Abstract
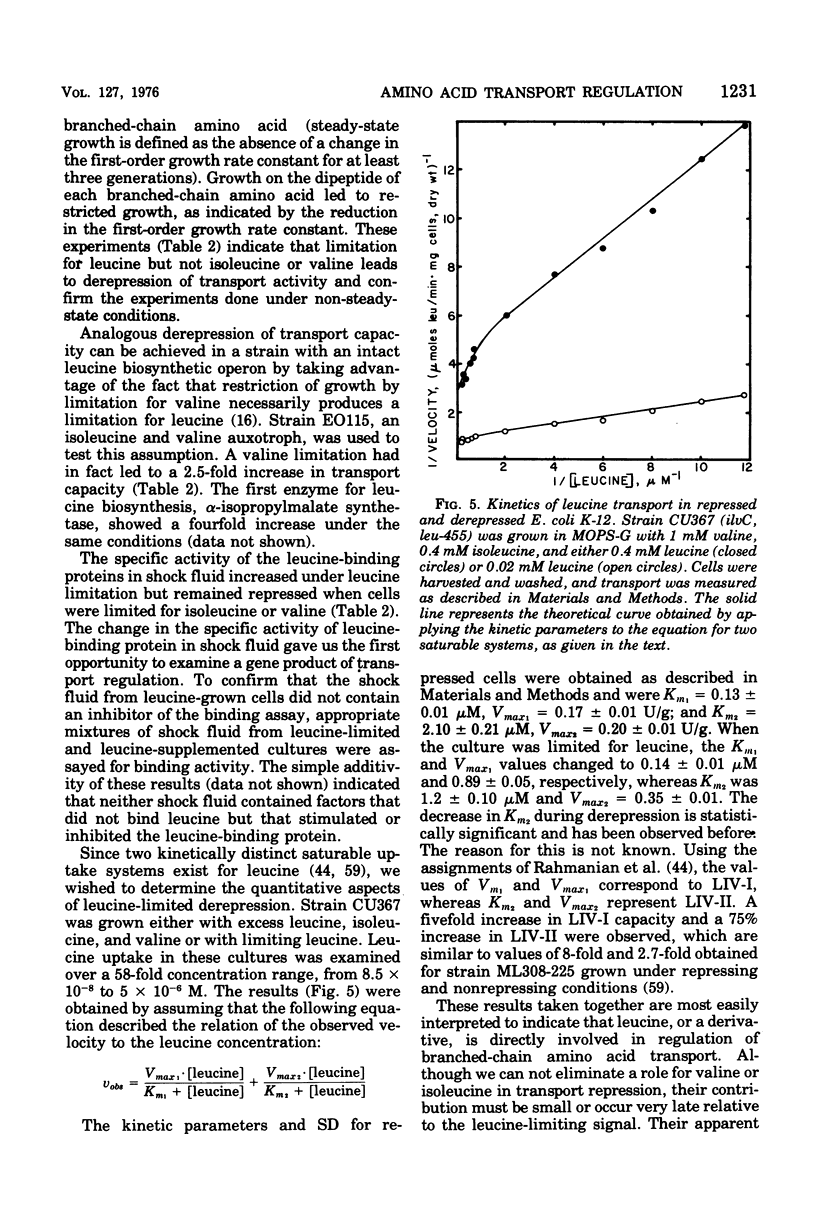
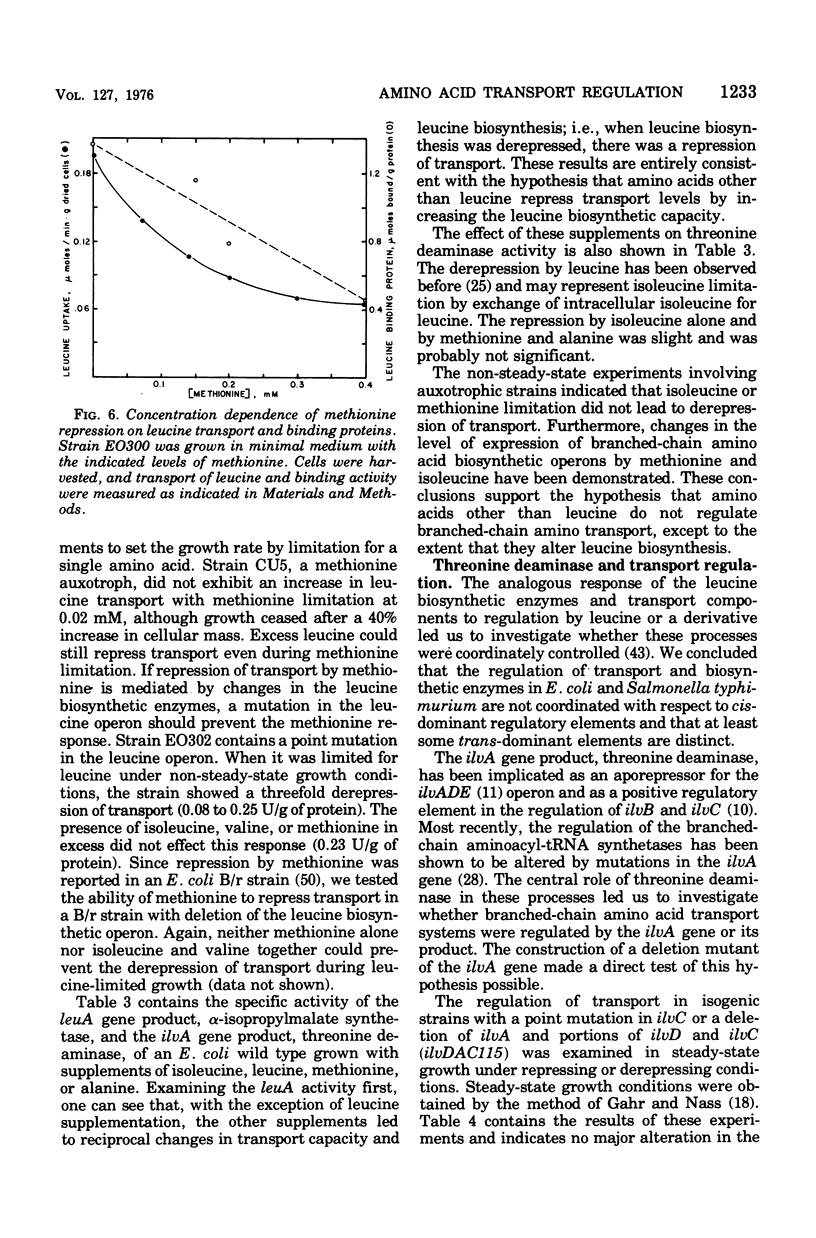
The repression and derepression of leucine, isoleucine, and valine transport in Escherichia coli K-12 was examined by using strains auxotrophic for leucine, isoleucine, valine, and methionine. In experiments designed to limit each of these amino acids separately, we demonstrate that leucine limitation alone derepressed the leucine-binding protein, the high-affinity branched-chain amino acid transport system (LIV-I), and the membrane-bound, low-affinity system (LIV-II). This regulation did not seem to involve inactivation of transport components, but represented an increase in the differential rate of synthesis of transport components relative to total cellular proteins. The apparent regulation of transport by isoleucine, valine, and methionine reported elsewhere was shown to require an intact leucine, biosynthetic operon and to result from changes in the level of leucine biosynthetic enzymes. A functional leucyl-transfer ribonucleic acid synthetase was also required for repression of transport. Transport regulation was shown to be essentially independent of ilvA or its gene product, threonine deaminase. The central role of leucine or its derivatives in cellular metabolism in general is discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALFOELDI L., KEREKES E. NEUTRALIZATION OF THE AMINO ACID SENSITIVITY OF RCREL ESCHERICHIA COLI. Biochim Biophys Acta. 1964 Sep 11;91:155–157. doi: 10.1016/0926-6550(64)90179-3. [DOI] [PubMed] [Google Scholar]
- Ames G. F., Lever J. Components of histidine transport: histidine-binding proteins and hisP protein. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1096–1103. doi: 10.1073/pnas.66.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand K., Korn L., Lee F., Platt T., Squires C. L., Squires C., Yanofsky C. New features of the regulation of the tryptophan operon. Science. 1975 Jul 4;189(4196):22–26. doi: 10.1126/science.1094538. [DOI] [PubMed] [Google Scholar]
- Broman R. L., Goldenbaum P. E., Dobrogosz W. J. The effect of amino acids on the ability of cyclic AMP to reverse catabolite repression in Escherichia coli. Biochem Biophys Res Commun. 1970 May 11;39(3):401–406. doi: 10.1016/0006-291x(70)90591-7. [DOI] [PubMed] [Google Scholar]
- Burns R. O., Calvo J., Margolin P., Umbarger H. E. Expression of the leucine operon. J Bacteriol. 1966 Apr;91(4):1570–1576. doi: 10.1128/jb.91.4.1570-1576.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns R. O., Zarlengo M. H. Threonine deaminase from Salmonella typhimurium. I. Purification and properties. J Biol Chem. 1968 Jan 10;243(1):178–185. [PubMed] [Google Scholar]
- Calhoun D. H., Hatfield G. W. Autoregulation: a role for a biosynthetic enzyme in the control of gene expression. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2757–2761. doi: 10.1073/pnas.70.10.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman W., Jr, Kline E. L., Brown C. S., Williams L. S. Regulation of branched-chain aminoacyl-transfer ribonucleic acid synthetases in an ilvDAC deletion strain of Escherichia coli K-12. J Bacteriol. 1975 Mar;121(3):785–793. doi: 10.1128/jb.121.3.785-793.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch C. E., Soffer R. L. Regulation of proline catabolism by leucyl,phenylalanyl-tRNA-protein transferase. Proc Natl Acad Sci U S A. 1975 Jan;72(1):405–408. doi: 10.1073/pnas.72.1.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. ROLE OF VALYL-SRNA SYNTHETASE IN ENZYME REPRESSION. Proc Natl Acad Sci U S A. 1965 Mar;53:539–543. doi: 10.1073/pnas.53.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUNDLICH M., BURNS R. O., UMBARGER H. E. Control of isoleucine, valine, and leucine biosynthesis. I. Multivalent repression. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1804–1808. doi: 10.1073/pnas.48.10.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M. J., Peterkofsky A. Formation of chromatographically unique species of transfer ribonucleic acid during amino acid starvation of relaxed-control Escherichia coli. J Bacteriol. 1975 May;122(2):538–548. doi: 10.1128/jb.122.2.538-548.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J., Newman E. B. Derivation of glycine from threonine in Escherichia coli K-12 mutants. J Bacteriol. 1975 Jun;122(3):810–817. doi: 10.1128/jb.122.3.810-817.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong C. E., Weiner J. H. Purification of a leucine-specific binding protein from Escherichia coli. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1076–1083. doi: 10.1016/0006-291x(70)90349-9. [DOI] [PubMed] [Google Scholar]
- Gahr M., Nass G. Regulation of the formation of isoleucyl-tRNA-synthetase and the level of isoleucine biosynthetic enzymes in E. coli K12. Mol Gen Genet. 1972;116(4):348–359. doi: 10.1007/BF00270091. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Dice J. F. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem. 1974;43(0):835–869. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- Greene R. C., Radovich C. Role of methionine in the regulation of serine hydroxymethyltransferase in Eschericia coli. J Bacteriol. 1975 Oct;124(1):269–278. doi: 10.1128/jb.124.1.269-278.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiola J., De Felice M., Klopotowski T., Iaccarino M. Multiplicity of isoleucine, leucine, and valine transport systems in Escherichia coli K-12. J Bacteriol. 1974 Feb;117(2):382–392. doi: 10.1128/jb.117.2.382-392.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiola J., De Felice M., Klopotowski T., Iaccarino M. Mutations affecting the different transport systems for isoleucine, leucine, and valine in Escherichia coli K-12. J Bacteriol. 1974 Feb;117(2):393–405. doi: 10.1128/jb.117.2.393-405.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield G. W., Burns R. O. Specific binding of leucyl transfer RNA to an immature form of L-threonine deaminase: its implications in repression. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1027–1035. doi: 10.1073/pnas.66.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield I. N., Yeh F. M., Sawyer L. E. Metabolites influence control of lysine transfer ribonucleic acid synthetase formation in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1364–1367. doi: 10.1073/pnas.72.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J., Williams L. S., Umbarger H. E. Regulation of synthesis of the branched-chain amino acids and cognate aminoacyl-transfer ribonucleic acid synthetases of Escherichia coli: a common regulatory element. J Bacteriol. 1974 Dec;120(3):1380–1386. doi: 10.1128/jb.120.3.1380-1386.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R., Stadtman E. R. Proline uptake by an isolated cytoplasmic membrane preparation of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Apr;55(4):920–927. doi: 10.1073/pnas.55.4.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki S., Anraku Y. Transport of sugars and amino acids in bacteria. IV. Regulation of valine transport activity by valine and cysteine. J Biochem. 1971 Aug;70(2):215–224. doi: 10.1093/oxfordjournals.jbchem.a129633. [DOI] [PubMed] [Google Scholar]
- Kline E. L., Brown C. S., Umbarger H. E. Effect of a leu-linked mutation on the valine sensitivity of acetohydroxy acid synthase activity in Escherichia coli. J Bacteriol. 1975 Feb;121(2):491–496. doi: 10.1128/jb.121.2.491-496.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L. Turbidity measurements of bacterial cultures in some available commercial instruments. Anal Biochem. 1970 Nov;38(1):252–259. doi: 10.1016/0003-2697(70)90174-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Low B., Gates F., Goldstein T., Söll D. Isolation and partial characterization of temperature-sensitive Escherichia coli mutants with altered leucyl- and seryl-transfer ribonucleic acid synthetases. J Bacteriol. 1971 Nov;108(2):742–750. doi: 10.1128/jb.108.2.742-750.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis E., Williams L. S. Regulation of synthesis of the aminoacyl-transfer ribonucleic acid synthetases for the branched-chain amino acids of Escherichia coli. J Bacteriol. 1971 Oct;108(1):254–262. doi: 10.1128/jb.108.1.254-262.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal J. L. Analysis of Michaelis kinetics for two independent, saturable membrane transport functions. J Theor Biol. 1972 Apr;35(1):113–118. doi: 10.1016/0022-5193(72)90196-8. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxender D. L., Quay S. Binding proteins and membrane transport. Ann N Y Acad Sci. 1975 Dec 30;264:358–372. doi: 10.1111/j.1749-6632.1975.tb31496.x. [DOI] [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. Induced formation of serine and threonine deaminases by Escherichia coli. J Bacteriol. 1955 Dec;70(6):667–674. doi: 10.1128/jb.70.6.667-674.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penrose W. R., Nichoalds G. E., Piperno J. R., Oxender D. L. Purification and properties of a leucine-binding protein from Escherichia coli. J Biol Chem. 1968 Nov 25;243(22):5921–5928. [PubMed] [Google Scholar]
- Pine M. J. Regulation of intracellular proteolysis in Escherichia coli. J Bacteriol. 1973 Jul;115(1):107–116. doi: 10.1128/jb.115.1.107-116.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino-acid-binding protein released from Escherichia coli by osmotic shock. J Biol Chem. 1966 Dec 10;241(23):5732–5734. [PubMed] [Google Scholar]
- Quay S. C., Kline E. L., Oxender D. L. Role of leucyl-tRNA synthetase in regulation of branched-chain amino-acid transport. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3921–3924. doi: 10.1073/pnas.72.10.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quay S. C., Oxender D. L., Tsuyumu S., Umbarger H. E. Separate regulation of transport and biosynthesis of leucine, isoleucine, and valine in bacteria. J Bacteriol. 1975 Jun;122(3):994–1000. doi: 10.1128/jb.122.3.994-1000.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmanian M., Claus D. R., Oxender D. L. Multiplicity of leucine transport systems in Escherichia coli K-12. J Bacteriol. 1973 Dec;116(3):1258–1266. doi: 10.1128/jb.116.3.1258-1266.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmanian M., Oxender D. L. Derepressed leucine transport activity in Escherichia coli. J Supramol Struct. 1972;1(1):55–59. doi: 10.1002/jss.400010108. [DOI] [PubMed] [Google Scholar]
- Richaud C., Mazat J. P., Felenbok B., Patte J. C. The role of lysine and leucine binding on the catalytical and structural properties of aspartokinase III of Escherichia coli K 12. Eur J Biochem. 1974 Oct 1;48(1):147–156. doi: 10.1111/j.1432-1033.1974.tb03752.x. [DOI] [PubMed] [Google Scholar]
- Stephens J. C., Artz S. W., Ames B. N. Guanosine 5'-diphosphate 3'-diphosphate (ppGpp): positive effector for histidine operon transcription and general signal for amino-acid deficiency. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4389–4393. doi: 10.1073/pnas.72.11.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmai A., Szentirmai M., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XV. Biochemical properties of mutants resistant to thiaisoleucine. J Bacteriol. 1968 May;95(5):1672–1679. doi: 10.1128/jb.95.5.1672-1679.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton B. A., Savageau M. A. Transport of biosynthetic intermediates: homoserine and threonine uptake in Escherichia coli. J Bacteriol. 1974 Mar;117(3):1002–1009. doi: 10.1128/jb.117.3.1002-1009.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton B. A., Savageau M. A. Transport of biosynthetic intermediates: regulation of homoserine and threonine uptake in Escherichia coli. J Bacteriol. 1974 Oct;120(1):114–120. doi: 10.1128/jb.120.1.114-120.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Isoleucine and valine metabolism in Escherichia coli. VII. A negative feedback mechanism controlling isoleucine biosynthesis. J Biol Chem. 1958 Aug;233(2):415–420. [PubMed] [Google Scholar]
- Umbarger H. E. Metabolite analogs as genetic and biochemical probes. Adv Genet. 1971;16:119–140. doi: 10.1016/s0065-2660(08)60356-9. [DOI] [PubMed] [Google Scholar]
- WILBRANDT W., ROSENBERG T. The concept of carrier transport and its corollaries in pharmacology. Pharmacol Rev. 1961 Jun;13:109–183. [PubMed] [Google Scholar]
- Williams L. S. Control of arginine biosynthesis in Escherichia coli: role of arginyl-transfer ribonucleic acid synthetase in repression. J Bacteriol. 1973 Mar;113(3):1419–1432. doi: 10.1128/jb.113.3.1419-1432.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. M. Leucine transport in Escherichia coli. The resolution of multiple transport systems and their coupling to metabolic energy. J Biol Chem. 1975 Jun 25;250(12):4477–4485. [PubMed] [Google Scholar]
- Yegian C. D., Stent G. S. An unusual condition of leucine transfer RNA appearing during leucine starvation of Escherichia coli. J Mol Biol. 1969 Jan 14;39(1):45–58. doi: 10.1016/0022-2836(69)90332-5. [DOI] [PubMed] [Google Scholar]


