Abstract
GnRH autoregulates GnRH neurons through an ultrashort feedback loop. One potential mechanism is the regulation of K+ channel activity through the GnRH receptor. Whereas GnRH inhibits the activity of the M-current in peripheral neurons, there is no direct evidence that the M-current is involved in the autoregulatory pathway of GnRH or if the M-current is expressed in GnRH neurons. The M-current is a noninactivating, subthreshold K+ current that inhibits cell excitability and is ubiquitously expressed in the central nervous system. We found that GnRH neurons expressed the neuronal M-current subunits, KCNQ2, -3, and -5 in addition to GnRH receptor (GnRH R1). Therefore, using whole-cell patch clamp recording and single-cell RT-PCR, we explored the effects of mammalian GnRH peptide on enhanced green fluorescent protein-tagged GnRH neurons acutely dispersed as well as in slice preparations. GnRH (100nm) inhibited GnRH neuronal excitability by hyperpolarizing the membrane. In the presence of CdCl2, BaCl2, and tetrodotoxin, GnRH activated an outward current in a dose-dependent manner (EC50 11 nm) in 30% of GnRH neurons. In voltage clamp, the selective M-channel blocker, XE-991, inhibited a K+ current in GnRH neurons. XE-991 also antagonized the outward K+ current induced by GnRH. Moreover, the GnRH effects on the M-current were blocked by the GnRH R1 antagonist antide. Therefore, these findings indicate that GnRH activates the M-current in a subpopulation of GnRH neurons via GnRH R1. This ultrashort circuit is one potential mechanism by which GnRH could modulate its own neuronal excitability through an autoreceptor.
THE PEPTIDE GnRH functions by a short-circuit feedback pathway to modulate the neuronal excitability of GnRH neurons (1,2), wherein GnRH and GnRH agonists reduce GnRH secretion and inhibit LH release in vivo (3) and in vitro (4). However, it is currently unclear which cellular mechanism(s) GnRH uses to modulate GnRH neuronal excitability. Several studies have reported that GnRH at high concentrations augments excitability (5,6,7), whereas others have indicated that GnRH inhibits GnRH neuronal excitability at low physiological concentrations (6,8,9). In the central nervous system, autoregulatory mechanisms often involve changes in neuronal excitability due to the activation of G protein-coupled inwardly rectifying K+ channels (10). Therefore, differential regulation of GnRH neuronal function may be due to concentration-dependent mechanisms that directly modulate GnRH neuronal excitability via activating or inhibiting K+ channel activity.
Potentially these divergent effects of GnRH can occur via the modulation of K+ channels that hyperpolarize the neurons and reduce excitability. In immortalized GnRH neurons (GT1–7), GnRH stimulates calcium-activated small conductance K+ (SK) channels (11). Another potential K+ channel candidate is the KCNQ family of channels that constitute the subthreshold, noninactivating M-current (12). GnRH was first identified as a modulator of the M-current in bullfrog sympathetic ganglia more than 25 yr ago (13), and multiple types of GnRH peptides (chicken I and II, salmon, mammalian) inhibit the M-current in bullfrog sympathetic neurons at low micromolar concentrations (14). The mechanism underlying the GnRH modulation of the M-current in these neurons is via a G protein-coupled receptor (GPCR) that activates phospholipase C (PLC) to hydrolyze phosphatidylinositol-3,4-bisphosphate (PIP2). The loss of PIP2 in the membrane is the primary mechanism for the attenuation of the M-current in ganglionic neurons (15). The KCNQ channels are also modulated by numerous neurotransmitters including acetylcholine, serotonin, and dopamine through GPCR signaling pathways (12).
However, the effects of GnRH on the M-current in GnRH neurons are not known, nor has the expression of the KCNQ channels in GnRH neurons been examined. Because GnRH modulates the M-current in other neuronal systems, we hypothesized that the modulation of the M-current by GnRH would alter the neuronal excitability of GnRH neurons via the GnRH receptor 1 (R1). To address this hypothesis, we recorded from acutely dispersed fluorescent protein (EGFP)-GnRH neurons and GnRH neurons in slice preparations from intact, adult male mice using whole-cell patch recording coupled with single cell RT-PCR to document the channel mRNA expression in individual GnRH neurons. Indeed, we found that in 30% of GnRH neurons, GnRH at low nanomolar concentrations activated the M-current and reduced neuronal excitability in a GnRH R1-dependent manner. Furthermore, we identified the presence of GnRH R1 in 25% of the GnRH neurons, which was coexpressed with KCNQ2, -3, and -5 subunits. These findings suggest that GnRH may inhibit GnRH release in a subpopulation of GnRH neurons by the activation of the M-current through a GnRH R1-mediated mechanism.
Materials and Methods
Animals
All animal treatments described in this study are in accordance with institutional guidelines based on National Institutes of Health standards and were performed with institutional Animal Care and Use Committee approval at the Oregon Health and Science University. For the electrophysiology, transgenic male mice expressing enhanced green fluorescent protein (EGFP) under the control of the GnRH promoter (EGFP-GnRH) were used in these studies (16). Animals were group housed and were maintained under controlled temperature and photoperiod (light on at 0600 h and off at 1800 h) and given free access to food and water.
Drugs
Mammalian GnRH (LHRH) was purchased from American Peptide Company, Inc. (Sunnyvale, CA). Tetrodotoxin (TTX) was from Alomone Labs (Jerusalem, Israel). The M-channel blockers, linopirdine and 10,10-bis (4-pyridinylmethyl)-9(10H)-anthracenone (XE-991), were purchased from Tocris (Ellisville, MO). The GnRH R1 antagonist, antide, the nonselective K+ channel blocker, tetraethylammonium (TEA), and the SK channel blocker, apamin, were all purchased from Sigma-Aldrich (St. Louis, MO).
Preparation of preoptic area (POA)-GnRH slices and dispersed GnRH neurons
Transgenic GnRH male mice expressing EGFP were used in our experiments. Brain slices were prepared by the following procedures: intact male mice, 6–12 wk old, were anesthetized with halothane and quickly decapitated. The brain was rapidly removed from the skull and a block containing the diagonal band-POA was immediately dissected and placed in oxygenated (95% O2-5% CO2), ice-cold artificial CSF (aCSF) containing 124 mm NaCl, 5 mm KCl, 1.25 mm NaH2PO4, 5 mm HEPES, 10 mm d-glucose, 26 mm NaHCO3, 2 mm MgSO4-7H2O, 2 mm CaCl2 (pH 7.4). Coronal brain slices (300 μm) were cut on a vibratome (VT 1000; Leica, Heidelberg, Germany). The slices were transferred to an auxiliary chamber in which they were kept in oxygenated aCSF solution at room temperature (25 C).
For slice recording, the brain slice was incubated in aCSF consisting of (in millimoles): 124 NaCl, 5 KCl, 2.6 NaH2PO4, 2 MgSO4, 2 CaCl2, 26 NaHCO3, 10 HEPES, 10 glucose (pH 7.4), until recording (recovery for 2 h). A single slice was transferred to the recording chamber at a time and was kept viable by continually perfusing with warm (35 C), oxygenated aCSF at 1.5 ml/min. For dispersal of GnRH neurons, a discrete region of the diagonal band-rostral POA containing a high density of GnRH neurons was microdissected and incubated in 5–10 ml aCSF [124 mm NaCl, 5 mm KCl, 2.6 mm NaH2PO4, 2 mm MgSO4, 2 mm CaCl2, 26 mm NaHCO3, 10 mm HEPES, 10 mm d-glucose, in diethyl pyrocarbonate (DEPC)-treated water (pH 7.3), 300 mOsm] containing 1 mg/ml protease (Protease; Sigma, St. Louis, MO) for approximately 17 min at 37 C. The partially digested brain slice was transferred to a 5-ml tube, washed four times with low Ca2+ (1 mm) aCSF, and then washed three times with regular aCSF. The cells were isolated by trituration with flame-polished Pasteur pipettes and were dispersed into the electrophysiological recording chamber and allowed to settle to the bottom. The cells were washed with aCSF for 15 min to remove any remaining debris. GnRH neurons were identified with an inverted fluorescent microscope (Nikon) equipped with Hoffman differential interference contrast optics using initially ×10 and then a ×40 objective for patching. EGFP-positive neurons with bipolar processes were preferentially used based on the established GnRH neuronal morphology. Normal aCSF was perfused through the recording chamber by the gravity perfusion system at a rate of 1.5 ml/min, and drugs were rapidly perfused using a Valvelink 8.2 pinch valve perfusion system (Automate Scientific, Inc., San Francisco, CA).
Single-cell RT-PCR of GnRH neurons
Seven intact male EGFP-GnRH mice were used for the single cell harvesting. The mice were processed as above for slicing, dispersal, and plating. The cells were isolated by triturating the tissue with flame-polished Pasteur pipettes, dispersed on a 35-mm glass-bottomed petri dish and perfused continuously with aCSF at a rate of 1.5 ml/min. Approximately 10–15 individual neurons from each male were patched and harvested by applying negative pressure to the electrode. Samples of aCSF from the petri dish were also collected before, during, and after cells were harvested. Single cell-negative control (no reverse transcriptase) and perfused aCSF samples were also collected and analyzed along with tissue (POA) positive and negative controls. The pipette contents were expelled into a siliconized microcentrifuge tube containing 1 μl 5 × buffer, 0.38 μl Rnasin, 0.5 μl 100 mm dithiothreitol, and 3.62 μl DEPC-water (Ambion, Austin, TX) and stored at −80 C as previously described (17).
The harvested cell solution was denatured for 5 min at 65 C and cooled on ice for 5 min, and then single-stranded cDNA was synthesized from cellular RNA by adding 50 U murine leukemia virus reverse transcriptase (Applied Biosystems, Foster City, CA), 4 μl 5 × buffer, 5 mm MgCl2, 0.625 mm deoxynucleotide triphosphate, 100 ng random hexamer primers, 40 U/μl Rnasin, and 10 mm dithiothreitol in DEPC-water in total volume of 20 μl as previously described (17). Reverse transcription was conducted using the following protocol: 60 min at 42 C, 5 min at 95 C, and 5 min at 4 C. PCR was performed using 2.5–3 μl cDNA template from each reverse transcription reaction in a 30-μl PCR mix containing the following: 6 μl 5× buffer (Promega, Madison, WI), 2–3 mm MgCl2, 0.33 mm deoxynucleotide triphosphate, 0.33 μm forward and reverse primers, 2 U TaqDNA polymerase, and TaqStart antibody (CLONTECH, Palo Alto, CA). TaqDNA polymerase and TaqStart antibody were combined and incubated at room temperature for 5 min, and the remainder of the reaction content was added to the tube. Primers for KCNQ2, -3, -4, and -5 and for GnRH R1 were designed and tested using known mouse sequences. All primers were designed to span introns and synthesized by Invitrogen (Carlsbad, CA) using Clone Manager 5 software (Sci Ed Software, Cary, NC). See Table 1 for a listing of all the primer sets used for single-cell RT-PCR. PCR products were verified by sequencing. Each reaction was amplified for 50 cycles using a PTC-100 thermocycler (MJ Research, Waltham, MA) in 0.5 ml thin-walled PCR tubes according to protocols optimized for each primer pair. Ten microliters of PCR product were visualized with ethidium bromide on a 2.5% agarose gel.
Table 1.
Primer sequences used for single-cell RT-PCR
| Name | Product size, bp | Primer sequence | Base pairs | Accession no. |
|---|---|---|---|---|
| KCNQ2 | 100 | GGCCTCGTTTCTGGTGTACT | 857–876 | NM_172107 |
| AATGGTTGTCAGGGTGAT CAG | 956–936 | |||
| KCNQ3 | 180 | CAGCAAAGAACTCATCACCG | 739–758 | BC128576.1 |
| ATGGTGGCCAGTGTGATCAG | 918–899 | |||
| KCNQ4 | 280 | CCGAATCCGCATAAGCAG | 1317–1334 | NM_001081142 |
| CTTCACAGCAGGCATGAC | 1596–1579 | |||
| KCNQ5 | 225 | CTCACCCCACCACTTAAA | 1623–1640 | NM_019842 |
| CTCTCGGCTCTTCTTATCTG | 1847–1828 | |||
| GnRH R1 | 377 | ATGCCACTGGATGGGATG | 328–345 | NM_010323 |
| ATGAAGAGGCAGCCGAAG | 704–687 | |||
| GnRH | 239 | CGGCATTCTACTGCTGACTG | 21–40 | NM_008145 |
| GCCTGGCTTCCTCTTCAATC | 259–240 |
The sense primer is listed first with the antisense primer below.
Electrophysiology recording of dispersed GnRH neurons
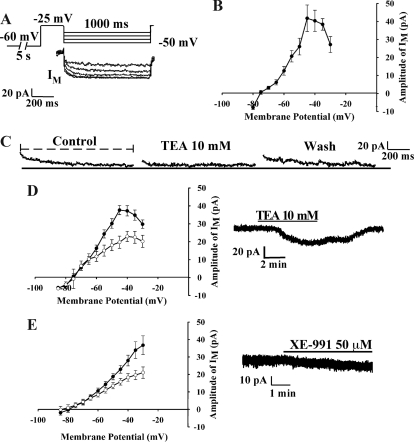
Isolated cell recordings were conducted using the Axopatch 200A patch clamp amplifier (Axon Instruments, Molecular Devices, Union City, CA). Digidata 1440A and PC clamp 10.0 software (Molecular Devices) were used for data acquisition and analysis. Patch pipettes (3–5 mΩ) were made of capillary glass (World Precision Instruments, Sarasota, FL) prepared with Flaming/Brown micropipette puller P-97 (Sutter Instrument Co., Novato, CA). Pipettes were filled with intracellular solution containing 115 mm K-gluconate, 10 mm HEPES, 20 mm KCl, 5 mm EGTA, 0.1 mm CaCl2, 4 mm MgATP, 0.4 mm NaGTP (pH 7.4). M-current recordings as shown in Fig. 1 were made in voltage-clamp mode with −60 mV holding potential for 5000 msec, depolarized to −25 mV for 300 msec, and then deactivated from −30 to −80 mV for 1000 msec, back to −25 mV.
Figure 1.
GnRH neurons exhibit a M-current (IM). A, The slow, delayed deactivation of the M-current (IM) of GnRH neurons dispersed from the male GnRH mice POA was recorded using whole-cell recording technique. In the presence of TTX and from a holding potential of −25 mV for 300 sec, the cell was stepped from −30 to −80 mV in 5-mV increments for a 1000-msec duration. Only the traces from −35 to −50 mV are shown. B, Using dispersed neurons, the I-V curve of A is shown where the M-current peaked at −45 mV (n = 34). The difference between the start (2–10 msec for dispersed cells and 10–20 msec for slice preparations) of the trace and the end is a measure of the relaxation of the M-current at −45 mV (the hatched line beneath control in C). This difference is graphed in the I-V plots as the amplitude of the IM [Schweitzer et al. (18)]. The measurement of the M-current differed between the cell preparations because of a greater capacitance artifact in the slice preparation (see Materials and Methods). C, TEA inhibits the M-current in GnRH neurons. TEA (10 mm), a nonselective K+ channel blocker, markedly inhibited M-current in GnRH neurons (47.5%) in a reversible manner (n = 12). D, The I-V curve of C is shown in which TEA (open circles) significantly inhibited the peak of the M-current in GnRH neurons (n = 12). In voltage clamp, TEA altered the K+ current in GnRH neurons. Vhold = −60 mV. E, Using preoptic slice preparations, the M-current in GnRH neurons was significantly inhibited by XE-991 (open circles) (50 μm; n = 12). In voltage clamp, XE-991 induced an inward current by inhibiting the M-current in GnRH neurons. Because of the pharmacokinetics of XE-991, the washout of a high concentration of XE-991 exceeded the period for reliable recording the M-current. Vhold = −60 mV.
In current clamp mode, the resting membrane potential of GnRH neurons was recorded in gap-free mode. The liquid junction potential was approximately −10 mV and corrected in the data analysis. BaCl2 (500 μm) and CdCl2 (200 μm) were added to the perfusion solution (aCSF) to minimize the contamination from G-protein coupled inward rectifying K+ and calcium currents. TTX (0.8 μm) was also added to the aCSF to block fast sodium currents.
Electrophysiology recording in the slice preparation
Recordings were made under a Axioskop FS (Zeiss, Jena, Germany) outfitted with epifluorescence (fluorescein isothiocyanate filter set) and infrared differential interference contrast (IR-DIC) video microscopy. The EGFP-tagged GnRH neurons in a slice were visualized through a ×40 water immersion objective (Achroplan; Zeiss). Patch pipettes (A-M Systems, Seattle, WA; 1.5 mm outer diameter borosilicate glass) were pulled on a Brown/Flaming puller (Sutter Instrument; model P-97). Pipette resistances were 4–5 mΩ when filled with pipette solutions. In whole-cell configuration, the access resistance was kept less than 15 mΩ and was 60–80% compensated. Current-clamp and voltage-clamp experiments were performed with an Axopatch 1D amplifier (Axon Instruments). Electrophysiological signals were digitized with Digidata 1322A (Axon Instruments). The voltage-clamp protocol used to measure M-current was the same as for isolated GnRH neurons (see below). HEPES-buffered CSF (in millimoles: 145 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, 10 glucose) was used in most cases for electrophysiological recording. Then 100 nm apamin, 500 μm Ba2+, and 200 μm Cd2+ were added to the CSF to block SK channels, inward rectifying potassium channels, and voltage-gated calcium channels, respectively. HEPES-buffered solutions were oxygenated by medical oxygen. Normal pipette solution contained (in millimoles): 125 potassium gluconate, 10 NaCl, 1 MgCl2, 11 EGTA, 10 HEPES, 2 MgATP, 2 K2ATP, and 0.25 NaGTP; adjusted to pH 7.3 with KOH; 295 mOsm. In whole-cell current-clamp and voltage-clamp recordings, 0.5–1 μm TTX was used to eliminate the effect of presynaptic input. Different drug stocks were 1000 times diluted into CSF to their final concentrations in a 20 ml syringe and were delivered by a Gilson Mini-Plus pump with a perfusion rate of 1.5 ml/min.
Measurement of the M-current and data analysis
The amplitude of the M-current was measured as the difference between the beginning and ending of the current trace (Fig. 1) elicited during 1000 msec voltage steps from −30 to −80 mV after a 300-msec prepulse to −25 mV, which included the membrane potential at which the maximal M-current could be obtained (18). In the slice preparation due to a greater membrane capacitance artifact and delayed membrane voltage clamping, the initial measurement of the M-current was later than in the dispersed cells. In the slice, the M-current was measured as the difference in the amplitude between the beginning (10–20 msec, after voltage stepping) and the ending (800–1000 msec) of the current trace elicited during 1000 msec voltage steps from −30 to −80 mV. In the dispersed cells, the beginning measurement was between 2 and 10 msec.
The Student’s t test was used for determining significant differences between treatments or concentrations. Differences were considered statistically significant if the probability of error was less than 5%. The drug effects on the M-current were derived from the composite concentration response curves/dose-response curves of the compound on the M-current generated from the following logistic equation using Sigma Plot 8.0 (San Jose, CA): ΔImax = 100 × [(agonist)n/([agonist]n + EC50n)], where Imax is the maximum outward current for a given agonist, EC50 represents the agonist potency, and n is the Hill slope.
Results
Characterization of M-current in GnRH neurons
Whole-cell recordings were made in dispersed EGFP-GnRH neurons from intact male mice (Fig. 1). For the electrophysiology analysis, only GnRH cells with gigaohm or better seals were included in this study. The mean resting membrane potential was −55.8 ± 3.8 mV at a 0 pA holding current (n = 26). The mean membrane input resistance was 922 ± 105 mΩ with a membrane capacitance of 13.2 ± 4.4 pF (n = 25). Series resistance was 15.4 ± 6.3 mΩ. Based on the current-voltage (I-V) relationship curve for the GnRH neurons, the M-current peaked around −40 mV (n = 34). In Fig. 1, 10 mm TEA, which selectively blocks KCNQ channels but also inhibits other K+ channels at higher concentrations, inhibited a noninactivating K+ current (M-current?) by 47.5% in GnRH neurons (Fig. 1C). TEA decreased the peak of the M-current in the I-V plot at −45 mV from 37.6 ± 2.5 to 19.8 ± 2.5 pA (n = 12). TEA application also induced an inward current (inhibited an outward current) in GnRH neurons by −17.8 ± 12.5 pA (n = 6; at a resting potential of −60 mV) (Fig. 1D). In the slice preparation in the presence of TTX and apamin, the specific KCNQ channel blocker, XE-991 (50 μm), significantly reduced the M-current at −30 mV from 36.8 ± 5.4 to 21.1 ± 3.2 pA (n = 7) and decreased the M-current amplitude by −9.4 ± 3.8 pA at −60 mV (n = 7, Fig. 1E). Apamin was used throughout the slice studies to block the effects of GnRH-induced SK channel activation (11).
Expression of KCNQ subunits in GnRH neurons
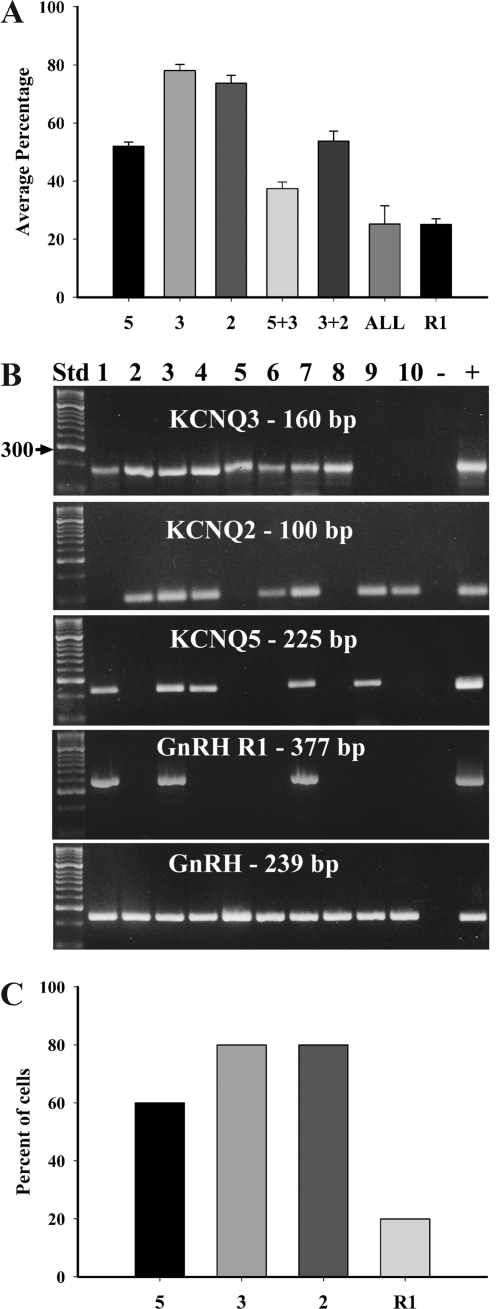
We used single-cell RT-PCR to ascertain the expression patterns of the KCNQ subunits in GnRH neurons. In preliminary experiments, we found that KCNQ4 mRNA was not detected in GnRH neurons (0 of 15), although there is expression in the preoptic area. Furthermore, both linopirdine and XE-991 were effective at inhibiting the GnRH response at concentrations five to 10 times lower than the EC50 (>200 μm) for KCNQ4-containing channels (12). Therefore, in these experiments only KCNQ2, -3, and -5 were measured. Of the 81 GnRH neurons collected, all but two expressed at least one KCNQ subunit. Of the three subunits examined, the average expression per animal was 78 ± 2.2% for KCNQ3, 74 ± 2.7% for KCNQ2, and 52 ± 1.4% for KCNQ5 (Fig. 2, A and B). The coexpression of KCNQ3 and KCNQ2, the primary functional M-current channel subunits (12), occurred in 54 ± 3.4% of GnRH neurons and the coexpression of KCNQ3 and KCNQ5 occurred in 37 ± 2.3%. Twenty-five percent (25 ± 6.3%) of the GnRH neurons expressed all three subunits. There were no harvested nonfluorescent neurons that expressed GnRH (n = 10), although these neurons did express KCNQ subunits (Fig. 2C). We also designed and optimized primers for the mouse GnRH R1 (Table 1). Using single-cell RT-PCR, the mRNA for GnRH R1 was expressed in 25 ± 2.0% of GnRH neurons and all neurons expressing R1 also expressed either KCNQ2/3 or KCNQ3/5 as illustrated in the representative gel (Fig. 2, A and B). In nonfluorescent neurons, GnRH R1 was expressed in 20% (two of 10) (Fig. 2C). Currently there is no published sequence for the mouse GnRH receptor 2.
Figure 2.
A, The summary graph shows the average percentage of GnRH neurons expressing or coexpressing KCNQ5, KCNQ3, and KCNQ2 mRNA and the coexpression with GnRH R1. The average percentage of neurons expressing the KCNQ channel subunits and GnRH R1 collected from the POA of seven intact male EGFP-GnRH mice. Bar graphs represent the mean ± sem. B, A representative gel illustrating the coexpression of K+ channel subunits in GnRH neurons harvested from intact male mice. The expected size of the PCR products is as follows (in base pairs): KCNQ3, 160; KCNQ2 100; KCNQ5, 225; GnRH R1, 377; and GnRH, 239. The positive (+) control was amplified using preoptic area or pituitary cDNA, and negative (−) control was amplified from a harvested cell without reverse transcriptase. Other controls included multiple aCSF samples from the dispersed cellular milieu collected during the cell harvesting, all of which were negative after RT-PCR (data not shown). C, The graph shows the percentage of nonfluorescent neurons (n = 10) expressing KCNQ5, KCNQ3, KCNQ2, and GnRH R1 mRNA collected from the POA of seven intact male EGFP-GnRH mice. No nonfluorescent cells expressed GnRH.
GnRH peptide activation of the M-current in GnRH neurons
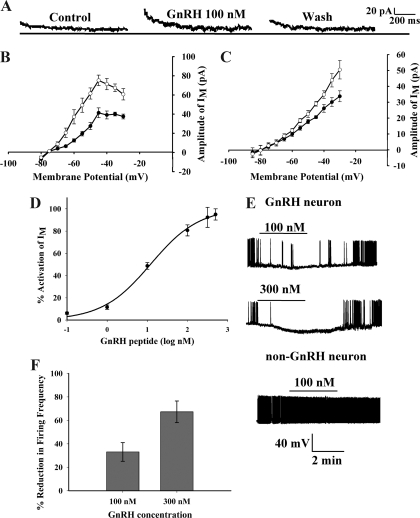
To determine whether GnRH modulates the M-current, we used whole-cell patch clamp recordings of EGFP-GnRH neurons and perfused GnRH peptide (100 nm) with a rapid perfusion system. In the presence of CdCl2, BaCl2 and TTX, GnRH activated an outward current that reversed at EK+ with a maximal outward current of 35.9 ± 5.7 pA (n = 25) at a membrane potential of −45 mV. The perfusion of GnRH increased the outward M-current in 30% of the GnRH neurons examined (Fig. 3A) and hyperpolarized the membrane from rest by −5 mV (n = 5; P < 0.05). The activation of the M-current was reversible because the M-current returned to the resting level after washout of GnRH. An I-V curve illustrates that GnRH (10 nm) significantly activated the M-current in GnRH neurons (n = 7) (Fig. 3). The maximum outward current for the control at −45 mV was 41.4 ± 13.6 pA, and for GnRH, the maximum outward current was 75.2 ± 14.5 pA (Fig. 3B). The same effect of GnRH (10–100 nm) on the M-current was found in approximately 33% (five of 15) of GnRH neurons from ovariectomized, adult female mice. The maximum outward current for the control at −45 mV was 48.0 ± 14.1 pA, and for GnRH the maximum outward current was 118.8 ± 27.5 pA. There was no statistical difference between the GnRH-induced maximum outward current from male and female GnRH neurons. In the slice preparation, GnRH increased the M-current at −30 mV from 33.7 ± 3.5 to 50.5 ± 5.8 pA (n = 4; Fig. 3C). The difference in the peak of the M-current between the dispersed cells and GnRH neurons recorded in the slice preparation is due to differences in the point of measurement as explained in Materials and Methods. The activation of the M-current in the subpopulation of GnRH neurons was dose dependent. The EC50 concentration for GnRH activation of the M-current was 11 nm with a maximum effect at 300 nm GnRH peptide (Fig. 3D). The hyperpolarization by GnRH (100 and 300 nm) occurred only in GnRH neurons and not in the population of tested non-GnRH (nonfluorescent) dispersed neurons from the same preoptic slice (n = 6; Fig. 3E), although some adjacent neurons do express GnRH R1 (Fig. 2C). The hyperpolarization caused a concentration-dependent inhibition in firing frequency in the GnRH neurons at both 100 and 300 nm (Fig. 3F).
Figure 3.
GnRH peptide activates the M-current in GnRH neurons. A, GnRH (100 nm) activates the M-current in a reversible manner. B, Using dispersed neurons, an I-V curve is shown in which GnRH (10 nm) significantly activated the M-current in GnRH neurons (n = 7). The maximum current for the control (filled circles) at −45 mV is 41.4 ± 13.6 pA, and in the presence of GnRH (open circles), the maximum current is 75.2 ± 14.5 pA. C, Using preoptic slice preparations, an I-V curve is shown in which GnRH (200 nm) significantly activated the M-current in GnRH neurons (n = 7). The maximum current for the control (filled circles) at −30 mV is 33.7 ± 3.5 pA, and in the presence of GnRH (open circles), the maximum current is 50.5 ± 5.8 pA. D, Using dispersed neurons, GnRH peptide activated M-current of GnRH neuron in a dose-dependent manner. One hundred percent of M-current (IM) was activated at 300 nm. The calculated EC50 was 11 nm. The number of cells tested per GnRH concentration was 11, 6, 8, 7, 7, and 6 from 0.1 to 500 nm, respectively. GnRH at concentrations 1 nm and higher was significantly different from control (P < 0.05–0.01). There was no significant difference between the 300- and 500-nm concentrations. The best fit curve was derived using the equation discussed in Materials and Methods. E, GnRH hyperpolarizes GnRH neurons but not non-GnRH neurons. GnRH hyperpolarized and inhibited cell firing. GnRH (100 nm) depressed GnRH neurons firing by hyperpolarizing cells from −58 ± 2.7 to −63.6 ± 4.5 mV (n = 5); by contrast, the spontaneous activity of non-GnRH neurons (n = 6) was not affected by GnRH administration. F, GnRH significantly reduced the firing frequency of GnRH neurons in a dose-dependent manner.
Pharmacological blockers of the M-current and GnRH R1 antagonist attenuate GnRH-induced activation
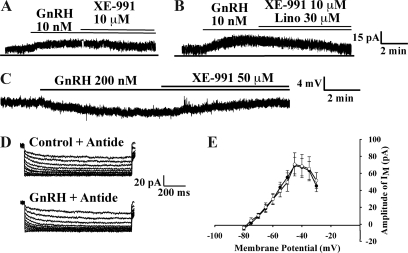
To confirm that the hyperpolarization of GnRH neurons by GnRH functions through the M-current, we used specific pharmacological blockers of KCNQ channels. The M-current blocker XE-991 at a low concentration (10 μm; n = 6) partially attenuated the GnRH-induced increase in the outward current (Fig. 4A). When 10 μm XE-991 was used in combination with linopirdine (30 μm; n = 6), another selective blocker of the M-current, these two compounds completely abrogated the GnRH-induced increase in the outward current (Fig. 4B). At a higher concentration and in the presence of TTX and apamin, XE-991 (50 μm) completely blocked the GnRH-induced hyperpolarization (n = 4; Fig. 4C) in GnRH neurons recorded in the slice preparation.
Figure 4.
M-channel blockers, XE-991 and linopirdine, attenuated the activation of the M-current by GnRH. A, Using dispersed neurons, XE991 (10 μm) partially inhibited the GnRH-induced outward current of GnRH neurons (n = 6; Vhold = −60 mV). B, XE-991 and linopirdine (30 μm) applied together completely blocked the GnRH-induced outward current of GnRH neurons (n = 6). C, Using the preoptic slice preparation and in the presence of TTX and apamin, XE-991 (50 μm) completely blocked the GnRH-induced hyperpolarization (n = 4). D, Antide, an antagonist of the GnRH R1, blocked the activation of the M-current by GnRH peptide in all 13 cells tested. The dispersed cells were preincubated with antide (1 μm) 20 min before perfusion with GnRH peptide. This concentration of antide was used to ensure complete block of the receptor during the short incubation period. E, The I-V curve of C is shown in which antide (open circles) significantly blocks the activation of the M-current by GnRH in GnRH neurons (n = 13). The I-V curves were generated as described in Fig. 1A.
To confirm that the activation of the M-current by GnRH transmits via the GnRH R1, we preincubated the dispersed cells with antide, a GnRH R1 antagonist, for 20 min before recording and coperfusion with GnRH. Due to the necessary short incubation period and the pharmacokinetics of antide, we used a higher concentration (1 μm) than reported for the long-term treatment of cell cultures (19). In all of the cells tested (n = 13), antide blocked the activation of the M-current by GnRH (10 nm) (Fig. 4, D and E). Based on the fact that 30% of the GnRH neurons responded to GnRH, at least four of the 13 cells should have expressed a GnRH-activated M-current.
Discussion
In the present study, we examined the activation of the M-current by GnRH peptide in a subpopulation of GnRH neurons. The activation of the M-current caused a significant hyperpolarization in the responsive neurons. To our knowledge, this is the first report of a potentiation of the M-current by GnRH to decrease neuronal excitability in hypothalamic neurons. The activation of the M-current by GnRH also provides a mechanism for the ultrashort negative feedback loop proposed by numerous investigators (2,3,4,8,9).
Centrally, GnRH induces a long-lasting depolarization in 72% of rat CA1 hippocampal neurons from slice preparations at low nanomolar concentrations. However, approximately 9% of CA1 hippocampal neurons examined responded with a small 2- to 5-mV hyperpolarization (20), similar to what is reported in this study. The receptor mediating this effect is the GnRH R1, which is expressed in a subpopulation of GnRH neurons (our data) (6,21). GnRH R1 is a Gq/11-coupled receptor that activates PLC and the subsequent hydrolysis of PIP2 followed by the activation of protein kinase C. It is well documented that multiple neurotransmitters including GnRH use this pathway to impinge on KCNQ channels to inhibit their activity (12,15,22) as well as through other signaling molecules including calcium and arachidonic acid (AA) metabolites (18,23,24).
We see a clear activation of the M-current by GnRH in GnRH neurons similar to what has been reported with somatostatin in hippocampal CA1 pyramidal neurons (18,23). In this cell type, somatostatin activates phospholipase A2 (PLA2) through a GPCR and increases the production of AA, which subsequently activates the M-current in a Ca2+-dependent mechanism. In immortalized GnRH neurons, GnRH in nanomolar concentrations activates GnRH R1, which results in Gq/11 activation and PLC signaling along with coupling to Gs and increased cAMP production (25) that may induce PLA2 and AA production. Furthermore, in a gonadotrope cell line, stimulation of GnRH R1 also activates PLA2 and induces AA production that involves the mobilization of Ca2+ via voltage-sensitive Ca2+ channel activation (26). Presently we do not know the mechanism by which GnRH R1 signals in GnRH neurons, but it could be via Gq activation of PLA2-AA-Ca2+ pathway as referred to above. This pathway will be examined in future experiments.
The primary function of GnRH is the stimulation of FSH and LH (1,27); however, GnRH also self-regulates its release in a dose-dependent ultrashort feedback loop. Bedran de Castro et al. (8) suggested such a feedback loop on GnRH-controlled GnRH release in 1985. Additional research in multiple species using GnRH receptor agonist and antagonist has further characterized the autoregulatory feedback loop (2,3,4,9). In many of these studies, GnRH at higher concentrations induced LH release, whereas lower concentrations suppressed LH release. Indeed, Xu et al. (6) showed that high concentrations of GnRH excited GnRH neurons (6). However, this is most likely due to spill over in the slice preparation to excite presynaptic neurons because the GnRH neurons were not synaptically isolated as they were in our single-cell preparation. In the present experiments, even higher concentrations of GnRH (1 μm) did not inhibit the M-current and/or increase neuronal excitability. In fact, GnRH at this concentration also activated the M-current (n = 3; data not shown). In other studies using perforated patch clamp recording, GnRH depolarized 50% of the neurons in slice preparations in the presence of TTX (21). This effect was dose dependent and blocked by antide with no cells exhibiting a hyperpolarization as reported presently in our study and in another study (6). We have been able to measure these effects in both slice and dispersed cell preparations, so we are uncertain as to what is the reason for the differences between the study by Todman et al. (21) and the former (6) or current studies. However, we do know that the M-current is pH sensitive and runs down quickly (28).
In this study, approximately one third of GnRH neurons responded to nanomolar concentrations of GnRH with an activation of the M-current. The activation of this persistent K+ current by GnRH caused a hyperpolarization that along with activation of the other K+ currents (11) would contribute to the inhibition of cell firing (Fig. 3D). The GnRH-induced inhibition of cell firing would decrease the contribution of this subpopulation of GnRH neurons to the overall release of GnRH. Furthermore, GnRH neurons have multiple synaptic connections with other GnRH neurons including gap junctions (1,29). These connections potentially would allow for a dampening of the neuronal excitability in other neighboring GnRH neurons. However, in this hyperpolarized state, GnRH neurons can rebound via activation of the hyperpolarization-activated cation (h-) current that would facilitate burst firing (17,30). In addition, GnRH may also activate presynaptic neurons (inhibition of the M-current) (16,21) to increase the excitatory synaptic drive to a pool of GnRH neurons (6) and facilitate GnRH release. Therefore, M-current activation may be viewed as an initial brake on GnRH neuronal activity that ultimately facilitates the synchronization and burst firing of GnRH neurons via synaptic drive (1). However, further studies are needed to determine GnRH actions on presynaptic neurons.
Acknowledgments
We thank Dr. Suzanne Moenter (University of Virginia, Charlottesville, VA) for providing the transgenic EGFP-GnRH mice and Martha A. Bosch for her technical expertise in cell harvesting.
Footnotes
This work was supported by Public Health Service Grants NS43330, NS38809, and DK68098. T.A.R. was supported by Public Health Service Training Grants 5T32 DA07262 and 1F32 DK079508.
Disclosure Statement: The authors have nothing to disclose.
First Published Online January 24, 2008
Abbreviations: AA, Arachidonic acid; aCSF, artificial cerebral spinal fluid; DEPC, diethyl pyrocarbonate; DTT, dithiothreitol; EGFP, enhanced GFP; GFP-GnRH, green fluorescent protein-GnRH neurons; GnRH R1, GnRH receptor 1; GPCR, G-protein coupled receptor; PIP2, phosphatidylinositol 4,5-bisphosphate; PLA2, phospholipase A2; PLC, phospholipase C; POA, preoptic area; SK, small conductance K+; TEA, tetraethylammonium; TTX, tetrodotoxin; XE-991, 10,10-bis (4-pyridinylmethyl)-9(10H)-anthracenone.
References
- Herbison AE 2006 Physiology of the gonadotropin-releasing hormone neuronal network. In: Neill JD, ed. Knobil and Neill’s physiology of reproduction. 3rd ed. Boston: Elsevier; 1415–1482 [Google Scholar]
- Krsmanovic LZ, Martinez-Fuentes AJ, Arora KK, Mores N, Navarro CE, Chen H-C, Stojilkovic SS, Catt KJ 1999 Autocrine regulation of gonadotropin-releasing hormone secretion in cultured hypothalamic neurons. Endocrinology 140:1423–1431 [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Evans NP, Dahl GE, McFadden KL, Mauger DT, Karsch FJ 1995 Evidence for short or ultrashort loop negative feedback of gonadotropin-releasing hormone secretion. Neuroendocrinology 62:248–258 [DOI] [PubMed] [Google Scholar]
- DePaolo LV, King RA, Carrillo AJ 1987 In vivo and in vitro examination of an autoregulatory mechanism for luteinizing hormone-releasing hormone. Endocrinology 120:272–279 [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, McNeilly AS 2001 Is there an FSH-releasing factor? Reproduction 121:21–30 [DOI] [PubMed] [Google Scholar]
- Xu C, Xu X-Z, Nunemaker CS, Moenter SM 2004 Dose-dependent switch in response of gonadotropin-releasing hormone (GnRH) neurons to GnRH mediated through the type 1 GnRH receptor. Endocrinology 145:728–735 [DOI] [PubMed] [Google Scholar]
- Yu WH, Karanth S, Walczewska A, Sower SA, McCann SM 1997 A hypothalamic follicle-stimulating hormone-releasing decapeptide in the rat. Proc Natl Acad Sci USA 94:9499–9503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedran de Castro JC, Khorram O, McCann SM 1985 Possible negative ultra-short loop feedback of luteinizing hormone releasing hormone (LHRH) in the ovariectomized rat. Proc Soc Exp Biol Med 179:132–135 [DOI] [PubMed] [Google Scholar]
- Valença MM, Johnston CA, Ching M, Negro-Vilar A 1987 Evidence for a negative ultrashort loop feedback mechanism operating on the luteinizing hormone-releasing hormone neuronal system. Endocrinology 121:2256–2259 [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Loose MD, Rønnekleiv OK 1990 Opioids hyperpolarize β-endorphin neurons via μ-receptor activation of a potassium conductance. Neuroendocrinology 52:268–275 [DOI] [PubMed] [Google Scholar]
- Van Goor F, Krsmanovic LZ, Catt KJ, Stojilkovic SS 1999 Coordinate regulation of gonadotropin-releasing hormone neuronal firing patterns by cytosolic calcium and store depletion. Proc Natl Acad Sci USA 96:4101–4106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J 2001 KCNQ potassium channels: physiology, pathophysiology, and pharmacology. Pharmacol Ther 90:1–19 [DOI] [PubMed] [Google Scholar]
- Adams PR, Brown DA 1980 Luteinizing hormone-releasing factor and mascarinic agonists act on the same voltage-sensitive neurones. Br J Pharmacol 68:353–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SW 1987 Chicken II luteinizing hormone-releasing hormone inhibits the M-current of bullfrog sympathetic neurons. Neurosci Lett 80:180–184 [DOI] [PubMed] [Google Scholar]
- Ford CP, Stemkowski PL, Smith PA 2007 Possible role of phosphatidylinositol 4,5, bisphosphate in luteinizing hormone releasing hormone-mediated M-current inhibition in bullfrog sympathetic neurons. Eur J Neurosci 20:2990–2998 [DOI] [PubMed] [Google Scholar]
- Suter KJ, Song WJ, Sampson TL, Wuarin J-P, Saunders JT, Dudek FE, Moenter SM 2000 Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: Characterization of whole-cell electrophysiological properties and morphology. Endocrinology 141:412–419 [DOI] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Levine JE, Rønnekleiv OK, Kelly MJ 2007 Gonadotropin-releasing hormone neurons express KATP channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci 27:10153–10164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer P, Madamba S, Siggins GR 1990 Arachidonic acid metabolites as mediators of somatostatin-induced increase of neuronal M-current. Nature 346:464–467 [DOI] [PubMed] [Google Scholar]
- Vitale AM, Abramovich D, Peluffo MC, Meresman G, Tesone M 2006 Effect of gonadotropin-releasing hormone agonist and antagonist on proliferation and apoptosis of human luteinized granulosa cells. Fertil Steril 85:1064–1067 [DOI] [PubMed] [Google Scholar]
- Wong M, Eaton MJ, Moss RL 1990 Electrophysiological actions of luteinizing hormone-releasing hormone: intracellular studies in the rat hippocampal slice preparation. Synapse 5:65–70 [DOI] [PubMed] [Google Scholar]
- Todman MG, Han S-K, Herbison AE 2005 Profiling neurotransmitter receptor expression in mouse gonadotropin-releasing hormone neurons using green fluorescent protein-promoter transgenics and microarrays. Neuroscience 132:703–712 [DOI] [PubMed] [Google Scholar]
- Pfaffinger PJ, Leibowitz MD, Subers EM, Nathanson NM, Almers W, Hille B 1988 Agonists that suppress M-current elicit phosphoinositide turnover and Ca2+ transients, but these events do not explain M-current suppression. Neuron 1:477–484 [DOI] [PubMed] [Google Scholar]
- Schweitzer P, Madamba S, Champagnat J, Siggins GR 1993 Somatostatin inhibition of hippocampal CA1 pyramidal neurons: mediation by arachidonic acid and its metabolites. J Neurosci 13:2033–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SP 1995 Roles of arachidonic acid, lipoxygenases and phosphatases in calcium-dependent modulation of M-current in bullfrog sympathetic neurons. J Physiol 487(Pt 3):797–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krsmanovic LZ, Mores N, Navarro CE, Arora KK, Catt KJ 2003 An agonist-induced switch in G protein coupling of the gonadotropin-releasing hormone receptor regulates pulsatile neuropeptide secretion. Proc Natl Acad Sci USA 100:2969–2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin B, Rich N, Mitev Y, Gautron JP, Kordon C, Enjalbert A, Drouva SV 1996 Differential involvement of calcium channels and protein kinase-C activity in GnRH-induced phospholipase-C, -A2 and -D activation in a gonadotrope cell line (αT3-1). Mol Cell Endocrinol 122:33–50 [DOI] [PubMed] [Google Scholar]
- Sherwood NM, Lovejoy DA, Coe IR 1993 Origin of mammalian gonadotropin-releasing hormones. Endocr Rev 14:241–254 [DOI] [PubMed] [Google Scholar]
- Prole DL, Lima PA, Marrion NV 2003 Mechanisms underlying modulation of neuronal KCNQ2/KCNQ3 potassium channels by extracellular protons. J Gen Physiol 122:775–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JW 1999 Synchronized neuronal networks: the GnRH system. Microsc Res Tech 44:11–18 [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Wagner EJ 2002 GnRH neurons and episodic bursting activity. Trends Endocrinol Metab 13:409–410 [DOI] [PubMed] [Google Scholar]