Abstract
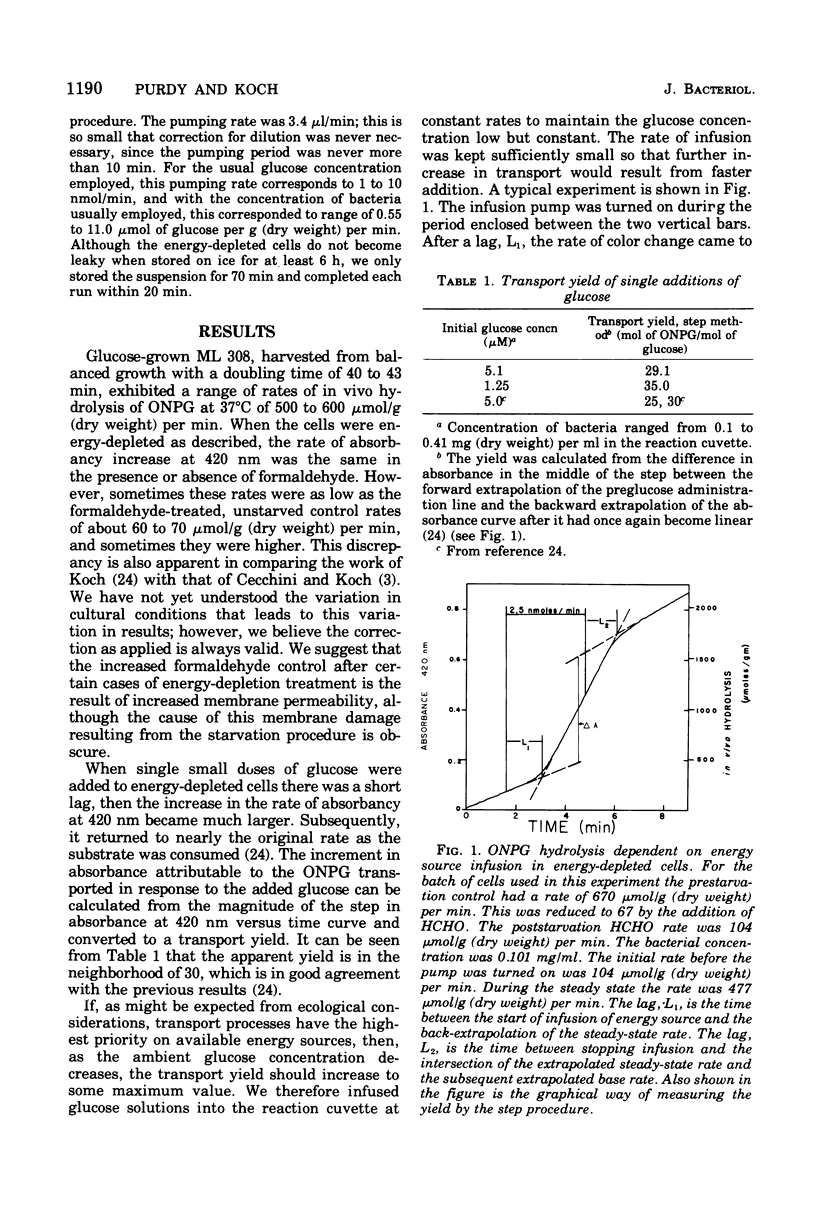
Energy reserves of Escherichia coli can be depleted by our previously reported procedure to a level such that even the "downhill" transport of o-nitrophenyl-beta-D-galactopyranoside (ONPG) is completely dependent upon the exogenous energy supply. The ONPG concentration is high externally to the cells and is low intracellular because of the action of cytoplasmic beta-galactosidase. In the present work, depleted cell suspensions have been infused at low, steady rates with glucose and other energy sources while measurements of transport were being made. Comparing the rate of ONPG transport with the rate of introduction of glucose under conditions where the chosen glucose infusion rate limits transport, we find that 89 molecules of ONPG are transported per molecule of fully oxidized glucose. This transport yield is constant over a 6.5-fold range in rate of glucose addition. This constancy over a range of infusion rates implies that transport is the major cellular function under these special conditions. The yield value if 89 is in the agreement with the predicitions of 76 from Mitchell's chemiosmotic theory and constitutes an independent proff of its validity, since all the other proposed mechanisms of engery coupling predict much smaller yields. The lag from the start of glucose infusion into the reaction cuvette, to the extrapolated time at which a steady rate of transport and concomitant hydrolysis are achieved, is short (approximately 1 min). Similarly, the time after the infusion is stopped until the rate of transport returns to the background rate is also short. The latter implies that the energy metabolism is directed almost entirely to transport and/or other ongoing cellular processes and not to repair or renewal of an energy-independent, facilitated diffusion system.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altendorf K., Hirata H., Harold F. M. Accumulation of lipid-soluble ions and of rubidium as indicators of the electrical potential in membrane vesicles of Escherichia coli. J Biol Chem. 1975 Feb 25;250(4):1405–1412. [PubMed] [Google Scholar]
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchini G., Koch A. L. Effect of uncouplers on "downhill" beta-galactoside transport in energy-depleted cells of Escherichia coli. J Bacteriol. 1975 Jul;123(1):187–195. doi: 10.1128/jb.123.1.187-195.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E., WATSON J. A., HOFFEE P. A. The glucose effect and the relationship between glucose permease, acid phosphatase, and glucose resistance. Cold Spring Harb Symp Quant Biol. 1961;26:261–276. doi: 10.1101/sqb.1961.026.01.033. [DOI] [PubMed] [Google Scholar]
- Fox C. F., Kennedy E. P. Specific labeling and partial purification of the M protein, a component of the beta-galactoside transport system of Escherichia coli. Proc Natl Acad Sci U S A. 1965 Sep;54(3):891–899. doi: 10.1073/pnas.54.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFEE P., ENGLESBERG E. Effect of metabolic activity on the glucose permease of bacterial cells. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1759–1765. doi: 10.1073/pnas.48.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFEE P., ENGLESBERG E., LAMY F. THE GLUCOSE PERMEASE SYSTEM IN BACTERIA. Biochim Biophys Acta. 1964 Mar 30;79:337–350. [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengstenberg W., Egan J. B., Morse M. L. Carbohydrate transport in Staphylococcus aureus. V. The accumulation of phosphorylated carbohydrate derivatives, and evidence for a new enzyme-splitting lactose phosphate. Proc Natl Acad Sci U S A. 1967 Jul;58(1):274–279. doi: 10.1073/pnas.58.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg E. L., Hinkle P. C. Oxidative phosphorylation and proton translocation in membrane vesicles prepared from Escherichia coli. Biochem Biophys Res Commun. 1974 May 7;58(1):178–184. doi: 10.1016/0006-291x(74)90908-5. [DOI] [PubMed] [Google Scholar]
- Hirata H., Altendorf K., Harold F. M. Role of an electrical potential in the coupling of metabolic energy to active transport by membrane vesicles of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1804–1808. doi: 10.1073/pnas.70.6.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. W., Brice J. M., Downs A. J., Drozd J. W. Bacterial respiration-linked proton translocation and its relationship to respiratory-chain composition. Eur J Biochem. 1975 Mar 17;52(2):265–271. doi: 10.1111/j.1432-1033.1975.tb03994.x. [DOI] [PubMed] [Google Scholar]
- KEPES A. [Kinetic studies on galactoside permease of Escherichia coli]. Biochim Biophys Acta. 1960 May 6;40:70–84. doi: 10.1016/0006-3002(60)91316-0. [DOI] [PubMed] [Google Scholar]
- KOCH A. L. THE ROLE OF PERMEASE IN TRANSPORT. Biochim Biophys Acta. 1964 Jan 27;79:177–200. doi: 10.1016/0926-6577(64)90050-6. [DOI] [PubMed] [Google Scholar]
- KOCH A. L. The inactivation of the transport mechanism for beta-galactosides of Escherichia coli under various physiological conditions. Ann N Y Acad Sci. 1963 Jan 21;102:602–620. doi: 10.1111/j.1749-6632.1963.tb13663.x. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Barnes E. M., Jr Mechanisms of active transport in isolated membrane vesicles. II. The mechanism of energy coupling between D-lactic dehydrogenase and beta-galactoside transport in membrane preparations from Escherichia coli. J Biol Chem. 1971 Sep 10;246(17):5523–5531. [PubMed] [Google Scholar]
- Kaback H. R. The role of the phosphoenolpyruvate-phosphotransferase system in the transport of sugars by isolated membrane preparations of Escherichia coli. J Biol Chem. 1968 Jul 10;243(13):3711–3724. [PubMed] [Google Scholar]
- Kaback H. R. Transport studies in bacterial membrane vesicles. Science. 1974 Dec 6;186(4167):882–892. doi: 10.1126/science.186.4167.882. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Proton-coupled accumulation of galactoside in Streptococcus lactis 7962. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2866–2869. doi: 10.1073/pnas.70.10.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy E. P., Scarborough G. A. Mechanism of hydrolysis of O-nitrophenyl-beta-galactoside in Staphylococcus aureus and its significance for theories of sugar transport. Proc Natl Acad Sci U S A. 1967 Jul;58(1):225–228. doi: 10.1073/pnas.58.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein W. L., Boyer P. D. Energization of active transport by Escherichia coli. J Biol Chem. 1972 Nov 25;247(22):7257–7265. [PubMed] [Google Scholar]
- Koch A. L. Energy expenditure is obligatory for the downhill transport of galactosides. J Mol Biol. 1971 Aug 14;59(3):447–459. doi: 10.1016/0022-2836(71)90309-3. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Unimportance of counterflux in the energetics of "downhill" transport. J Bacteriol. 1974 Nov;120(2):895–901. doi: 10.1128/jb.120.2.895-901.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundig W. Molecular interactions in the bacterial phosphoenolpyruvate-phosphotransferase system (PTS). J Supramol Struct. 1974;2(5-6):695–814. doi: 10.1002/jss.400020514. [DOI] [PubMed] [Google Scholar]
- Laue P., MacDonald R. E. Studies on the relation of thiomethyl-beta-D-galactoside accumulation to thiomethyl-beta-D-galactoside phosphorylation in Staphylococcus aureus HS1159. Biochim Biophys Acta. 1968 Oct 15;165(3):410–418. doi: 10.1016/0304-4165(68)90220-1. [DOI] [PubMed] [Google Scholar]
- Lawford H. G., Haddock B. A. Respiration-driven proton translocation in Escherichia coli. Biochem J. 1973 Sep;136(1):217–220. doi: 10.1042/bj1360217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M. A., Hong J. S. Energization of osmotic shock-sensitive transport systems in Escherichia coli requires more than ATP. Arch Biochem Biophys. 1976 Jan;172(1):312–315. doi: 10.1016/0003-9861(76)90080-1. [DOI] [PubMed] [Google Scholar]
- Meyer D. J., Jones C. W. Oxidative phosphorylation in bacteria which contain different cytochrome oxidases. Eur J Biochem. 1973 Jul 2;36(1):144–151. doi: 10.1111/j.1432-1033.1973.tb02894.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Performance and conservation of osmotic work by proton-coupled solute porter systems. J Bioenerg. 1973 Jan;4(1):63–91. doi: 10.1007/BF01516051. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Kaback H. R. Membrane potential and active transport in membrane vesicles from Escherichia coli. Biochemistry. 1975 Dec 16;14(25):5451–5461. doi: 10.1021/bi00696a011. [DOI] [PubMed] [Google Scholar]
- West I., Mitchell P. Proton-coupled beta-galactoside translocation in non-metabolizing Escherichia coli. J Bioenerg. 1972 Aug;3(5):445–462. doi: 10.1007/BF01516082. [DOI] [PubMed] [Google Scholar]
- Winkler H. H. Efflux and the steady state in alpha-methylglucoside transport in Escherichia coli. J Bacteriol. 1971 May;106(2):362–368. doi: 10.1128/jb.106.2.362-368.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


