Abstract
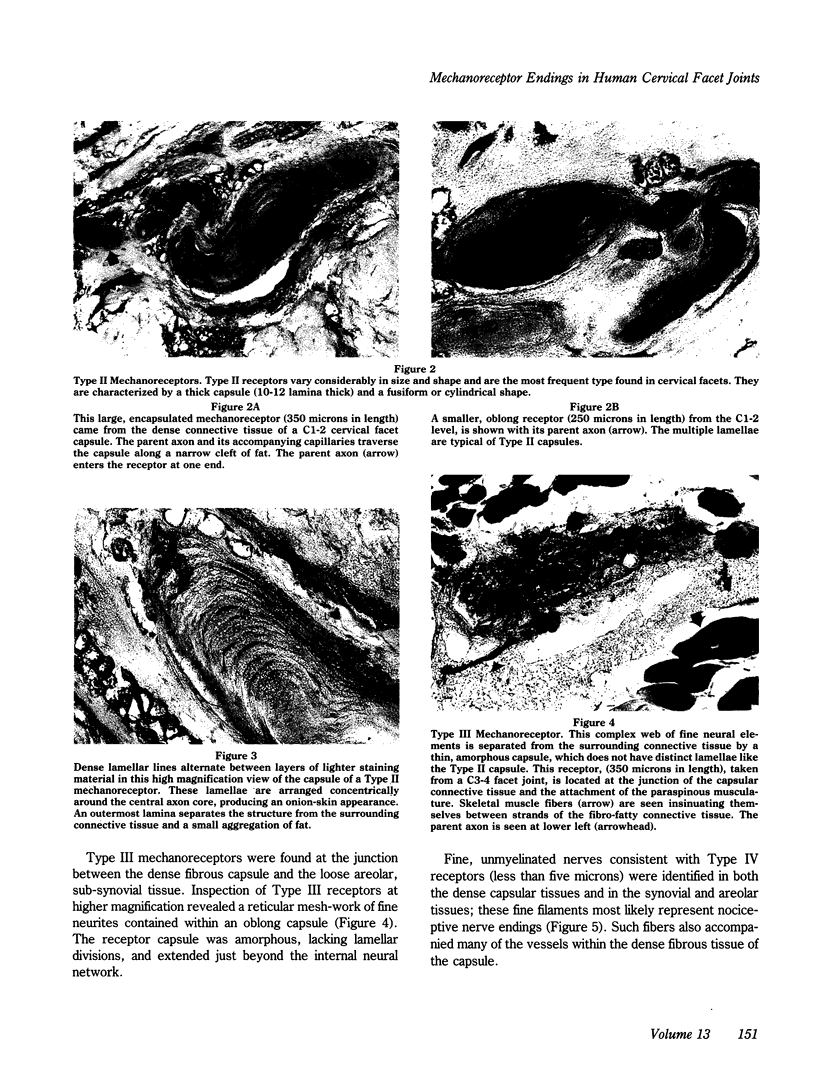
Normal cervical facet capsules, taken from three human subjects, were examined to determine the density and distribution of three types of mechanoreceptive nerve endings. Clearly identifiable mechanoreceptors were found in 80% of the specimens and were categorized according to the classification of Freeman and Wyke. Eleven Type I, twenty Type II, and five Type III receptors were identified, as well as a number of small, unencapsulated nerve endings. Type I receptors were small, globular structures measuring 25-50 microns in diameter. Type II receptors varied in size and contour, but were characterized by their oblong shape and broad, lamellar capsule. Type III receptors were relatively large, oblong structures with a thin, amorphous capsule, within which a reticular mesh-work of fine neurites was embedded. Free (nociceptive) nerve endings were found in sub-synovial, loose areolar, and dense capsular tissues. The presence of mechanoreceptive and nociceptive nerve endings demonstrates that cervical facet capsules are monitored by the central nervous system, and implies that neural input from the facets is important to the function of the cervical spine. Previous studies have suggested that protective muscular reflexes modulated by these types of mechanoreceptors are important in preventing joint instability and degeneration. The complex neural elements identified in this study may be damaged inadvertently when surgically exposing the cervical spine. Care should be taken, during posterior approaches, to avoid excessive stripping of the facets in portions of the spine not being fused.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxendale R. H., Ferrell W. R. Modulation of transmission in flexion reflex pathways by knee joint afferent discharge in the decerebrate cat. Brain Res. 1980 Dec 8;202(2):497–500. doi: 10.1016/0006-8993(80)90163-8. [DOI] [PubMed] [Google Scholar]
- Baxendale R. H., Ferrell W. R. Modulation of transmission in forelimb flexion reflex pathways by elbow joint afferent discharge in decerebrate cats. Brain Res. 1981 Sep 28;221(2):393–396. doi: 10.1016/0006-8993(81)90789-7. [DOI] [PubMed] [Google Scholar]
- Burgess P. R., Clark F. J. Characteristics of knee joint receptors in the cat. J Physiol. 1969 Aug;203(2):317–335. doi: 10.1113/jphysiol.1969.sp008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark F. J., Burgess P. R. Slowly adapting receptors in cat knee joint: can they signal joint angle? J Neurophysiol. 1975 Nov;38(6):1448–1463. doi: 10.1152/jn.1975.38.6.1448. [DOI] [PubMed] [Google Scholar]
- EKHOLM J., EKLUND G., SKOGLUND S. On the reflex effects from the knee joint of the cat. Acta Physiol Scand. 1960 Oct 31;50:167–174. doi: 10.1111/j.1748-1716.1960.tb02087.x. [DOI] [PubMed] [Google Scholar]
- Freeman M. A., Wyke B. The innervation of the knee joint. An anatomical and histological study in the cat. J Anat. 1967 Jun;101(Pt 3):505–532. [PMC free article] [PubMed] [Google Scholar]
- Giles L. G., Harvey A. R. Immunohistochemical demonstration of nociceptors in the capsule and synovial folds of human zygapophyseal joints. Br J Rheumatol. 1987 Oct;26(5):362–364. doi: 10.1093/rheumatology/26.5.362. [DOI] [PubMed] [Google Scholar]
- Giles L. G., Taylor J. R. Innervation of lumbar zygapophyseal joint synovial folds. Acta Orthop Scand. 1987 Feb;58(1):43–46. doi: 10.3109/17453678709146341. [DOI] [PubMed] [Google Scholar]
- Grigg A., Hoffman A. H., Fogarty K. E. Properties of Golgi-Mazzoni afferents in cat knee joint capsule, as revealed by mechanical studies of isolated joint capsule. J Neurophysiol. 1982 Jan;47(1):31–40. doi: 10.1152/jn.1982.47.1.31. [DOI] [PubMed] [Google Scholar]
- Grigg P., Hoffman A. H. Properties of Ruffini afferents revealed by stress analysis of isolated sections of cat knee capsule. J Neurophysiol. 1982 Jan;47(1):41–54. doi: 10.1152/jn.1982.47.1.41. [DOI] [PubMed] [Google Scholar]
- Grigg P. Mechanical factors influencing response of joint afferent neurons from cat knee. J Neurophysiol. 1975 Nov;38(6):1473–1484. doi: 10.1152/jn.1975.38.6.1473. [DOI] [PubMed] [Google Scholar]
- Grönblad M., Konttinen Y. T., Korkala O., Liesi P., Hukkanen M., Polak J. M. Neuropeptides in synovium of patients with rheumatoid arthritis and osteoarthritis. J Rheumatol. 1988 Dec;15(12):1807–1810. [PubMed] [Google Scholar]
- Grönblad M., Korkala O., Konttinen Y. T., Nederström A., Hukkanen M., Tolvanen E., Polak J. M. Silver impregnation and immunohistochemical study of nerves in lumbar facet joint plical tissue. Spine (Phila Pa 1976) 1991 Jan;16(1):34–38. doi: 10.1097/00007632-199101000-00006. [DOI] [PubMed] [Google Scholar]
- Grönblad M., Liesi P., Korkala O., Karaharju E., Polak J. Innervation of human bone periosteum by peptidergic nerves. Anat Rec. 1984 Jul;209(3):297–299. doi: 10.1002/ar.1092090306. [DOI] [PubMed] [Google Scholar]
- Kennedy J. C., Alexander I. J., Hayes K. C. Nerve supply of the human knee and its functional importance. Am J Sports Med. 1982 Nov-Dec;10(6):329–335. doi: 10.1177/036354658201000601. [DOI] [PubMed] [Google Scholar]
- Kidd B. L., Mapp P. I., Blake D. R., Gibson S. J., Polak J. M. Neurogenic influences in arthritis. Ann Rheum Dis. 1990 Aug;49(8):649–652. doi: 10.1136/ard.49.8.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P., Grönblad M., Korkala O., Karaharju E., Rusanen M. Substance P: a neuropeptide involved in low back pain? Lancet. 1983 Jun 11;1(8337):1328–1329. doi: 10.1016/s0140-6736(83)92435-2. [DOI] [PubMed] [Google Scholar]
- O'Connor B. L., Gonzales J. Mechanoreceptors of the medial collateral ligament of the cat knee joint. J Anat. 1979 Dec;129(Pt 4):719–729. [PMC free article] [PubMed] [Google Scholar]
- O'Connor B. L., McConnaughey J. S. The structure and innervation of cat knee menisci, and their relation to a "sensory hypothesis" of meniscal function. Am J Anat. 1978 Nov;153(3):431–442. doi: 10.1002/aja.1001530306. [DOI] [PubMed] [Google Scholar]
- PALMER I. Pathophysiology of the medical ligament of the knee joint. Acta Chir Scand. 1958 Aug 30;115(4):312–318. [PubMed] [Google Scholar]
- Ramcharan J. E., Wyke B. Articular reflexes at the knee joint: an electromyographic study. Am J Physiol. 1972 Dec;223(6):1276–1280. doi: 10.1152/ajplegacy.1972.223.6.1276. [DOI] [PubMed] [Google Scholar]
- Schutte M. J., Dabezies E. J., Zimny M. L., Happel L. T. Neural anatomy of the human anterior cruciate ligament. J Bone Joint Surg Am. 1987 Feb;69(2):243–247. [PubMed] [Google Scholar]
- Solomonow M., Baratta R., Zhou B. H., Shoji H., Bose W., Beck C., D'Ambrosia R. The synergistic action of the anterior cruciate ligament and thigh muscles in maintaining joint stability. Am J Sports Med. 1987 May-Jun;15(3):207–213. doi: 10.1177/036354658701500302. [DOI] [PubMed] [Google Scholar]
- Wyke B. Articular neurology--a review. Physiotherapy. 1972 Mar 10;58(3):94–99. [PubMed] [Google Scholar]
- Wyke B. The neurology of joints. Ann R Coll Surg Engl. 1967 Jul;41(1):25–50. [PMC free article] [PubMed] [Google Scholar]
- el-Bohy A., Cavanaugh J. M., Getchell M. L., Bulas T., Getchell T. V., King A. I. Localization of substance P and neurofilament immunoreactive fibers in the lumbar facet joint capsule and supraspinous ligament of the rabbit. Brain Res. 1988 Sep 20;460(2):379–382. doi: 10.1016/0006-8993(88)90386-1. [DOI] [PubMed] [Google Scholar]