Abstract
The topology of signal transduction is particularly important for neurons. Neurotrophic factors such as nerve growth factor (NGF) interact with receptors at distal axons and a signal is transduced by retrograde transport to the cell body to ensure survival of the neuron. We have discovered an organelle that may account for the retrograde transport of the neurotrophin signal. This organelle is derived from endocytosis of the receptor tyrosine kinase for NGF, TrkA. In vitro reactions containing semi-intact PC12 cells and ATP were used to enhance recovery of a novel organelle: small vesicles containing internalized NGF bound to activated TrkA. These vesicles were distinct from clathrin coated vesicles, uncoated primary endocytic vesicles, and synaptic vesicles, and resembled transport vesicles in their sedimentation velocity. They contained 10% of the total bound NGF and almost one-third of the total tyrosine phosphorylated TrkA. These small vesicles are compelling candidates for the organelles through which the neurotrophin signal is conveyed down the axon.
A long-standing hypothesis predicts that neurons exploit retrograde transport of “signaling vesicles” to convey the nerve growth factor (NGF) signal from the distal axon, where receptors bind NGF, to the cell body to evoke changes in gene expression (1–3). Putative signaling vesicles may arise after endocytosis of neurotrophin receptors (4). It is known that endocytosis occurs at neurite tips (5, 6). TrkA, the receptor tyrosine kinase for the neurotrophin NGF, follows the pattern of other receptor tyrosine kinases, which dimerize upon ligand binding, with resulting activation of the intracellular kinase domain and rapid internalization (7). It is known that tyrosine phosphorylated TrkA is retrogradely transported in axons and that the amount increases in response to NGF treatment (8). Significantly, however, the organelles containing TrkA that convey signal transduction down the axon have not been isolated. We set out to capture such an organelle.
PC12 cells have been used as a model for signal transduction events that occur at neurite tips (9). PC12 cells mimic quite closely signal transduction events that occur in sympathetic neurons; in their undifferentiated state they express TrkA and respond to NGF by inducing expression of neuronal proteins and adopting a neuronal morphology (10, 11). Prolonged NGF treatment, followed by its withdrawal, induces these cells to undergo programmed cell death closely resembling that seen in sympathetic neurons (12–15). Undifferentiated PC12 cells also contain synaptic vesicles (16, 17) whose biogenesis and trafficking are controlled by regulated secretory processes similar to those in nerve terminals (18, 19). These observations suggest that the membrane traffic that occurs at axon tips, including formation of other organelles that are specific to neurons, may be modeled in undifferentiated PC12 cells. We recently examined TrkA receptors in intracellular organelles after a brief period of internalization in PC12 cells (20). These organelles contained tyrosine phosphorylated TrkA, bound to both NGF and to phospholipase C-γ (PLC-γ) (20). These findings are evidence that internalized TrkA receptors continue to be activated after endocytosis.
A number of organelles are involved in trafficking proteins to and from endosomes (21, 22), and any one of these could in principle be used to convey a signal. Activated TrkA is internalized via clathrin-mediated endocytosis (20) from the plasma membrane into primary endocytic vesicles, which might in theory be retrogradely transported. However, primary endocytic vesicles also contain synaptic vesicle and housekeeping proteins. Sorting of receptors away from these proteins at the plasma membrane could be invoked to explain their specific retrograde transport, but the existence of a distinct class of primary endocytic vesicles for receptors has not been established. The endosome, in contrast, is known to be a sorting organelle (21, 22). For example, synaptic vesicle proteins are delivered by clathrin-mediated endocytosis to the endosome. From there they are sorted into synaptic vesicles, away from other proteins destined for recycling as well as degradation (23). We hypothesized that neurons evolved a mechanism for sending a signal to the cell body by building on existing sorting machinery to create a novel class of transport vesicles (3). Transport vesicles would be ideal for retrograde signaling, having a high surface to volume ratio, and a topology that would allow the cytoplasmic tail of receptors to easily interact with the appropriate proteins. Small transport vesicles derived from endosomes have not yet been isolated. However, at least two different types of coats have recently been shown to be associated with the sorting endosome (24–27). Distinct coats are presumably required for the formation of different types of vesicles. Neurons express more types of coat proteins than other cells (28), and it is possible that these are used to form different types of transport vesicles.
In vitro reconstitution of membrane traffic has been used to characterize short-lived intermediates, such as transport vesicles, that convey proteins from one compartment to another (29–32), as well as formation of synaptic vesicles (33). In vitro reactions were used here to identify and characterize membrane traffic intermediates formed through endocytosis, which contained NGF and TrkA. The data suggest that a unique transport vesicle derived from endosomes containing activated neurotrophin receptors may well serve the purpose of conveying signal transduction down the axon.
MATERIALS AND METHODS
PC12 cells were obtained from Lloyd Greene (Columbia University, New York). They were grown on collagen-coated plates in RPMI 1640 medium, 10% horse serum, 5% fetal calf serum exactly as described (9). NGF or 125I-NGF (1 nM) was bound to PC12 cells at 4°C as described (20). Cells were then warmed to 37°C for 10 min (warmed in vivo) to initiate membrane traffic, then chilled, washed, and permeabilized by passage through a Balch homogenizer in a cytoplasm-like buffer containing 38 mM each of the potassium salts of aspartic, gluconic and glutamic acids, 20 mM Mops, 5 mM reduced glutathione, 10 mM potassium bicarbonate, 0.5 mM magnesium carbonate, 1 mM EGTA, 1 mM EDTA; pH adjusted to 7.1 at 37°C with potassium hydroxide (31, 34, 35). In vitro reactions were performed with hexokinase and glucose (−ATP) or creatine phosphate, creatine kinase, and ATP (+ATP) for 15 min at 37°C as described (31). Where indicated (see Fig. 3F), 1 mM sodium orthovanadate was added to in vitro reactions. Cytosol and organelles released from the cells were separated from the cell ghosts by centrifugation at 1,000 × g. Organelles that emerged from permeabilized cells (the supernatant of the 1,000 × g spin) were separated from the cytosol and free NGF by layering over a 0.4 ml pad of 10% sucrose and centrifuging 100,000 × g for 1 hr (Fig. 1 A and B). Alternatively, an 8,000 × g centrifugation (P2, Fig. 1 C–E) preceded the one at 100,000 × g (P3, Fig. 2). Vesicles in the pellets were resuspended and applied to 10–40% (wt/wt, for velocity sedimentation) or 15–50% (for equilibrium density) sucrose gradients with a 60% sucrose pad and centrifuged at 100,000 × g for 1 hr (velocity) or 16 hr (equilibrium) in a Beckman TiSW50.1 rotor. For Fig. 2 A and B the P3 was applied to a 5–25% glycerol gradient in buffer B with a 50% glycerol pad (16, 31). Gradient fractions were trichloroacetic acid-precipitated and counted and/or submitted to SDS/PAGE and Western blotting (20). NGF crosslinking to TrkA was carried out with disuccinimidyl suberate (Pierce), as described (20). In some experiments intact 125I-NGF (14 kDa) was quantified by SDS/PAGE and a Molecular Dynamics PhosphorImager (data not shown). The profile of total 125I cpm in gradients corresponded to that of intact NGF, indicating that there was no degradation of NGF. For equilibrium gradients, the density of each fraction was calculated from refractometry measurements.
Figure 3.
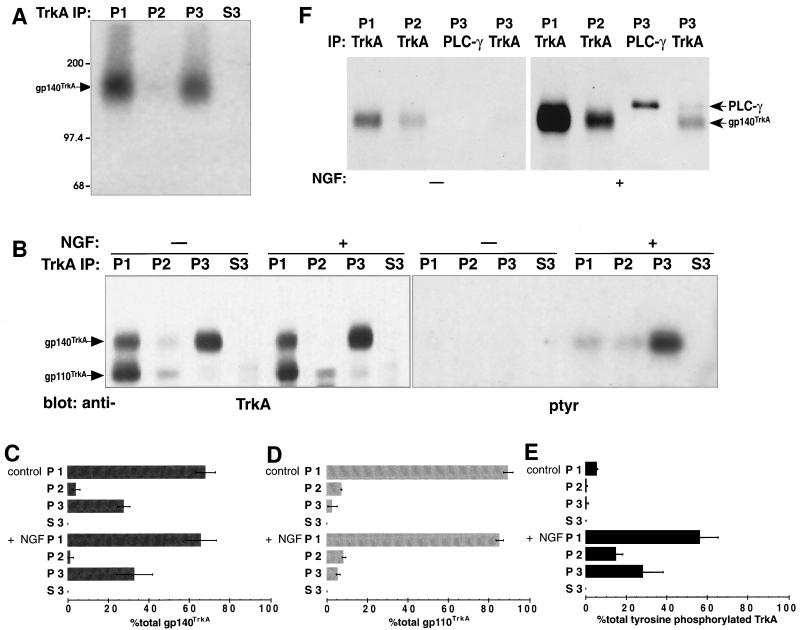
(A) TrkA was crosslinked to NGF in small endocytic vesicles. Cells were bound to 125I-NGF, washed, warmed 10 min, chilled and analyzed after an in vitro reaction with ATP. The membrane permeable crosslinking reagent disuccinimidyl suberate was added (2 mM, 4°C, 30 min) to the permeabilized cell suspension before fractionation of membranes. One-fifth of the cell ghost membranes (1,000 × g pellet, P1), one-half of the 8,000 × g pellet (P2) one-half of the 100,000 × g pellet (P3), and one-tenth of the 100,000 × g supernatant (S3) were immunoprecipitated from samples with anti-TrkA (1088) (20, 42) and analyzed by SDS/PAGE and autoradiography (22 day exposure). The position of molecular weight markers (kDa) is indicated. (B) TrkA is still activated in intracellular organelles after in vitro reactions. Untreated PC12 cells or PC12 cells bound to NGF (1 nM, 4°C) were warmed 10 min and fractionated and immunoprecipitated as in A after in vitro incubation with ATP. The presence of TrkA was assessed by immunoprecipitation, followed by Western blotting, with anti-TrkA and anti-phosphotyrosine (indicated). RTA (a gift of D. Clary, ref. 72) was used for immunoprecipitations. Western blots were probed with RTA followed by horseradish peroxidase-conjugated anti-rabbit IgG and ECL (Left, 1 min exposure). Two proteins were identified, gp140TrkA and gp110TrkA (indicated). The latter is a precursor to gp140TrkA (41, 42), and remained mostly with the cell ghosts after in vitro reactions with ATP. TrkA immunoprecipitates were also probed with anti-phosphotyrosine (4G10, a gift of S. Robbins and M. Bishop, University of California, San Francisco) followed by 125I-goat anti-mouse IgG (Right, 34 day exposure). Mature 140-kDa TrkA was tyrosine phosphorylated, while the 110-kDa immature glycosylated TrkA was not. Equal amounts of cells were used to compare conditions. The top and bottom edges of the panels mark the position of the 200- and 97.4-kDa molecular weight markers, respectively. (C–E) Quantification of TrkA and tyrosine phosphorylated TrkA in intracellular organelles. Data for gp140TrkA (C) and gp110TrkA (D) from 4 experiments as in B (Left) were quantified by densitometry and plotted as a percent of the total in all cell fractions with error bars showing standard deviations. The conditions and fractions are labeled at the left. Data for tyrosine phosphorylated TrkA (E) from four experiments as in (B Right) were quantified by phosphorimaging or densitometry and plotted in reference to total tyrosine phosphorylated TrkA following NGF treatment with error bars as in C. (F) Sodium orthovanadate was added to in vitro incubations with ATP. Untreated cells (Left) and NGF-treated cells (Right) were permeabilized and incubated in vitro with ATP in the presence of 1 mM sodium orthovanadate. TrkA was immunoprecipitated as in A with anti-TrkA (1,088) (20, 42). In the P3 fraction, PLC-γ, then TrkA was immunoprecipitated from the P3 fractions (indicated by IP). Proteins were Western blotted with antiphosphotyrosine followed by horseradish peroxidase-conjugated antimouse and detected using ECL (20 sec exposure). Equal amounts of cells were used to compare conditions. The top and bottom edges of the panels mark the position of the 200- and 97.4-kDa molecular weight markers, respectively.
Figure 1.
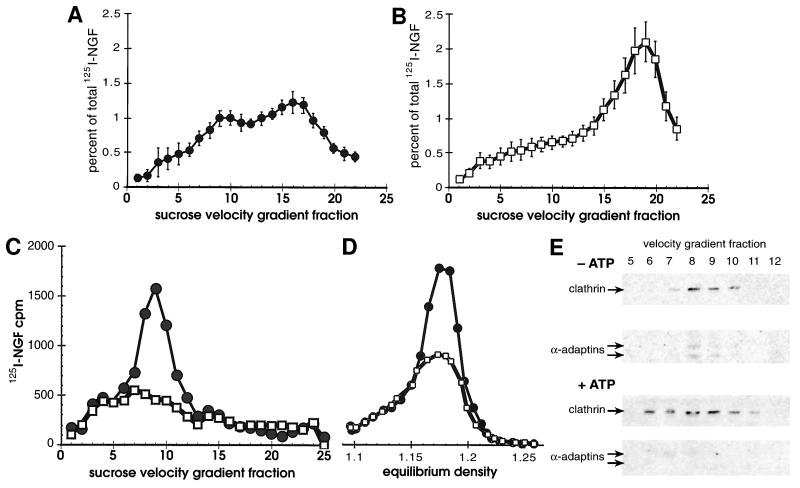
(A and B) Sucrose velocity sedimentation resolved different classes of vesicles containing 125I-NGF. Cells bound to 1 nM 125I-NGF at 4°C were washed, warmed 10 min in vivo, chilled, permeabilized, then subjected to in vitro reactions by warming for 15 min either in the presence of (A) an ATP depleting system (−ATP, •) or (B) an ATP regenerating system (+ATP, □). Released vesicles were separated from cell ghosts, concentrated by 100,000 × g centrifugation, and applied to sucrose velocity gradients. Gradient fractions were collected from the bottom of the tube, so that the largest organelles were in the lowest numbered fractions. Shown is the amount of 125I-NGF in gradient fractions expressed as a percent of the total from all cell fractions (20). Error bars indicate the SD of measurements from four (A) or six (B) experiments. (C and D) Large vesicles containing internalized NGF are clathrin-coated primary endocytic vesicles. Vesicles that emerged from permeabilized cells were fractionated by centrifuging 8,000 × g for 35 min (P2), either directly after warming 10 min in vivo, (•), or after warming and an in vitro reaction with ATP (+ATP, □). Pellets were resuspended and applied to sucrose velocity (C) and equilibrium (D) gradients. Representative experiments are plotted on the same y-axis scale. (E) Clathrin and α-adaptins were localized in the P2 in velocity gradient fractions 6–11 with or without in vitro reactions under all conditions. Shown is a Western blot of positive gradient fractions from in vitro reactions with an ATP-depleting system (−ATP) and ATP regenerating system (+ATP). Monoclonal antibodies TD.1 (70) and AP.6 (71) (a gift of F. Brodsky, University of California, San Francisco) detected clathrin (180 kDa) and two α-adaptins (a doublet centered around 100 kDa), respectively, using enhanced chemiluminescence (Amersham) for detection (1 hr exposure).
Figure 2.
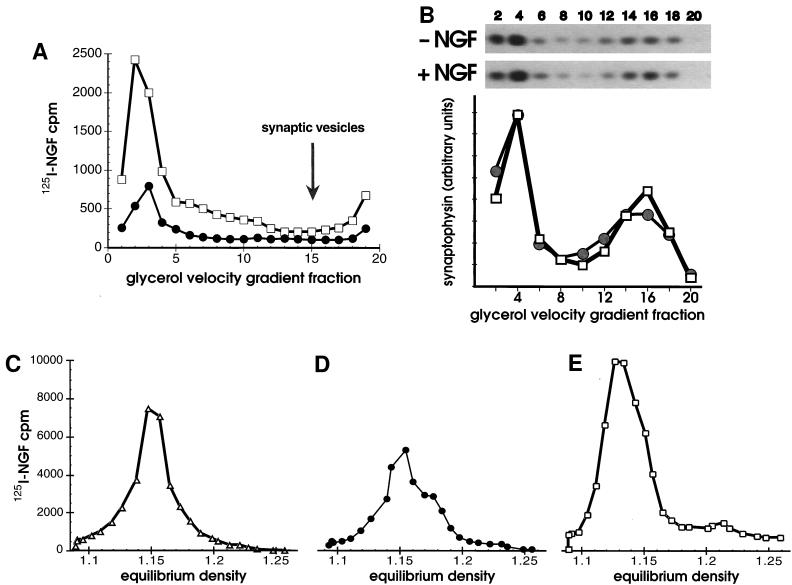
(A and B) Synaptic vesicles did not contain NGF. (A) Cells bound to 125I-NGF were washed, warmed 10 min in vivo, chilled, washed, then permeabilized. Cells were fractionated by successive 1,000 × g (P1), 8,000 × g (P2), and 100,000 × g (P3) centrifugations before or after in vitro reactions with ATP. Vesicles in the P3 without an in vitro reaction, (•) or after an in vitro reaction with ATP (+ATP, □) were applied to 5–25% glycerol velocity gradients and centrifuged 200,000 × g for 1 hr. (B) Synaptophysin in cell fractions was quantified by Western blotting. After incubating with 1 nM NGF or the vehicle alone at 4°C for 1 hr, PC12 cells were warmed 10 min at 37°C, chilled, washed, and permeabilized without in vitro reactions. Glycerol velocity gradient fractions (as in A except that fractions were pooled in pairs) were analyzed by SDS/PAGE, proteins were transferred to nitrocellulose and probed with anti-synaptophysin (SY38), followed by goat anti-mouse and 125I-protein A. Autoradiograms of Western blots (above, 3 day exposure) and PhosphorImager quantification (below) show the amount of synaptophysin present in fractions in the absence of NGF (−NGF, •) or its presence (+NGF, □). The distribution of rab3A (a gift of L. Elferink and R. Scheller, Stanford University, Stanford; not shown) and synaptophysin identified synaptic vesicles in a peak at fractions 14–16. (C–E) In vitro reactions capture intermediates at different stages of endocytosis. Equilibrium density of 125I-NGF-containing vesicles in the 100,000 × g P3 (plotted on the same y-axis scale) after 2 min internalization in vivo plus a 15 min in vitro reaction with ATP (C, +ATP, ▵), or after 10 min internalization in vivo, plus a 15 min in vitro reaction without ATP (D, −ATP, •), or after 10 min internalization in vivo, plus a 15 min in vitro reaction with ATP (E, +ATP, □).
Immunoprecipitation of cell fractions was performed on 100 μl P1M, P2, P3 or S3 fractions by the addition of 0.5 ml IP buffer (150 mM NaCl/1% Nonidet P-40/0.5% deoxycholate/20 mM Tris, pH 8.0/1 mM EDTA/1 mM sodium orthovanadate), 100 μl 2% BSA, and 100 μl 10% glycerol. Then 10 μg 1088 (IgG), 10 μg anti-rat TrkA (RTA; ref. 72; IgG) or 5 μg anti PLC-γ mixed monoclonal (Upstate Biotechnology, Lake Placid, NY) was added, incubated overnight and recovered with protein-A- or protein-A/G Sepharose (Pierce). Sepharose beads were washed twice in IP buffer and once with water, then resuspended in 50 μl 7 M urea sample buffer (7 M urea/2% SDS/125 mM Tris, pH 6.95/20 mM DTT/0.1% Bromophenol blue) and heated (55°C for 15–30 min). Gradient gels (from 5% acrylamide, 0.1% bisacrylamide to 12% acrylamide, 0.5% bisacrylamide, with a 4% acrylamide, 0.1% bisacrylamide stacking gel) were run (36) and gels were dried for autoradiography or proteins were transferred to nitrocellulose. For blots probed with monoclonal antibodies against synaptophysin (SY38, Boehringer Mannheim), incubation with goat anti-mouse IgG preceded 125I-protein A. Blots probed with anti-phosphotyrosine (4G10) were detected with 125I-goat anti-mouse IgG that was prepared with Iodobeads according to the commercial protocol (Pierce). Blots probed with anti-PLC-γ or anti-TrkA antibodies were detected with horseradish peroxidase conjugated anti-mouse or anti-rabbit IgG and chemiluminescence (Amersham).
RESULTS AND DISCUSSION
We used mechanical permeabilization, which allows isolation of intracellular organelles free from plasma membrane, to examine NGF and TrkA-containing endocytic organelles in PC12 cells (20). In vitro reconstitution of membrane traffic in mechanically permeabilized PC12 cells (31) was used to enhance recovery of short-lived organelles, such as transport vesicles (37), that contained internalized NGF and TrkA. 125I-NGF was bound to cells at 4°C. Warming was used to allow internalization of bound NGF. After a short period of internalization (10 min at 37°C) about 10% of the total bound NGF was released from permeabilized cells in the form of intracellular organelles (20). In vitro reactions were then performed for 15 min at 37°C with permeabilized cells in a cytoplasm-like buffer containing either hexokinase plus glucose, which depletes ATP, or creatine phosphate, creatine kinase and ATP, which regenerates ATP. The amount of bound NGF in released organelles increased to 16–18% after in vitro reactions.
Organelles that emerged from permeabilized cells were separated from cell ghosts, concentrated by 100,000 × g centrifugation, and applied to sucrose velocity gradients (Fig. 1 A and B). After an in vitro reaction without ATP (Fig. 1A) organelles that contained internalized NGF were heterogeneous and mostly in two peaks at fractions 9 and 16. When reactions were performed in the presence of ATP, the amount of 125I-NGF in the larger vesicles (fraction 9) decreased, and that in small vesicles increased dramatically (Fig. 1B, +ATP). The new peak did not reflect organelle lysis during the in vitro reaction because free NGF had been removed in an earlier step. The small vesicles whose release was ATP-dependent formed a peak around fraction 19, migrating more slowly than those present without ATP. The increase in 125I-NGF in the small vesicles was greater than the decrease in the large ones, which suggests that donor compartment(s) in the cell ghosts contributed to their formation.
We expected to find NGF in clathrin-coated vesicles, since NGF induced colocalization of intracellular clathrin and TrkA in PC12 cells (20). To separate different vesicle species, released organelles were centrifuged at 8,000 × g prior to centrifugation at 100,000 × g. The 8,000 × g pellet (P2) contained the large vesicles that peaked at fraction 9 on sucrose velocity gradients (Fig. 1C). After an in vitro reaction with ATP, NGF was depleted from large vesicles that were present before the reaction (Fig. 1C). The large vesicles in the pellet of an 8,000 × g centrifugation had an density of 1.18 g/ml on sucrose equilibrium gradients under all conditions (Fig. 1D). Both clathrin and α-adaptins were found in the P2 fraction, peaking at fractions 8–9 on sucrose velocity gradients (Fig. 1E) and at 1.18 g/ml on equilibrium gradients (not shown). α-adaptins were depleted from this peak after in vitro reactions with ATP (Fig. 1E). We conclude that the vesicles containing NGF in the 8,000 × g P2 represent clathrin-coated vesicles derived from the plasma membrane (28).
The small vesicles released with or without ATP in vitro were found in the 100,000 × g pellet (P3). We asked if they were synaptic vesicles, a neuronal organelle whose composition is relatively well defined (38). The P3 was subjected to glycerol velocity sedimentation (16) because synaptic vesicles are not well resolved from transport vesicles on sucrose velocity gradients (31). The small vesicles containing internalized NGF migrated to the bottom of glycerol velocity gradients (fractions 1–5, Fig. 2A). As expected, an in vitro reaction with ATP increased the amount of NGF in the vesicles that migrated to the bottom of the glycerol gradient (Fig. 2A). In contrast, synaptic vesicles formed a peak around fraction 15; they were identified by Western blots of glycerol gradient fractions with antibodies to the synaptic vesicle antigens synaptophysin (Fig. 2B) and rab3A (not shown). Both markers also colocalized with vesicles at the bottom of the glycerol gradient (fractions 1–4), but it is only the lighter peak at fraction 15 that corresponds to synaptic vesicles (16, 17, 39). Furthermore, under no conditions was a peak of 125I-NGF (or TrkA, not shown) observed in synaptic vesicle fractions.
The data suggested that the in vitro reactions could be used to capture intermediates at different stages of endocytosis. One such intermediate is clathrin-coated vesicles, as characterized above. Uncoated primary endocytic vesicles are derived from clathrin-coated vesicles (28). Formation of these vesicles should be favored after a very short internalization in vivo, followed by an in vitro reaction with ATP to activate the uncoating ATPase (28). If internalization of NGF in vivo was allowed to proceed only 2 min instead of 10 min, the vesicles released following in vitro reactions with ATP had an equilibrium density of 1.15 g/ml (Fig. 2C). Vesicles of this same density were produced after 10 min internalization and in vitro reactions in the absence of ATP (Fig. 2D). Significantly, none of these vesicles in the P3 contained clathrin or α-adaptins (data not shown). The data support the view that NGF and TrkA are internalized via clathrin coated vesicles (20), which are subsequently uncoated to form vesicles that migrate to fraction 16 on velocity gradients (Fig. 1A) and peak at 1.15 g/ml on sucrose equilibrium gradients.
It is possible that vesicles derived from endosomes serve to transport the neurotrophin signal (3). One would expect these vesicles to have a lower sedimentation velocity and equilibrium density than primary endocytic vesicles (21, 22). Vesicles produced in vitro with ATP after a 10 min internalization may correspond to vesicles derived from endosomes. Under these conditions, when the 100,000 × g P3 was centrifuged to equilibrium on sucrose gradients the NGF peak had an equilibrium density of 1.13 g/ml (Fig. 2E). This value is different from that for vesicles derived in the absence of ATP (Fig. 2D), and only slightly less than that for constitutive secretory vesicles, which in PC12 cells have a similar sedimentation velocity (31, 40). Thus, we appear to have detected a novel class of small endocytic vesicles that are downstream from both clathrin-coated and uncoated primary endocytic vesicles, and are distinct from synaptic vesicles.
The “signaling vesicle” hypothesis (1–3) motivated analysis of TrkA receptors in these organelles. We examined TrkA in the following cell fractions after in vitro reactions in the presence of ATP: 1) the NP40-soluble cell ghost membranes (P1), containing plasma membrane; 2) the 8,000 × g pellet (P2), containing clathrin-coated vesicles; 3) the 100,000 × g pellet (P3), containing small vesicles, and 4) supernatant (S3) containing cytosol (20). We asked if NGF was bound to TrkA in these fractions and if TrkA was activated (i.e., tyrosine phosphorylated). A membrane permeable crosslinking reagent, disuccinimidyl suberate, crosslinked 125I-NGF to TrkA in the small vesicle (P3) fraction (Fig. 3A). The amount of NGF crosslinked to TrkA in the small vesicles was 10% of total crosslinked NGF; this is in good agreement with the amount of NGF in these vesicles (10.1% ± 1 of total bound NGF, n = 10), suggesting that NGF in the small vesicles remained bound to TrkA.
Continued receptor occupancy suggested that TrkA would be activated in the small vesicles. TrkA was immunoprecipitated after a 10 min internalization in the absence and presence of NGF, followed by in vitro reactions with ATP (Fig. 3B). Under these conditions about a third of the mature form of the receptor, gp140TrkA, was recovered in the P3 (Fig. 3C). This represents TrkA in both the biosynthetic and endocytic pathways. The P2 and P3 fractions together contained 10–15% of the immature form of the receptor, gp110TrkA (41, 42), and the amount changed little with NGF (Fig. 3 B and D). Activated TrkA, which must be derived from the plasma membrane where it was exposed to NGF, was assessed by blotting immunoprecipitates with anti-phosphotyrosine (Fig. 3B Right). Fractions prepared from cells not exposed to NGF contained very little tyrosine phosphorylated TrkA, indicating that in vitro reactions did not artificially activate the receptor (Fig. 3 B and E). The small vesicle (P3) fraction contained almost one-third (28%) of the total tyrosine phosphorylated TrkA after NGF treatment (Fig. 3E). TrkA activation was persistent in these vesicles since it was detected 25 min after initiating NGF treatment (i.e., 10 min in vivo, 15 min in vitro).
Since the reactions above were performed in the absence of phophatase inhibitors, it is possible that tyrosine phosphatases were acting on TrkA. Without in vitro reactions, NGF induced a 17-fold increase in total cellular tyrosine phosphorylated TrkA (20). After in vitro reactions with ATP, stimulation was reduced to 14-fold. If the phosphatase inhibitor orthovanadate was added to in vitro reactions, a large increase in background tyrosine phosphorylation of TrkA was observed in cells not treated with NGF, and more tyrosine phosphorylated TrkA was detected in the P2 than in the P3, with or without NGF (Fig. 3F and data not shown). In spite of the increase in background, NGF induced a 5-fold increase in total tyrosine phosphorylated TrkA (Fig. 3F Right).
PLC-γ has been shown to bind to TrkA after NGF activation (43, 44). We have shown previously that PLC-γ was bound to TrkA in cell ghosts and intracellular organelles after NGF treatment (20). We asked whether this binding persisted in small vesicles after in vitro reactions. PLC-γ was immunoprecipitated from the small vesicle (P3) fractions after a 10 min internalization followed by in vitro reactions in the presence of ATP and orthovanadate (Fig. 3F). Under these conditions tyrosine phosphorylated PLC-γ was detected in the small vesicle fraction only in NGF treated cells (Fig. 3F, PLC-γ immunoprecipitation) and some was precipitated by anti-TrkA (P3, TrkA immunoprecipitation). The presence of activated TrkA and TrkA bound to PLC-γ in small vesicles suggests that signal transduction could be initiated from receptors in these organelles.
Our studies have identified one possible organelle that may convey the NGF signal down axons. Important signaling events may be mediated through TrkA in other organelles, through p75NTR, or through signal transduction mediators downstream from receptors (45). Because it was the first NGF receptor to be discovered, p75NTR was invoked in earlier versions of the “signaling vesicle” hypothesis (1, 2). The role of p75NTR, which binds other neurotrophins as well as NGF, has been controversial (46–49). The importance of this receptor is demonstrated by the fact that mice in whom the gene has been disrupted show abnormalities in both the peripheral and central nervous system (50). p75NTR has been shown to enhance NGF binding and signaling through TrkA (51–54). p75NTR also initiates signal transduction separately from TrkA (55–57). There is increasing evidence that the signals generated by p75NTR promote programmed cell death (58–61) rather than prevent it, as does TrkA (62). p75NTR is internalized and retrogradely transported, but it does not appear to be down-regulated from the cell surface in response to NGF (M.G., D. Hall, A. Schroepfel, and W.M., unpublished observations) (63–65). Moreover, p75NTR does not appear to play a significant role in the retrograde transport of NGF, but does transport other neurotrophins (66, 67). These findings suggest that p75NTR does not play a role in survival-promoting retrograde signaling by NGF.
In previous work (20) on NGF-treated PC12 cells, we showed that NGF was internalized bound to TrkA. Different endocytic organelles were separated by differential sedimentation and both large and small organelles were found to contain activated TrkA. In the present study we used in vitro reactions containing semi-intact PC12 cells and ATP to examine the kinetics of formation and physical characteristics of a novel organelle: small vesicles containing internalized NGF bound to activated TrkA. These vesicles were distinct from clathrin-coated vesicles, uncoated primary endocytic vesicles, and synaptic vesicles, and resembled transport vesicles in their sedimentation velocity and equilibrium density. They contained at least 10% of the total bound NGF and almost one-third of the total tyrosine phosphorylated TrkA. Because not all of the organelles may have emerged from permeabilized cells, we regard these values as a lower limit for the content of NGF and activated TrkA. Receptor activation was persistent in the vesicles. Sustained activation of receptors in intracellular organelles may explain prolonged activation of downstream kinases by TrkA (4, 68, 69). These findings provide strong evidence for the existence of a distinct class of vesicles derived from endosomes. Furthermore, the small vesicles are a compelling candidate for retrograde NGF signaling. They support the hypothesis that internalized, activated TrkA receptors could transduce the NGF signal from the platform of a small vesicle (1–3). It remains to be seen if these organelles are used to retrogradely transport activated TrkA from distal axons to neuron cell bodies (8).
Acknowledgments
We thank Gretchen McCaffrey for technical assistance. M.G. was supported by the National Alliance for Research on Schizophrenia and Depression, Whitehall Foundation, Cancer Society of New Zealand, Lottery Health and Science, National Child Health Research Foundation, and the Palmerston North Medical Research Foundation. E.B. was supported by National Institutes of Health Grant T32 NS 07219. W.C.M. acknowledges support by the National Institutes of Health (NS 24054), the Adler Foundation, the McGowan Charitable Trust, and the March of Dimes.
ABBREVIATIONS
- NGF
nerve growth factor
- TrkA
the receptor tyrosine kinase for NGF
- PLC-γ
phospholipase-C-γ1
References
- 1.Misko T P, Radeke M J, Shooter E M. J Exp Biol. 1987;132:177–190. doi: 10.1242/jeb.132.1.177. [DOI] [PubMed] [Google Scholar]
- 2.Halegoua S, Armstrong R C, Kremer N E. Curr Top Microbiol Immunol. 1991;165:119–170. doi: 10.1007/978-3-642-75747-1_7. [DOI] [PubMed] [Google Scholar]
- 3.Grimes M, Zhou J, Li Y, Holtzman D, Mobley W C. Semin Neurosci. 1993;5:239–247. [Google Scholar]
- 4.Baass P C, Di Guglielmo B M, Authier F, Posner B I, Bergeron J J. Trends Cell Biol. 1995;5:465–475. doi: 10.1016/s0962-8924(00)89116-3. [DOI] [PubMed] [Google Scholar]
- 5.Tooze J, Hollinshead M, Fuller S D, Tooze S A, Huttner W B. Eur J Cell Biol. 1989;49:259–273. [PubMed] [Google Scholar]
- 6.Sulzer D, Holtzman E. J Neurocytol. 1989;18:529–540. doi: 10.1007/BF01474548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Geer P, Hunter T, Lindberg R A. Annu Rev Cell Biol. 1994;10:251–337. doi: 10.1146/annurev.cb.10.110194.001343. [DOI] [PubMed] [Google Scholar]
- 8.Ehlers M D, Kaplan D R, Price D L, Koliatsos V E. J Cell Biol. 1995;130:149–156. doi: 10.1083/jcb.130.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greene L A, Tischler A S. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan D R, Stephens R M. J Neurobiol. 1994;25:1404–1417. doi: 10.1002/neu.480251108. [DOI] [PubMed] [Google Scholar]
- 11.Levi A, Biocca S, Cattaneo A, Calissano P. Mol Neurobiol. 1988;2:201–226. doi: 10.1007/BF02935346. [DOI] [PubMed] [Google Scholar]
- 12.Mills J C, Wang S, Erecinska M, Pittman R N. Methods Cell Biol. 1995;46:217–242. doi: 10.1016/s0091-679x(08)61931-7. [DOI] [PubMed] [Google Scholar]
- 13.Mesner P W, Winters T R, Green S H. J Cell Biol. 1992;119:1669–1680. doi: 10.1083/jcb.119.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batistatou A, Greene L A. J Cell Biol. 1993;122:523–532. doi: 10.1083/jcb.122.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards S N, Tolkovsky A M. J Cell Biol. 1994;124:537–546. doi: 10.1083/jcb.124.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clift-O’Grady L, Linstedt A D, Lowe A W, Grote E, Kelly R B. J Cell Biol. 1990;110:1693–1703. doi: 10.1083/jcb.110.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cameron P L, Sudhof T C, Jahn R, De Camilli P. J Cell Biol. 1991;115:151–164. doi: 10.1083/jcb.115.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly R B. Cell/Neuron. 1993;72/10:43–53. doi: 10.1016/s0092-8674(05)80027-3. [DOI] [PubMed] [Google Scholar]
- 19.Bean A J, Seifert R, Chen Y A, Sacks R, Scheller R H. Nature (London) 1997;385:826–829. doi: 10.1038/385826a0. [DOI] [PubMed] [Google Scholar]
- 20.Grimes M L, Zhou J, Beattie E, Yuen E C, Hall D E, Valletta J S, Topp K S, LaVail J H, Bunnett N W, Mobley W C. J Neurosci. 1996;16:7950–7964. doi: 10.1523/JNEUROSCI.16-24-07950.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trowbridge I S, Collawn J F, Hopkins C R. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- 22.Gruenberg J, Maxfield F R. Curr Opin Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- 23.Sudhof T C. Nature (London) 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 24.Takei K, Mundigl O, Daniell L, De Camilli P. J Cell Biol. 1996;133:1237–1250. doi: 10.1083/jcb.133.6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitney J A, Gomez M, Sheff D, Kreis T E, Mellman I. Cell. 1995;83:703–713. doi: 10.1016/0092-8674(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 26.Aniento F, Gu F, Parton R G, Gruenberg J. J Cell Biol. 1996;133:29–41. doi: 10.1083/jcb.133.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoorvogel W, Oorschot V, Geuze H J. J Cell Biol. 1996;132:21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmid S L, Damke H. FASEB J. 1995;9:1445–1453. doi: 10.1096/fasebj.9.14.7589986. [DOI] [PubMed] [Google Scholar]
- 29.Podbilewicz B, Mellman I. EMBO J. 1990;9:3477–3487. doi: 10.1002/j.1460-2075.1990.tb07556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett M K, Wandinger-Ness A, Simons K. EMBO J. 1988;7:4075–4085. doi: 10.1002/j.1460-2075.1988.tb03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grimes M, Kelly R B. J Cell Biol. 1992;117:539–549. doi: 10.1083/jcb.117.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Curtis I, Simons K. Cell. 1989;58:719–727. doi: 10.1016/0092-8674(89)90106-2. [DOI] [PubMed] [Google Scholar]
- 33.Desnos C, Clift O G L, Kelly R B. J Cell Biol. 1995;130:1041–1049. doi: 10.1083/jcb.130.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin T F. Methods Enzymol. 1989;168:225–233. doi: 10.1016/0076-6879(89)68016-0. [DOI] [PubMed] [Google Scholar]
- 35.Balch W E, Rothman J E. Arch Biochem Biophys. 1985;240:413–425. doi: 10.1016/0003-9861(85)90046-3. [DOI] [PubMed] [Google Scholar]
- 36.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 37.Rothman J E. Nature (London) 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 38.Calakos N, Scheller R H. Physiol Rev. 1996;76:1–29. doi: 10.1152/physrev.1996.76.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Linstedt A D, Kelly R B. Neuron. 1991;7:309–317. doi: 10.1016/0896-6273(91)90269-6. [DOI] [PubMed] [Google Scholar]
- 40.Grimes M, Kelly R B. Ann NY Acad Sci. 1992;674:38–52. doi: 10.1111/j.1749-6632.1992.tb27475.x. [DOI] [PubMed] [Google Scholar]
- 41.Martin-Zanca D, Oskam R, Mitra G, Copeland T, Barbacid M. Mol Cell Biol. 1989;9:24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou J, Valletta J S, Grimes M L, Mobley W C. J Neurochem. 1995;65:1146–1156. doi: 10.1046/j.1471-4159.1995.65031146.x. [DOI] [PubMed] [Google Scholar]
- 43.Stephens R M, Loeb D M, Copeland T D, Pawson T, Greene L A, Kaplan D R. Neuron. 1994;12:691–705. doi: 10.1016/0896-6273(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 44.Obermeier A, Bradshaw R A, Seedorf K, Choidas A, Schlessinger J, Ullrich A. EMBO J. 1994;13:1585–1590. doi: 10.1002/j.1460-2075.1994.tb06421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crouch M F, Heydon K, Garnaut S M, Milburn P J, Hendry I A. Eur J Neurosci. 1994;6:626–631. doi: 10.1111/j.1460-9568.1994.tb00307.x. [DOI] [PubMed] [Google Scholar]
- 46.Chao M V. J Neurobiol. 1994;25:1373–1385. doi: 10.1002/neu.480251106. [DOI] [PubMed] [Google Scholar]
- 47.Greene L A, Kaplan D R. Curr Opin Neurobiol. 1995;5:579–587. doi: 10.1016/0959-4388(95)80062-x. [DOI] [PubMed] [Google Scholar]
- 48.Bothwell M. Annu Rev Neurosci. 1995;18:223–253. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- 49.Bothwell M. Science. 1996;272:506–507. doi: 10.1126/science.272.5261.506. [DOI] [PubMed] [Google Scholar]
- 50.Lee K F, Li E, Huber L J, Landis S C, Sharpe A H, Chao M V, Jaenisch R. Cell. 1992;69:737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- 51.Barker P A, Shooter E M. Neuron. 1994;13:203–215. doi: 10.1016/0896-6273(94)90470-7. [DOI] [PubMed] [Google Scholar]
- 52.Hantzopoulos P A, Suri C, Glass D J, Goldfarb M P, Yancopoulos G D. Neuron. 1994;13:187–201. doi: 10.1016/0896-6273(94)90469-3. [DOI] [PubMed] [Google Scholar]
- 53.Benedetti M, Levi A, Chao M V. Proc Natl Acad Sci USA. 1993;90:7859–7863. doi: 10.1073/pnas.90.16.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hempstead B L, Martin Z D, Kaplan D R, Parada L F, Chao M V. Nature (London) 1991;350:678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- 55.Carter B D, Kaltschmidt C, Kaltschmidt B, Offenhauser N, Bohm-Matthaei R, Baeuerle P A, Barde Y A. Science. 1996;272:542–545. doi: 10.1126/science.272.5261.542. [DOI] [PubMed] [Google Scholar]
- 56.Dobrowsky R T, Werner M H, Castellino A M, Chao M V, Hannun Y A. Science. 1994;265:1596–1599. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- 57.Canossa M, Twiss J L, Verity A N, Shooter E M. EMBO J. 1996;15:3369–3376. [PMC free article] [PubMed] [Google Scholar]
- 58.Casaccia-Bonnefil P, Carter B D, Dobrowsky R T, Chao M V. Nature (London) 1996;383:716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- 59.Frade J M, Rodriguez-Tebar A, Barde Y A. Nature (London) 1996;383:166–168. doi: 10.1038/383166a0. [DOI] [PubMed] [Google Scholar]
- 60.Van der Zee C E, Ross G M, Riopelle R J, Hagg T. Science. 1996;274:1729–1732. doi: 10.1126/science.274.5293.1729. [DOI] [PubMed] [Google Scholar]
- 61.Carter B D, Lewin G R. Neuron. 1997;18:187–190. doi: 10.1016/s0896-6273(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 62.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 63.Johnson E M J, Andres R Y, Bradshaw R A. Brain Res. 1978;150:319–331. doi: 10.1016/0006-8993(78)90283-4. [DOI] [PubMed] [Google Scholar]
- 64.Hosang M, Shooter E M. EMBO J. 1987;6:1197–202. doi: 10.1002/j.1460-2075.1987.tb02354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eveleth D D, Bradshaw R A. J Cell Biol. 1992;117:291–299. doi: 10.1083/jcb.117.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Curtis R, Adryan K M, Stark J L, Park J S, Compton D L, Weskamp G, Huber L J, Chao M V, Jaenisch R, Lee K F. Neuron. 1995;14:1201–1211. doi: 10.1016/0896-6273(95)90267-8. [DOI] [PubMed] [Google Scholar]
- 67.von Bartheld C S, Williams R, Lefcort F, Clary D O, Reichardt L F, Bothwell M. J Neurosci. 1996;16:2995–3008. doi: 10.1523/JNEUROSCI.16-09-02995.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marshall C J. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 69.Qui M S, Green S H. Neuron. 1992;9:705–717. doi: 10.1016/0896-6273(92)90033-a. [DOI] [PubMed] [Google Scholar]
- 70.Nathke I S, Heuser J, Lupas A, Stock J, Turck C W, Brodsky F M. Cell. 1992;68:899–910. doi: 10.1016/0092-8674(92)90033-9. [DOI] [PubMed] [Google Scholar]
- 71.Chin D J, Straubinger R M, Acton S, Nathke I, Brodsky F M. Proc Natl Acad Sci USA. 1989;86:9289–9293. doi: 10.1073/pnas.86.23.9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clary D O, Weskamp G, Austin L R, Reichardt L F. Mol Biol Cell. 1994;5:549–563. doi: 10.1091/mbc.5.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]