Abstract
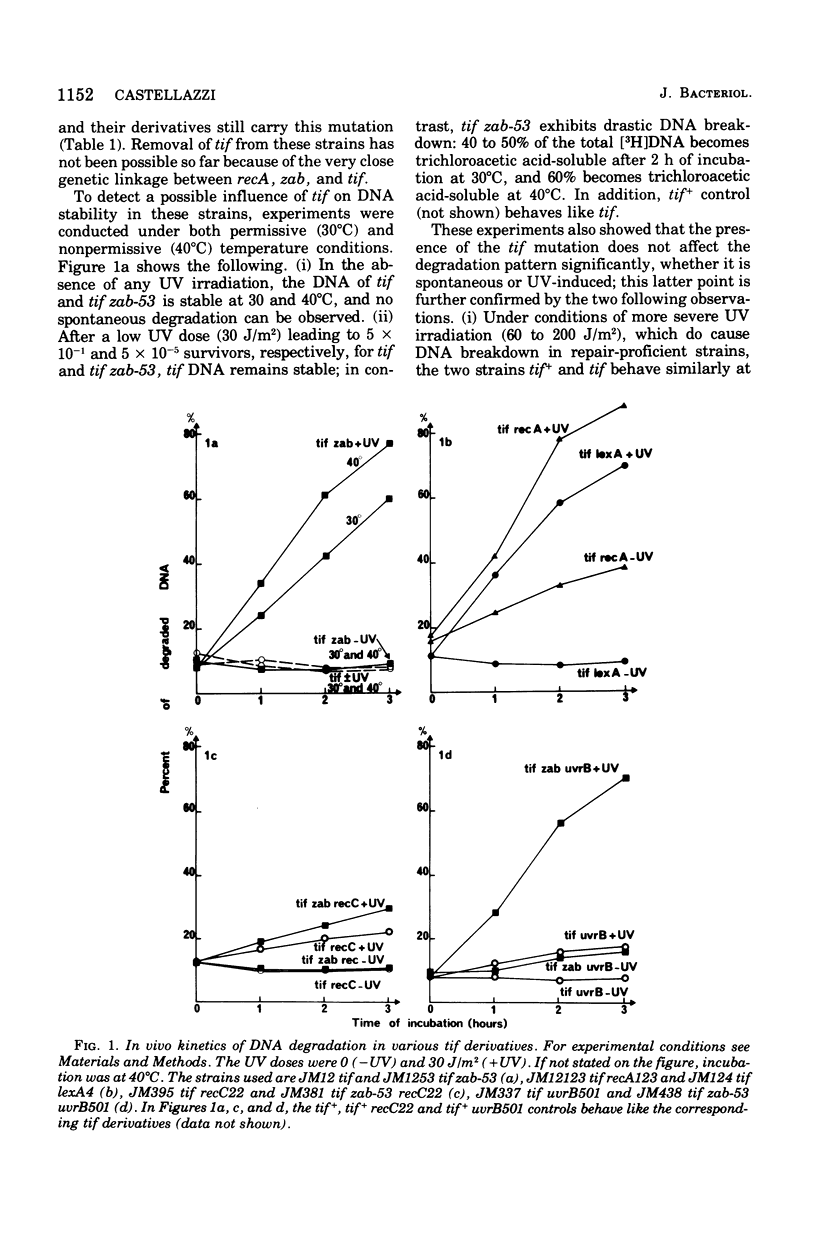
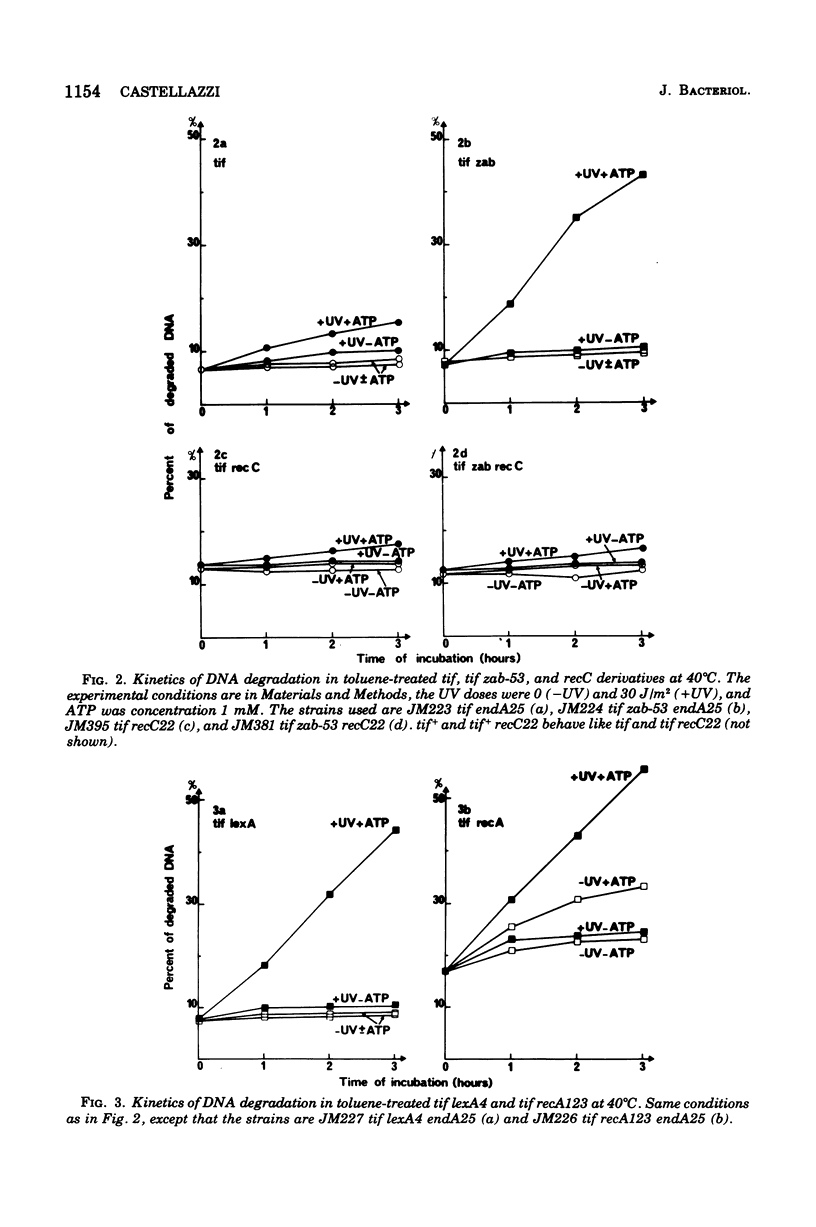
There are three mutations (recA, zab, and lexA) each of which suppresses the expression of the Escherichia coli tif mutation and causes high deoxyribonucleic acid (DNA) repair deficiency (Castellazi et al., 1972). The effect of the zab mutation on DNA stability was investigated. In vivo, a strain carrying the zab-53 mutation shows (i) no spontaneous DNA degradation and (ii) rapid DNA degradation after ultraviolet irradiation, which depends upon the exonuclease V activity coded by recB+/C+genes and which is independent from the correndonuclease II activity coded by uvrA+/B+. Thus, in regard to DNA stability, the zab mutant behaves like lexA and recA (Howard-Flanders and Boyce, 1966), the latter mutant showing in addition spontaneous DNA breakdown. The degradation patterns of these tif-suppressed strains are shown to be remarkably reproducible in bacteria made permeable to metabolites, by toluene or toluene plus Triton X-100. The degradation properties reflect the activity of the same biochemical system that works in vivo, in that degradation depends upon the presence of recA, zab, or lexA, ultraviolet irradiation, and exonuclease V activity. In addition, adenosine 5'-triphosphate (1 mM) is required. This assay with permeabilized cells offers a useful tool for studying degradation under controlled conditions, especially by permitting the dissociation of energy-dependent from energy-independent steps.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanco M., Levine A., Devoret R. IexB: a new gene governing radiation sensitivity and lysogenic induction in Escherichia coli K12. Basic Life Sci. 1975;5A:379–382. doi: 10.1007/978-1-4684-2895-7_50. [DOI] [PubMed] [Google Scholar]
- Braun A., Hopper P., Grossman L. The Escherichia coli UV endonuclease (correndonuclease II). Basic Life Sci. 1975;5A:183–190. doi: 10.1007/978-1-4684-2895-7_22. [DOI] [PubMed] [Google Scholar]
- Buttin G., Wright M. Enzymatic DNA degradation in E. coli: its relationship to synthetic processes at the chromosome level. Cold Spring Harb Symp Quant Biol. 1968;33:259–269. doi: 10.1101/sqb.1968.033.01.030. [DOI] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Capaldo F. N., Barbour S. D. DNA content, synthesis and integrity in dividing and non-dividing cells of rec- strains of Escherichia coli K12. J Mol Biol. 1975 Jan 5;91(1):53–66. doi: 10.1016/0022-2836(75)90371-x. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. Prophage induction and cell division in E. coli. I. Further characterization of the thermosensitive mutation tif-1 whose expression mimics the effect of UV irradiation. Mol Gen Genet. 1972;119(2):139–152. doi: 10.1007/BF00269133. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. [Prophage induction and cell division in E. coli. II. Linked (recA, zab) and unlinked (lex) suppressors of tif-1-mediated induction and filamentation]. Mol Gen Genet. 1972;119(2):153–174. doi: 10.1007/BF00269134. [DOI] [PubMed] [Google Scholar]
- Clark A. J., Chamberlin M., Boyce R. P., Howard-Flanders P. Abnormal metabolic response to ultraviolet light of a recombination deficient mutant of Escherichia coli K12. J Mol Biol. 1966 Aug;19(2):442–454. doi: 10.1016/s0022-2836(66)80015-3. [DOI] [PubMed] [Google Scholar]
- GOLDTHWAIT D., JACOB F. SUR LE M'ECANISME DE L'INDUCTION DU D'EVELOPPEMENT DU PROPHAGE CHEZ LES BACT'ERIES LYSOG'ENES. C R Hebd Seances Acad Sci. 1964 Jul 20;259:661–664. [PubMed] [Google Scholar]
- George J., Castellazzi M., Buttin G. Prophage induction and cell division in E. coli. III. Mutations sfiA and sfiB restore division in tif and lon strains and permit the expression of mutator properties of tif. Mol Gen Genet. 1975 Oct 22;140(4):309–332. [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P. DNA repair and genetic recombination: studies on mutants of Escherichia coli defective in these processes. Radiat Res. 1966;(Suppl):156+–156+. [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. W., DeMoss J. A. Effects of toluene on Escherichia coli. J Bacteriol. 1965 Nov;90(5):1420–1425. doi: 10.1128/jb.90.5.1420-1425.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karu A. E., MacKay V., Goldmark P. J., Linn S. The recBC deoxyribonuclease of Escherichia coli K-12. Substrate specificity and reaction intermediates. J Biol Chem. 1973 Jul 25;248(14):4874–4884. [PubMed] [Google Scholar]
- Kirby E. P., Jacob F., Goldthwait D. A. Prophage induction and filament formation in a mutant strain of Escherichia coli. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1903–1910. doi: 10.1073/pnas.58.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman R. P., Oishi M. Formation of the recB-recC DNase by in vitro complementation and evidence concerning its subunit nature. Nat New Biol. 1973 May 16;243(124):75–77. [PubMed] [Google Scholar]
- Monk M., Kinross J. Conditional lethality of recA and recB derivatives of a strain of Escherichia coli K-12 with a temperature-sensitive deoxyribonucleic acid polymerase I. J Bacteriol. 1972 Mar;109(3):971–978. doi: 10.1128/jb.109.3.971-978.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses R. E. Replicative deoixyribonucleic acid synthesis in a system diffusible for macromolecules. J Biol Chem. 1972 Oct 10;247(19):6031–6038. [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. Replication and repair of DNA in cells of Escherichia coli treated with toluene. Proc Natl Acad Sci U S A. 1970 Oct;67(2):674–681. doi: 10.1073/pnas.67.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radman M. Endonuclease III: an endonuclease from Escherichia coli that introduces single polynucleotide chain scissions in ultraviolet-irradiated DNA. Basic Life Sci. 1975;5A:197–200. doi: 10.1007/978-1-4684-2895-7_24. [DOI] [PubMed] [Google Scholar]
- SHORTMAN K., LEHMAN I. R. THE DEOXYRIBONUCLEASES OF ESCHERICHIA COLI. VI. CHANGES IN ENZYME LEVELS IN RESPONSE TO ALTERATIONS IN PHYSIOLOGICAL STATE. J Biol Chem. 1964 Sep;239:2964–2974. [PubMed] [Google Scholar]
- Schaller H., Otto B., Nüsslein V., Huf J., Herrmann R., Bonhoeffer F. Deoxyribonucleic acid replication in vitro. J Mol Biol. 1972 Jan 28;63(2):183–200. doi: 10.1016/0022-2836(72)90369-5. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J. Characteristics of some multiply recombination-deficient strains of Escherichia coli. J Bacteriol. 1969 Oct;100(1):231–239. doi: 10.1128/jb.100.1.231-239.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Persistence and decay of thermoinducible error-prone repair activity in nonfilamentous derivatives of tif-1, Escherichia coli B/r: the timing of some critical events in ultraviolet mutagenesis. Mol Gen Genet. 1975 Dec 29;142(2):87–103. doi: 10.1007/BF00266092. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Thermal enhancement of ultraviolet mutability in a tif-1 uvrA derivative of Escherichia coli B-r: evidence that ultraviolet mutagenesis depends upon an inducible function. Proc Natl Acad Sci U S A. 1974 May;71(5):1930–1934. doi: 10.1073/pnas.71.5.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]


