Abstract
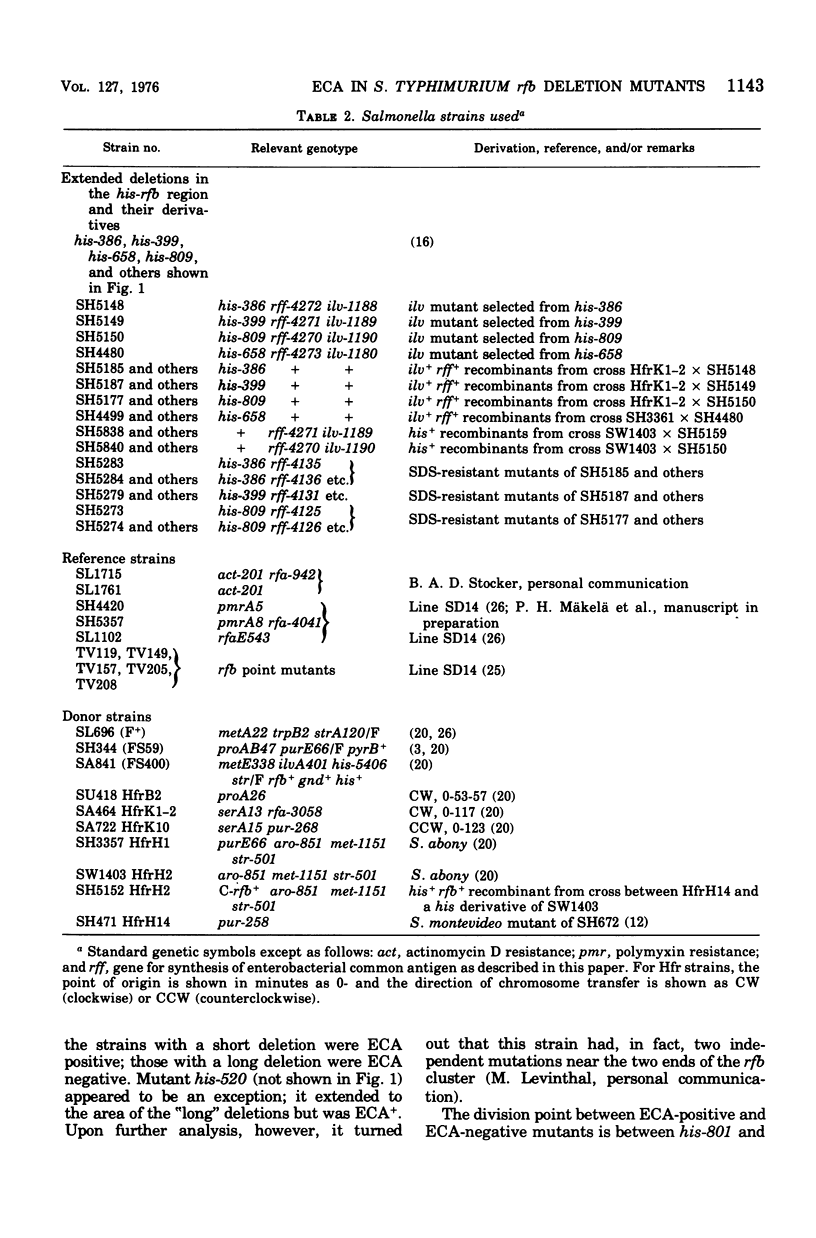
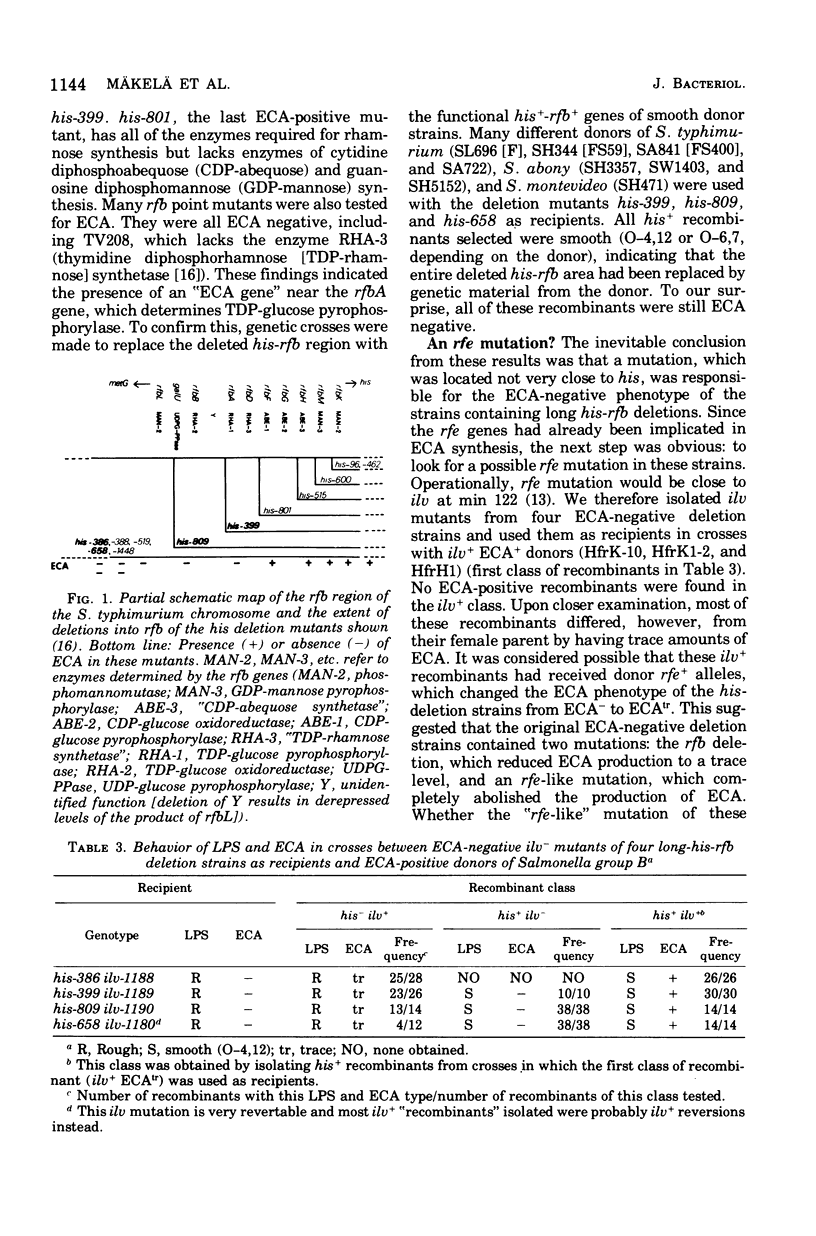
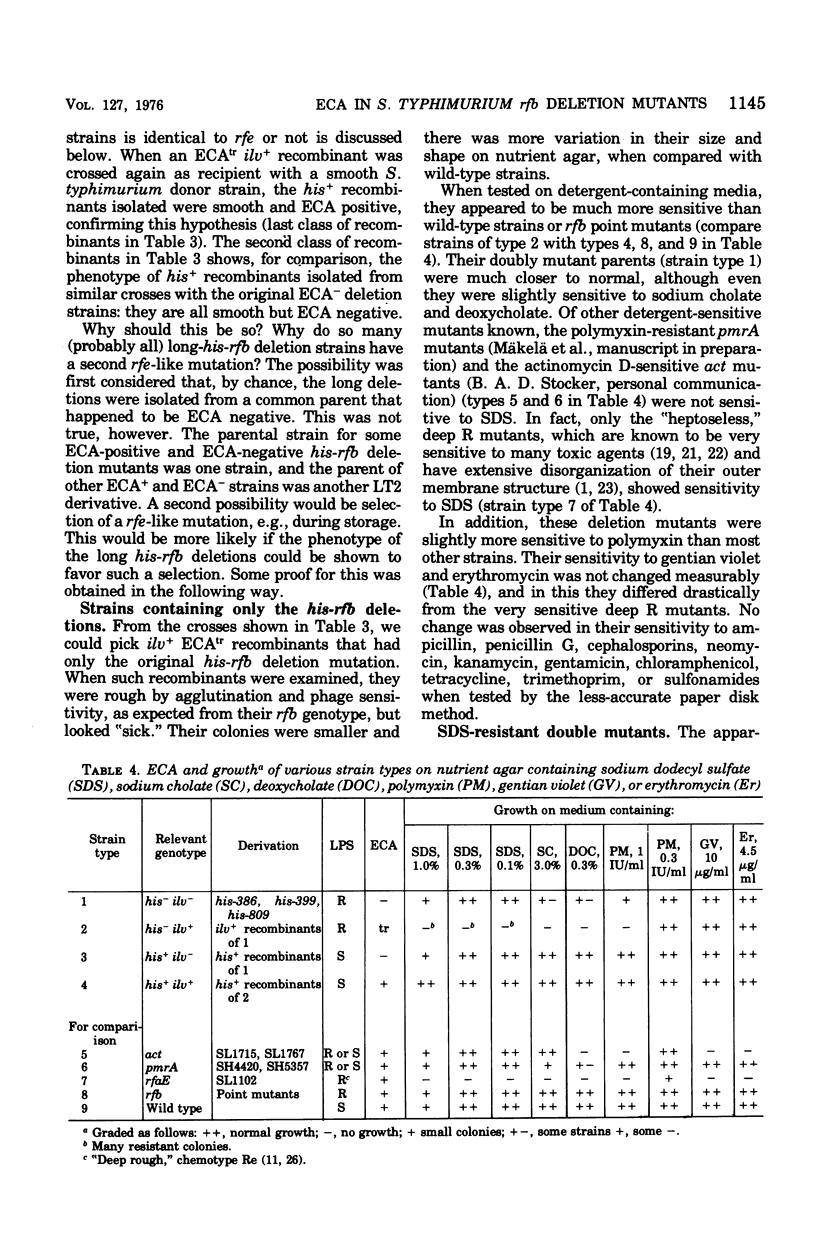
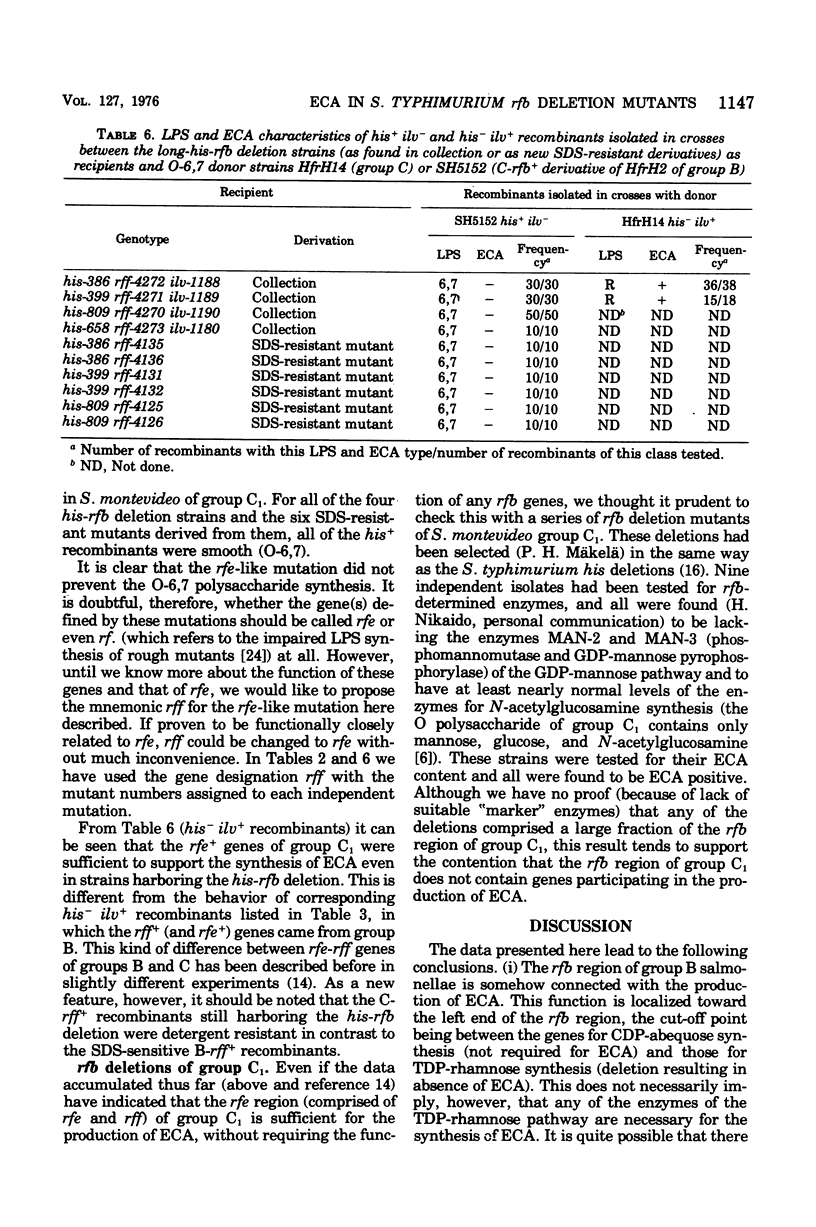
The his-rfb deletion series of Salmonella typhimurium mutants characterized previously by Nikaido et al. was examined for the presence of the enterobacterial common antigen (ECA). All deletions not extending further to the left than the genes for cytidine phosphoabequose synthesis were ECA positive, whereas longer deletions (extending to the genes for thymidine diphosphorhamnose synthesis or further) were ECA negative. When these long-his-rfb deletion strains were studied further, it became clear that they (four out of four studied) had accumulated a second mutation, called rff, close to ilv, which prevented the synthesis of ECA. When rff- was replaced by rff+, the recombinants, now having the his-rfb deletion only, produced traces of ECA, showed reduced viability, increased sensitivity to sodium dodecyl sulfate (SDS) and to a lesser extent, to other anionic detergents, and accumulated secondary "suppressor" mutations upon storage. Such suppressor-containing mutants could be isolated by selecting for resistance to 1% SDS. Thirty of 46 SDS-resistant mutants studied had a second mutation, which alone prevented the synthesis of ECA, close to ilv. This ilv-linked mutation was similar to the rff mutation of the strains studied originally. The new rff mutation was similar to previously described rfe mutations in its close linkage to ilv and association with an ECA-negative phenotype. It differed from rfe, however, by not affecting the synthesis of the O antigens (O-6,7) of group C1. In Salmonella group C1, all ECA genes identified thus far are linked to ilv (rfe and/or rff) and none is linked to rfb.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki S., Merkel M., McCabe W. R. Immunofluorescent demonstration of the common enterobacterial antigen. Proc Soc Exp Biol Med. 1966 Jan;121(1):230–234. doi: 10.3181/00379727-121-30744. [DOI] [PubMed] [Google Scholar]
- Bagdian G., Mäkelä P. H. Antigenic conversion by phage P27. I. Mapping of the prophage attachment site on the Salmonella chromosome. Virology. 1971 Feb;43(2):403–411. doi: 10.1016/0042-6822(71)90312-6. [DOI] [PubMed] [Google Scholar]
- FUKASAWA T., NIKAIDO H. Galactose-sensitive mutants of Salmonella. II. Bacteriolysis induced by galactose. Biochim Biophys Acta. 1961 Apr 15;48:470–483. doi: 10.1016/0006-3002(61)90045-2. [DOI] [PubMed] [Google Scholar]
- Fuller N. A., Staub A. M. Immunochemical studies on Salmonella. 13. Chemical changes appearing on the specific polysaccharide of S. cholerae suis (6-2,7) after its conversion by phage 14(6,7). Eur J Biochem. 1968 Apr;4(3):286–300. doi: 10.1111/j.1432-1033.1968.tb00207.x. [DOI] [PubMed] [Google Scholar]
- Hammarström S., Carlsson H. E., Perlmann P., Svensson S. Immunochemistry of the common antigen of Enterobacteriaceae (Kunin). Relation to lipopolysaccharide core structure. J Exp Med. 1971 Sep 1;134(3 Pt 1):565–576. doi: 10.1084/jem.134.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns M. A., Whiteside R. E., Baker E. E., McCabe W. R. Common enterobacterial antigen. I. Isolation and purification from Salmonella typhose 0:901. J Immunol. 1973 Mar;110(3):781–790. [PubMed] [Google Scholar]
- KUNIN C. M. SEPARATION, CHARACTERIZATION, AND BIOLOGICAL SIGNIFICANCE OF A COMMON ANTIGEN IN ENTEROBACTERIACEAE. J Exp Med. 1963 Oct 1;118:565–586. doi: 10.1084/jem.118.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüderitz O., Galanos C., Risse H. J., Ruschmann E., Schlecht S., Schmidt G., Schulte-Holthausen H., Wheat R., Westphal O., Schlosshardt J. Structural relationship of Salmonella O and R antigens. Ann N Y Acad Sci. 1966 Jun 30;133(2):349–374. doi: 10.1111/j.1749-6632.1966.tb52376.x. [DOI] [PubMed] [Google Scholar]
- Mäkelä P. H. Genetic determination of the O antigens of Salmonella groups B (4,5,12) and C1(6,7). J Bacteriol. 1966 Mar;91(3):1115–1125. doi: 10.1128/jb.91.3.1115-1125.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä P. H., Jahkola M., Lüderitz O. A new gene cluster rfe concerned with the biosynthesis of Salmonella lipopolysaccharide. J Gen Microbiol. 1970 Jan;60(1):91–106. doi: 10.1099/00221287-60-1-91. [DOI] [PubMed] [Google Scholar]
- Mäkelä P. H., Mayer H., Whang H. Y., Neter E. Participation of lipopolysaccharide genes in the determination of the enterobacterial common antigen: analysis of R mutants of Salmonella minnesota. J Bacteriol. 1974 Sep;119(3):760–764. doi: 10.1128/jb.119.3.760-764.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIKAIDO H., MIKAIDO K., SUBBAIAH T. V., STOCKER B. A. ROUGH MUTANTS OF SALMONELLA TYPHIMURIUM. III. ENZYMATIC SYNTHESIS OF NUCLEOTIDE-SUGAR COMPOUNDS. Nature. 1964 Mar 28;201:1301–1302. doi: 10.1038/2011301a0. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Levinthal M., Nikaido K., Nakane K. Extended deletions in the histidine-rough-B region of the Salmonella chromosome. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1825–1832. doi: 10.1073/pnas.57.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Nikaido K., Mäkelä P. H. Genetic determination of enzymes synthesizing O-specific sugars of Salmonella lipopolysaccharides. J Bacteriol. 1966 Mar;91(3):1126–1135. doi: 10.1128/jb.91.3.1126-1135.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUBBAIAH T. V., STOCKER B. A. ROUGH MUTANTS OF SALMONELLA TYPHIMURIUM. I. GENETICS. Nature. 1964 Mar 28;201:1298–1299. doi: 10.1038/2011298a0. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., MacAlister T., Costerton J. W., Cheng K. J. Permeability of lipopolysaccharide-deficient (rough) mutants of Salmonella typhimurium to antibiotics, lysozyme, and other agents. Can J Microbiol. 1974 Aug;20(8):1135–1145. doi: 10.1139/m74-176. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Ross H., Ziegler L., Mäkelä P. H. F + , Hfr, and F' strains of Salmonella typhimurium and Salmonella abony. Bacteriol Rev. 1972 Dec;36(4):608–637. doi: 10.1128/br.36.4.608-637.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G., Schlecht S., Westphal O. Untersuchungen zur Typisierung von Salmonella-R-Formen. 3. Typisierung von S. minnesota-Mutanten mittels chemischer Agenzien. Zentralbl Bakteriol Orig. 1969 Dec;212(1):88–96. [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]


