 3H-Hyperfilm for 50 or 2 days, respectively. CPu, caudate putamen; GP, globus pallidus; S, subiculum; SN, substantia nigra; SuG, superficial gray layer of the superior colliculus. (Bar = 2 mm.)
DISCUSSION
In the present study, we combined digital and film autoradiography to characterize [3H]-5-HT-moduline binding sites in rat and mouse brain. Digital autoradiography experiments were carried out by using the newly developed high resolution β-radio imager (22), which allows the rapid detection and accurate quantification of the binding of radioactive ligands to tissue sections. Whereas several months would have been required with the film autoradiography method, results of the [3H]-5-HT-moduline saturation curve were available within 6 days using β-imager. Nevertheless, we used the former technique as a complementary method since the film provides a better spacial resolution that is required for accurate identification of brain structures.
Binding conditions were adapted from those described previously (21). The binding of [3H]-5-HT-moduline to rat brain slices reached an equilibrium after 6 hr at 4°C. The temperature was maintained at 4°C in these experiments to protect the tetrapeptide from degradation. These results were similar to those reported previously with rat cortical membrane preparations (21).
The saturation studies showed that [3H]-5-HT-moduline binds to a single population of binding sites with high affinity (Kd = 0.77 nM). This value is similar to those reported previously in rat and guinea pig brain homogenates as well as in transfected cells expressing rat 5-HT1B or human 5-HT1B receptors (21). [3H]-5-HT-moduline binding was totally displaced by unlabeled 5-HT-moduline, whereas other peptides such as enkephalines did not displace the binding significantly. Similar data have been obtained in guinea pig cortical homogenates, indicating that native 5-HT-moduline among all the other structural derivatives tested was the most efficient peptide in displacing the binding of the labeled molecule (21). This indicates that the binding site is specific for 5-HT-moduline.
Using both film and digital autoradiographic approaches, our data showed that 5-HT-moduline binding sites were expressed in several regions of rat and mouse brain. Previous binding studies using rat brain cortical homogenates indicated that 5-HT1B receptors were the specific targets for this tetrapeptide (21). Since the 5-HT1B receptors are predominantly expressed on the terminals of serotonergic and nonserotonergic neurons, we were interested in comparing the regional distribution of 5-HT-moduline sites with that of 5-HT1B receptors. In a first series of experiments, the distribution of 5-HT1B receptors was examined; our results were in good agreement with previous data (11, 29), showing the presence and distribution of 5-HT1B binding sites in various regions of the brain. The second series of experiments showed that 5-HT-moduline binding sites and 5-HT1B receptors follow similar patterns of distribution (Fig. 5 and Table 1). This observation is particularly true in regions that contain intermediate levels of 5-HT1B receptors. These regions included nucleus accumbens, caudate putamen, ventral pallidum, hypothalamus, amygdala, superficial gray layer of the superior colliculi, central gray, thalamic nuclei, and septum. 5-HT-moduline binding sites were also found in fiber tracts such as anterior commissure and stria terminalis in accordance with previous data regarding the 5-HT1B recognition sites (11). Some discrepancies were observed, however, both in regions containing high levels of 5-HT1B receptors such as globus pallidus, subiculum, and subthalamic and entopeduncular nuclei, which were found to be poorly labeled by [3H]-5-HT-moduline, and in regions such as hippocampus and some cortical areas where 5-HT-moduline binding sites were more dense than 5-HT1B receptors as related to the total amount of 5-HT-moduline binding. Among the regions studied, we did not observe any exclusive expression of either 5-HT-moduline or 5-HT1B recognition sites in any region. However, the distribution of 5-HT-moduline binding between hippocampal layers did not entirely correspond to that of 5-HT1B receptors. For example, we found that the stratum lacunosum moleculare of the CA1 and CA3 fields and the dentate gyrus contained significant amount of 5-HT-moduline binding sites, whereas the 5-HT1B receptors were rather concentrated in the stratum lacunosum moleculare of the CA1 field of Ammon’s horn (Fig. 5 B–B′).
The 5-HT1B receptors are located on the terminals of both serotonergic and nonserotonergic neurons that represent auto- and heteroreceptors, respectively. To determine whether both of these receptors represent targets for 5-HT-moduline, we compared the binding site distribution of this tetrapeptide with that of 5-HT terminals that were previously identified by the specific labeling of the serotonin transporter (30, 31). We observed the 5-HT-moduline binding sites in brain regions such as frontal cortex, septum, superior colliculus, amygdala, substantia nigra, hypothalamus, and raphe nuclei that have been reported to be rich in serotonergic innervation (32). In addition, other structures such as hippocampus, caudate putamen, and globus pallidus, which contain less 5-HT terminals than these former regions, also exhibited significant [3H]-5-HT-moduline binding. This suggests that 5-HT-moduline targets are present on both the serotonergic and the nonserotonergic neurons. However, as discussed earlier, we did not find a complete overlap between 5-HT-moduline binding sites and 5-HT1B receptors. One hypothesis to explain the differences in the distribution of 5-HT-moduline binding sites with that of 5-HT1B receptors could be that 5-HT-moduline, in addition to its interaction with 5-HT1B receptors, may also interact with other unknown target sites. Our data show that the 5-HT-moduline binding is absent in 5-HT1B receptor knock-out mice that lack 5-HT1B receptors and therefore exclude this hypothesis. These results demonstrate that 5-HT-moduline interacts specifically with 5-HT1B receptors and indicate that not only presynaptic 5-HT1B receptors but also postsynaptic 5-HT1B receptors are the targets for 5-HT-moduline. Another possible explanation, as reported previously, is that the constitutive knock-out mice may have suffered compensatory changes during development that led to misinterpretation of their phenotype (33). Although earlier autoradiographic studies have suggested that r5-HT1D (previously called 5-HT1Dα) receptors are present in 5-HT1B knock-out mice (34), it is unlikely that 5-HT-moduline interacts with these receptors since no significant residual labeling was observed on 5-HT1B knock-out brain sections in our experiments.
Using rat brain membranes under similar experimental conditions, it was shown that the total number of binding sites for 5-HT-moduline was ten times lower than that of 5-HT1B receptors (21). Our results show the same lack of correlation between Bmax values for 5-HT-moduline and 5-HT1B receptors when binding values were expressed in fmol/mg protein using standard scintillation techniques (data not shown). The Bmax values of [3H]-5-HT-moduline and [125I]cyanopindolol bindings were reported to be in the same order of magnitude in experiments conducted on brain homogenates at room temperature (21). Although we did not observe changes in Bmax values due to variation in incubation temperature when frozen tissue sections were used (data not shown), we cannot exclude the possibility that the 5-HT1B receptor protein may exist under different conformations such as those reported for other G-protein-coupled receptors that bind allosteric modulators (35). Therefore, 5-HT-moduline binding may depend on the state of conformation of the 5-HT1B receptor that in turn constitutes the interaction site for 5-HT-moduline. However, this does not explain why the proportion of receptors in the conformation that binds 5-HT-moduline differs between regions. It is reasonable to assume that receptors with high affinity for [3H]-5-HT-moduline may not have an even distribution among all brain regions. Another possibility would be that the existing pool of endogenous 5-HT-moduline prevents the exogenous radioactive ligand from binding by partial occupation of its target sites. Moreover, the endogenous 5-HT-moduline that is present locally at different concentrations may play an inhibitory role and provide an explanation for the incomplete patterns and overlap of 5-HT-moduline binding sites with that of 5-HT1B receptors.
It has been reported that the interaction of 5-HT-moduline with 5-HT1B receptors leads to a rapid decrease in receptor response and sensitivity (21). By measuring the inhibitory effect of a 5-HT1B agonist on forskolin-stimulated adenylyl cyclase activity, it was shown that the intracerebroventricular injection of 5-HT-moduline decreases the sensitivity of 5-HT1B receptors within 15 min (36). Using the same experimental approach to measure 5-HT1B receptor function, such rapid desensitization has also been observed under acute stress conditions (15). The desensitization of 5-HT1B receptors may reduce the efficacy of the negative feedback mechanisms on 5-HT neurotransmission. This will lead to an increased level of extracellular 5-HT as observed under acute stress conditions. These observations suggest that desensitization of the 5-HT1B receptors is likely due to the interaction of 5-HT-moduline with these receptors under these conditions. However, further studies are necessary to fully understand the precise mechanism underlying this effect.
In conclusion, the present autoradiographic studies clearly extend the results obtained in biochemical studies and further demonstrate an interaction between 5-HT-moduline and 5-HT1B receptors; they also indicate that the interaction affects the 5-HT1B autoreceptors and the heteroreceptors. The functional consequences of this interaction leading to a control of the 5-HT activity are physiologically significant. The serotonergic system is known to play an important role in diverse physiological functions such as behavior, learning, and memory as well as in pathologies such as stress, anxiety and depression. The β-imager technique used in the present work provides the most rapid and accurate method for the assessment of changes in 5-HT-moduline binding sites and allows visualization of structures potentially involved in stress mechanisms in the central nervous system.
ABBREVIATION
- 5-HT
5-hydroxytryptamine (serotonin)
References
- 1.Zifa E, Fillion G. Pharmacol Rev. 1992;44:401–457. [PubMed] [Google Scholar]
- 2.Boess F G, Martin I L. Neuropharmacology. 1994;33:275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 3.Hoyer D, Clarke D E, Fozard J R, Hartig P R, Martin G R, Mylecharane E J, Saxena P R, Humphrey P P A. Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 4.Hartig P R, Hoyer D, Humphrey P P A, Martin G R. Trends Pharmacol Sci. 1996;17:103–105. doi: 10.1016/0165-6147(96)30002-3. [DOI] [PubMed] [Google Scholar]
- 5.Engel G, Göthert M, Hoyer D, Schlicker E, Hillenbrand K. Naunyn-Schmiedeberg’s Arch Pharmacol. 1986;332:1–7. doi: 10.1007/BF00633189. [DOI] [PubMed] [Google Scholar]
- 6.Maura G, Roccatagliata E, Raiteri M. Naunyn-Schmiedeberg’s Arch Pharmacol. 1986;334:323–326. doi: 10.1007/BF00569364. [DOI] [PubMed] [Google Scholar]
- 7.Maura G, Raiteri M. Eur J Pharmacol. 1986;129:333–337. doi: 10.1016/0014-2999(86)90443-7. [DOI] [PubMed] [Google Scholar]
- 8.Harel-Dupas C, Cloëz I, Fillion G. J Neurochem. 1991;56:221–227. doi: 10.1111/j.1471-4159.1991.tb02584.x. [DOI] [PubMed] [Google Scholar]
- 9.Boschert U, Aït-Amara D, Segu L, Hen R. Neuroscience. 1994;58:167–182. doi: 10.1016/0306-4522(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 10.Bruinvels A T, Landwehrmeyer B, Gustafson E L, Durkin M M, Mengod G, Branchek T A, Hoyer D, Palacios J M. Neuropharmacology. 1994;33:367–386. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 11.Bruinvels A T, Palacios J M, Hoyer D. Naunyn-Schmiedeberg’s Arch Pharmacol. 1993;347:569–582. doi: 10.1007/BF00166939. [DOI] [PubMed] [Google Scholar]
- 12.Eison M S. J Clin Psychopharmacol. 1990;10:26S–30S. [PubMed] [Google Scholar]
- 13.Anisman H, Zacharko R M. Br J Psychiatry. 1992;160:36–43. [PubMed] [Google Scholar]
- 14.Chaouloff F. Brain Res Rev. 1993;18:1–32. doi: 10.1016/0165-0173(93)90005-k. [DOI] [PubMed] [Google Scholar]
- 15.Bolanos-Jimenez F, Manhaes de Castro R, Seguin L, Cloëz-Tayarani I, Monneret V, Drieu K, Fillion G. Eur J Pharmacol. 1995;294:531–540. doi: 10.1016/0014-2999(95)00590-0. [DOI] [PubMed] [Google Scholar]
- 16.Kennet G A, Joseph M H. Neuropharmacology. 1981;20:39–43. doi: 10.1016/0028-3908(81)90039-3. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu N, Take S, Hori T, Oomura Y. Brain Res Bull. 1992;28:727–734. doi: 10.1016/0361-9230(92)90252-s. [DOI] [PubMed] [Google Scholar]
- 18.Joseph M H, Kennet G A. Brain Res. 1983;270:251–257. doi: 10.1016/0006-8993(83)90598-x. [DOI] [PubMed] [Google Scholar]
- 19.Dunn A J. Life Sci. 1988;42:1847–1853. doi: 10.1016/0024-3205(88)90023-9. [DOI] [PubMed] [Google Scholar]
- 20.Rousselle J C, Massot O, Delepierre M, Zifa E, Rousseau B, Fillion G. J Biol Chem. 1996;271:726–735. doi: 10.1074/jbc.271.2.726. [DOI] [PubMed] [Google Scholar]
- 21.Massot O, Rousselle J C, Fillion M-P, Grimaldi B, Cloëz-Tayarani I, Fugelli A, Prudhomme N, Seguin L, Rousseau B, Plantefol M, Hen R, Fillion G. Mol Pharmacol. 1996;50:752–762. [PubMed] [Google Scholar]
- 22.Charpak G, Dominick W, Zaganidis N. Proc Natl Acad Sci USA. 1989;86:1741–1745. doi: 10.1073/pnas.86.6.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubois-Dauphin M, Théler J-M, Zaganidis N, Dominick W, Tribollet E, Pévet P, Charpak G, Dreifuss J-J. Proc Natl Acad Sci USA. 1991;88:11163–11167. doi: 10.1073/pnas.88.24.11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Théler J M, Lakhdar-Ghazal N, Pévet P, Charpak G, Dominick W, Zaganidis N, Dreifuss J-J, Dubois-Dauphin M. J Neurosci Methods. 1993;49:231–240. doi: 10.1016/0165-0270(93)90128-e. [DOI] [PubMed] [Google Scholar]
- 25.Saudou F, Aït Amara D, Dierich A, LeMeur M, Ramboz S, Segu L, Buhot M-C, Hen R. Science. 1994;265:1875–1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- 27.Swanson L W. Brain Maps: Structure of the Brain. Amsterdam: Elsevier; 1992. [Google Scholar]
- 28.Sidman R L, Angevine J B, Jr, Taber Pierce E. Atlas of the Mouse Brain and Spinal Cord. Cambridge, MA: Harvard Univ. Press; 1971. [Google Scholar]
- 29.Pazos A, Palacios J M. Brain Res. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- 30.De Souza E B, Kuyatt B. Synapse. 1987;1:488–496. doi: 10.1002/syn.890010513. [DOI] [PubMed] [Google Scholar]
- 31.Fuxe K, Calza L, Benfenati F, Zini I, Agnati L F. Proc Natl Acad Sci USA. 1983;80:3836–3840. doi: 10.1073/pnas.80.12.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinbush H W M. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- 33.Grailhe R, Hen R. Semin Neurosci. 1995;7:395–400. [Google Scholar]
- 34.Ramboz S, Saudou F, Aït Amara D, Betzung C, Segu L, Misslin R, Buhot M-C, Hen R. Behav Brain Res. 1996;73:305–312. doi: 10.1016/0166-4328(96)00119-2. [DOI] [PubMed] [Google Scholar]
- 35.Tucek S, Proska J. Trends Pharmacol Sci. 1995;16:205–212. doi: 10.1016/s0165-6147(00)89023-9. [DOI] [PubMed] [Google Scholar]
- 36.Seguin L, Seznec J-C, Fillion G. Neurosci Res. 1997;27:277–280. doi: 10.1016/s0168-0102(96)01150-9. [DOI] [PubMed] [Google Scholar]