Abstract
To determine whether the p44/p42 MAPK (ERK1/2) signaling pathway is involved in the activation of CRH-containing neurons in the hypothalamic paraventricular nucleus (PVN) after bacterial lipopolysaccharide (LPS) administration, Sprague Dawley rats were injected with LPS, and studied after 2, 6, 9, and 12 h. In saline-treated controls, isolated weak phosphorylated (phospho)ERK1/2 immunoreactive neurons were observed in the PVN. However, a dramatic increase in phospho-ERK1/2 immunoreactivity was apparent in the PVN 2 h after LPS administration, and gradually declined to baseline levels 9–12 h after injection. By double-labeling immunofluorescence, all CRH-containing neurons in the PVN contained phospho-ERK1/2 2 h after LPS. Intracerebroventricular administration of the MAPK inhibitor, PD98059, prevented LPS-induced ERK1/2 phosphorylation, c-fos activation, and the increase of CRH gene expression in the PVN but had no effect on c-fos activation in brainstem A2-C1/C2 regions. We conclude that LPS rapidly increases the phospho-ERK1/2 in CRH-containing neurons in the PVN and that activation of MAPKs is necessary for LPS-induced activation of the hypothalamic-pituitary-adrenal axis.
THE HYPOTHALAMIC-PITUITARY-adrenal (HPA) axis has a critical role in preparedness for bacterial infection and stress responses in a number of animal species (1). This response is regulated primarily by CRH-producing neurons, residing in the medial parvocellular subdivision of the hypothalamic paraventricular nucleus (PVN) (2,3,4,5,6,7). Thus, after the administration of bacterial lipopolysaccharide (LPS), an endotoxin that simulates bacterial infection, there is a rapid increase in CRH gene expression in the PVN (8,9,10,11,12), and ultimately, an increase in circulating levels of glucocorticoids (4,8,12,13).
Recent studies have demonstrated that the response of the CRH neurons in the PVN to hypoglycemic stress involves the ERK1/2 pathway (5,14). MAPKs, including ERK1/2, p38, and c-Jun N-terminal kinase, comprise a group of serine/threonine protein kinases that are involved in transduction of neurotropic signals from the surface of the cell to the nucleus. Phosphorylation of MAPK at critical threonine and tyrosine residues lead to MAPK activation and its translocation to the nucleus, where it phosphorylates other transcription factors that regulate immediate early gene expression (15,16). Thus, the phosphorylation ERK1/2 constitutes an important step in the pathways for induction of c-fos mediated by Elk-1 (17,18,19) and cAMP response element (CRE) binding protein (CREB) (20). Because LPS is well recognized to increase c-fos expression in CRH neurons in the PVN (9), and CREB is involved in the regulation of CRH gene transcription (21,22,23), we raised the possibility that phospho-ERK1/2 may be linked to the activating effects of endotoxin on CRH neurons. Therefore, in the present study, we determined whether LPS stimulates phospho-ERK1/2 in CRH neurons in the parvocellular subdivision of the PVN and whether inhibition of MAPK can prevent the LPS-induced increase in CRH mRNA expression in this region.
Materials and Methods
Animals
The experiments were performed on adult, male Sprague Dawley rats (Taconic Farms, German Town, NY) weighing 250–285 g. All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Tufts-New England Medical Center and Tufts University School of Medicine. Animals were housed individually in cages under standard environmental conditions (light between 0600 and 1800 h; temperature, 22 ± 1 C; rat chow and water ad libitum), and subjected to mock injections and accustomed to handling twice daily for at least 1 wk before experimentation to reduce stress.
Effect of LPS administration on ERK1/2 activation in the PVN
To determine whether LPS stimulates ERK1/2 activation in the PVN, animals were divided into five groups (n = 4 each). The control group received an ip injection of sterile saline, whereas the experimental groups received an equal volume of bacterial LPS [Sigma-Aldrich Corp., St. Louis, MO; 0127:B8; 250 μg/100 g body weight (BW), ip, in sterile saline]. All injections were given between 0900 and 1200 h. Two, 6, 9, and 12 h after injection, the animals were overdosed with pentobarbital (50 mg/kg; Ovation Pharmaceuticals, Inc., Deerfield, IL) and perfused transcardially with 20 ml 0.01 m PBS (pH 7.4) containing 15,000 U/liter heparin sulfate, followed by 150 ml 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.4). After perfusion, the brains were removed from the calvarium and postfixed by immersion in the same fixative overnight at 4 C. The brainstem and a segment of the forebrain containing the hypothalamus were removed using a coronal rat brain matrix (Ted Pella, Redding, CA) and cryoprotected in 25% sucrose in PBS at 4 C for 1 d. A series of coronal sections of the brain were cut at 20 μm through the PVN on a cryostat (Leica CM3050 S; Leica Microsystems, Nussloch GmbH, Germany) and collected in PBS. After washing in PBS, the sections were pretreated with 0.5% H2O2 in PBS for 15 min to remove endogenous peroxidase activity, followed by 0.5% Triton X-100 in PBS for 20 min to improve the antibody penetration. After preincubation in 10% normal horse serum for 30 min, the sections were incubated for 2 d at 4 C in mouse, monoclonal, primary antibody against phospho-ERK1/2 (ERK1/2; Cell Signaling Technology, Inc., Danvers, MA) diluted 1:5000. This antibody recognizes p44/p42 MAPK (ERK1 and ERK2) when phosphorylated at threonine 202 and tyrosine 204, and does not cross-react with phosphorylated SAPK/c-Jun N-terminal kinase or p38 MAPKs (24). The primary antibody was diluted in 1% normal horse serum in PBS containing 0.08% sodium azide and 0.2% Kodak PhotoFlo (Eastman Kodak Co., Rochester, NY). After washing in PBS, sections were incubated in biotinylated, horse, antimouse IgG (1:400; Vector Laboratories, Burlingame, CA) for 2 h. The sections were washed in PBS and immersed in avidin-biotin-peroxidase complex (ABC) (Vector Elite Kit, 1:100; Vector Laboratories) for 2 h at room temperature, rinsed in PBS followed by Tris buffer (pH 7.6), and developed in 0.025% diaminobenzedine/0.15% Ni-ammonium-sulfate/0.0036% H2O2 in 0.05 m Tris buffer (pH 7.6) for 5 min. The reaction developing time was kept uniform for all sections from all animals and stopped by immersion of the sections in 0.05 m Tris buffer. The sections were mounted on SuperFrost slides (Fisher Scientific, Pittsburgh, PA), air dried, dehydrated in an ascending series of alcohol, cleared in Histosol, and then coverslipped with DPX histology mounting medium (Fluka, Buchs, Switzerland).
Specificity of the immunoreaction product was determined by replacement of the primary antibody with normal horse serum, BSA, or PBS and replacement of the biotinylated secondary antibody with PBS during the immunohistochemistry protocol. All resulted in the complete loss of immunoreactivity.
Double-labeling immunofluorescence
Double-labeling immunofluorescence was used to study the association between phospho-ERK1/2 and CRH-containing neurons in the PVN. Sections through the rostrocaudal axis of the PVN of controls and 2-h LPS-treated animals were incubated in a mixture of rabbit antiserum against CRH (generous gift of Dr. Paul Sawchenko, The Salk Institute for Biological Studies, La Jolla, CA) at 1:2500 dilution, and mouse monoclonal antibody against phospho-ERK1/2 (Cell Signaling Technology) at 1:2500 for 2 d at 4 C. The sections were rinsed in PBS and incubated in biotinylated, goat antirabbit IgG (1:400; Vector Laboratories) for 2 h, followed by ABC (Vector Laboratories) at 1:100 dilution for 2 h. The CRH immunoreaction product was amplified using the Tyramide Signal Amplification kit according to the manufacturer’s instructions (New England Nuclear Life Science Products, Boston, MA) for 10 min, after which the sections were incubated in a mixture of 7-amino-4-methyl-coumarin-3-acetic acid (AMCA)-Avidin D (1:250; Vector Laboratories) and Cy3 conjugated antimouse IgG (1:250, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) overnight. After subsequent washes in PBS, the sections were mounted on SuperFrost/Plus slides and coverslipped with Vectashield mounting medium (Vector Laboratories).
Sections were analyzed under a Zeiss Axioplan 2 fluorescence microscope (Carl Zeiss Microimaging Inc., Thornwood, NY) using the following filter sets: Cy3, excitation 540–590 nm, bandpass 595 nm, and emission 600–660 nm; 7-amino-4-methyl-coumarin-3-acetic acid, excitation 395–410 nm, bandpass 415 nm, and emission 450–475 nm. Images were captured using a Spot digital camera (Diagnostic Instrument, Sterling Heights, MI), double exposed while switching filter sets for each fluorochrome, and superimposed in Adobe Photoshop CS 8.0 (Adobe Systems, Inc., San Jose, CA) using a Macintosh G4 computer (Apple Computer, Inc., Cupertino, CA) to create a composite image of the same field. The blue fluorescence color was pseudocolored green in Adobe Photoshop to facilitate identification of doubly labeled cells (yellow).
Efficacy of MAPK inhibition on LPS-induced activation of ERK1/2 and c-fos in the PVN, and c-fos in the brainstem
Eight days before the study, animals were anesthetized with ketamine (80 mg/kg BW ip) and xylazine (9 mg/kg BW ip), and a 22-gauge stainless steel guide cannula (Plastics One Inc., Roanoke, VA) was implanted into the lateral ventricle under stereotaxic control (coordinates from bregma, anteroposterior: −0.8 mm; lateral: −1.2 mm; and ventral: 3.5 mm) through a burr hole in the skull. The cannula was secured to the skull with three stainless steel screws and dental cement, and temporarily occluded with a dummy cannula. The rats were made accustomed to handling and given daily mock injections consisting of removal and reinsertion of the dummy cannula for at least 1 wk before experimentation. Animals weighing 265–285 g were divided into three groups (n = 4 each). The animals in the first two groups received 5 μl 15% dimethyl sulfoxide (DMSO) (Sigma-Aldrich) intracerebroventricularly (icv), followed 15 min later by an ip injection of either bacterial LPS (250 μg/100 g BW, in sterile saline) or the same volume of saline. The animals in the third group were administered 2 μg of the MAPK inhibitor, PD98059 (Calbiochem, San Diego, CA), icv in 5 μl 15% DMSO, followed 15 min later by an ip injection of bacterial LPS (250 μg/100 g BW, in sterile saline). PD98059 has been successfully used to inhibit MAPK in a dose range of 2–5 μg by icv administration with good tolerance (25,26,27). Because of the low solubility of PD98059 in aqueous solutions, 15% DMSO was used as a solvent. DMSO in a range of 10–40% has been widely used as a vehicle (Veh) for icv administration and is not known to have adverse effects on the brain (25,27,28). All the icv injections were made in freely moving animals through a 28-gauge needle that extended 1 mm below the guide cannula. The needle was connected by polyethylene tubing to a 1-cc GlassPak syringe (BD Diagnostic Systems, Sparks, MD), and injections were made over 2 min by a microprocessor controlled infusion pump (Bee Electronic Minipump; Bioanalytical Systems, West Lafayette, IN). Two hours after the LPS or saline injection, all animals were overdosed with pentobarbital (50 mg/kg) and perfused through the ascending aorta with heparinized PBS, followed by 4% paraformaldehyde. The brains were dissected from the calvarium, postfixed in the same fixative overnight, cryoprotected in 25% sucrose solution, and sections through the PVN and lower brainstem were cut on a cryostat at 20-μm thickness. The brain sections were processed for phospho-ERK1/2 immunolabeling as per the procedures described previously. The phospho-ERK1/2 immunoreactivity in the PVN of each animal was analyzed as described below.
One set of sections of the PVN and lower brainstem was processed for c-fos immunolabeling. Sections were washed in PBS and pretreated with 0.5% H2O2 in PBS for 15 min to remove endogenous peroxidase activity. After treating the sections in 0.5% Triton X-100 in PBS for 20 min, sections were preincubated in 10% normal horse serum for 30 min, followed by incubation in rabbit primary antiserum against c-fos, diluted 1:50,000 (Ab5; Oncogene Science, Cambridge, MA), for 2 d at 4 C with continuous agitation on a rotary shaker. Sections were washed in PBS and incubated in biotinylated, goat antirabbit IgG (1:400; Vector Laboratories) for 2 h. After washing in PBS, sections were immersed in ABC (Vector Elite Kit, 1:100) for 2 h at room temperature, rinsed in PBS, followed by Tris buffer (pH 7.6), and developed in 0.025% diaminobenzedine/0.15% Ni-ammonium-sulfate/0.0036% H2O2 in 0.05 m Tris buffer (pH 7.6) for 5 min. The time for developing the immunoreaction product was kept uniform for all sections from all animals. Reaction was stopped by immersion of the sections in 0.05 m Tris buffer. The sections were mounted on SuperFrost slides, air dried, dehydrated in an ascending series of alcohol, cleared in Histosol, and then coverslipped with DPX histology mounting medium.
Image analysis of phospho-ERK1/2, CRH, and c-fos immunoreactivity in the PVN
Density values of phospho-ERK1/2 immunoreactivity were determined using a computerized morphometric image analysis system consisting of a Carl Zeiss Axioplan 2 microscope and Cohu high-performance charged coupled device camera (Cohu, Inc., San Diego, CA) connected to a Macintosh G4 computer using Scion Image software (Scion Corp., Frederick, MD). Three phospho-ERK1/2 labeled sections through the rostrocaudal axis of the PVN from each animal were used for image analysis at ×100 magnification. Background density points were removed by thresholding the image, and integrated density values (density × area) of phospho-ERK1/2-immunoreactivity on each side of the PVN measured for each animal. The data are presented as mean ± sem, and were analyzed using Prism 4 software (GraphPad Software Inc., San Diego, CA) by one-way ANOVA and the Newman-Keuls multiple comparison test. P < 0.05 was considered significant.
For semiquantitative analysis of the number of CRH neurons in the PVN that contained phospho-ERK1/2 after LPS, three fluorescent-labeled sections through the rostrocaudal axis of the PVN from each animal were analyzed under a Zeiss Axioplan 2 epifluorescence microscope. Sections were visualized under ×100 magnification. While switching the filter sets, blue for CRH and red for phospho-ERK1/2, the CRH neurons that co-contained phospho-ERK1/2 on either side of the PVN were counted.
For the semiquantitative analysis of c-fos immunoreactivity in the PVN, three consecutive sections through the rostrocaudal axis of the PVN were visualized with a Zeiss Axioplan microscope equipped with a Cohu video camera. Images were projected onto the monitor of a Macintosh computer and analyzed using Scion Image software. All nuclei with intense or medium intensity c-fos labeling in the medial parvocellular subdivision of the PVN where CRH neurons are located were counted on each side for each animal. The data from all the animals in each group were pooled separately, and the mean ± sem was calculated. The data were statistically analyzed using one-way ANOVA, followed by the Newman-Keuls test using Prism 4 software. A probability of P < 0.05 was considered significant.
Effect of MAPK inhibition on LPS-induced increase in CRH mRNA in the PVN
To determine whether inhibition of MAPK alters LPS-induced CRH gene expression, a 22-gauge stainless steel guide cannula was placed into the lateral cerebral ventricle of rats weighing 250–280 g as described previously. After recovery from surgery, animals were accustomed to handling and mock injections to reduce stress. Animals were divided into four groups (n = 4 each). The animals in the first two groups received 15% DMSO icv, followed by an ip injection of LPS (250 μg/100 g BW, in sterile saline) or the same volume of sterile saline 15 min later. The animals in the other two groups were administered 2 μg PD98059 (Calbiochem) dissolved in 15% DMSO icv (5 μl), followed 15 min later by an ip injection of either LPS (250 μg/100 g BW, in sterile saline) or the same volume of sterile saline. All icv injections were given in freely moving animals through a 28-gauge needle that extended 1 mm below the guide cannula. The needle was connected by polyethylene tubing to a 1-cc GlassPak syringe, and injections were made over 2 min by a microprocessor controlled infusion pump (Bee Electronic Minipump). Six hours after the ip injections of either saline or LPS, the animals were anesthetized with an overdose of pentobarbital (50 mg/kg) and perfused transcardially with diethyl pyrocarbonate treated 20 ml 0.01 m PBS (BD Diagnostic Systems), containing 15,000 U/liter heparin sulfate, followed by 150 ml 4% paraformaldehyde in diethyl pyrocarbonate treated PBS. The brains were removed from the calvarium and postfixed in the same fixative overnight at 4 C. Hypothalamic tissue blocks were cryoprotected in 25% sucrose solution in PBS at 4 C overnight. A series of 18-μm thick coronal sections through the rostrocaudal extent of the PVN were cut on the cryostat, and every fourth section through the PVN was mounted onto SuperFrost Plus glass slides to obtain four sets of slides. Sections were desiccated overnight at 42 C and stored at −80 C until processed for in situ hybridization.
In situ hybridization
Every fourth section through the hypothalamus containing PVN was processed for in situ hybridization histochemistry as previously described (29). A single-stranded, [35S]uridine 5′-triphosphate-labeled cRNA probe for CRH generated from a 976-bp cDNA (30) was used. Hybridization was performed under plastic coverslips in buffer containing 50% formamide, a 2-fold concentration of standard sodium citrate, 10% dextran sulfate, 0.25% BSA, 0.25% Ficoll 400, 0.25% polyvinyl pyrrolidine, 250 mm Tris (pH 7.5), 0.5% sodium pyrophosphate, 0.5% sodium dodecyl sulfate, 250 μg/ml denatured salmon sperm DNA, and 0.5 × 106 cpm radiolabeled probes for 16 h at 55 C. Slides were washed and dipped into Kodak NTB autoradiography emulsion diluted 1:1 with distilled H2O, and all the autoradiograms were developed after 10-d exposure at 4 C. Tissue sections were dehydrated in ascending series of ethanol, cleared in Histosol, and coverslipped with DPX. The specificity of CRH mRNA hybridization in the PVN using this probe has been previously demonstrated (10,31).
Image analysis of in situ hybridization
In situ hybridization autoradiograms were observed under dark-field illumination using a Zeiss Axioplan 2 microscope fitted with a Cohu video camera. The CRH mRNA signal was analyzed with a Macintosh G4 computer using Scion Image software. Background density values were removed by thresholding, and integrated density values (density × area) of hybridized neurons in the same region on each side of the PVN were measured in three consecutive sections from each animal. The data are presented as mean ± sem, and were analyzed using Prism 4 software by one-way ANOVA and the Newman-Keuls multiple comparison test. P < 0.05 was considered significant.
Results
Effect of LPS administration on expression of phospho-ERK1/2 in the PVN
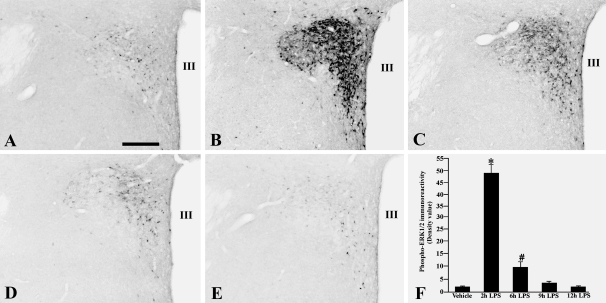
In saline-treated control animals, isolated weakly positive phospho-ERK1/2 immunoreactive neurons were observed on both sides of the PVN (Fig. 1A). No immunoreactivity was observed in other regions of the hypothalamus. Two hours after LPS administration, a dramatic increase in phospho-ERK1/2 immunoreactivity was apparent in the PVN, particularly in medial parvocellular subdivision neurons (Fig. 1B). Only a few phospho-ERK1/2 immunoreactive neurons were observed in the magnocellular subdivision of the PVN. A gradual reduction in the phospho-ERK1/2 immunoreactivity occurred by 6 h after LPS administration (Fig. 1C), and compared with earlier time points, continued to decline to basal levels by 9–12 h (Fig. 1, D–F) after injection.
Figure 1.
Photomicrographs showing the effect of LPS administration on phospho-ERK1/2 immunoreactivity in the PVN of saline-treated control animals (A), and 2 (B), 6 (C), 9 (D), and 12 h (E) after LPS. Note maximal increase in phospho-ERK1/2 immunoreactivity 2 h after LPS administration. F, Semiquantitative image analysis of the phospho-ERK1/2 immunoreactivity (OD) in the PVN. *, P < 0.001 compared with control, 6-, 9-, and 12-h LPS. #, P < 0.05 compared with control, 9- and 12-h LPS. Scale bar, 200 μm. III, Third ventricle.
Effect of LPS on ERK1/2 phosphorylation in CRH-containing neurons in the PVN
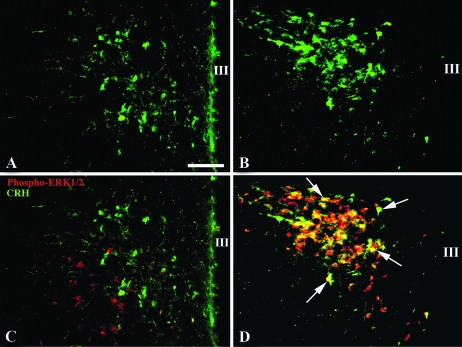
In saline-treated control animals, only a few CRH-containing neurons were observed in the PVN (Fig. 2A). After LPS treatment, there was an increase in both the number and intensity of CRH-immunoreactive neurons that organized primarily in rostral and midportions of the medial parvocellular subdivision of the PVN (Fig. 2B). Although no evidence for the coexistence of phospho-ERK1/2 and CRH immunoreactivity was observed in the PVN of saline-treated control animals (Fig. 2C), all visible CRH-containing neurons in the PVN co-contained phospho-ERK1/2 within 2-h LPS administration (Fig. 2D). Of the total number of phopho-ERK1/2 immunoreactive cells in the parvocellular subdivision of the PVN, only 19 ± 1.2% did not co-contain CRH.
Figure 2.
CRH immunofluorescence in the PVN of saline-treated controls (A) and LPS-treated animals (B). C, Doubly labeled phospho-ERK1/2- (red) and CRH-(green) immunoreactive neurons are not detected in the PVN of saline-treated control animals. D, Two hours after LPS administration, all CRH neurons in the PVN co-contain phospho-ERK1/2 and appear yellow due to color mixing (arrows). Scale bar, 200 μm. III, Third ventricle.
Effect of MAPK inhibitor PD98059 on LPS-induced phospho-ERK1/2 expression in the PVN
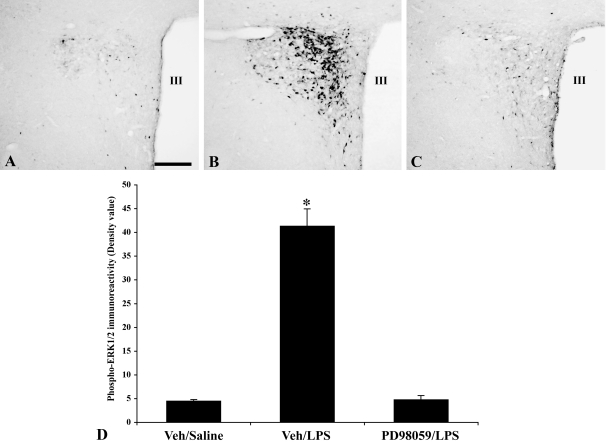
In icv Veh (15% DMSO)-treated animals injected with ip saline, a few isolated weakly positive phospho-ERK1/2 immunoreactive neurons were present in the PVN (Fig. 3, A and D), whereas a marked increase in phospho-ERK1/2 immunoreactivity was observed in the LPS-treated animals (Fig. 3, B and D). Pretreatment of the animals with the MAPK inhibitor, PD98059, significantly reduced LPS-induced phospho-ERK1/2 activation in the PVN, with only a few isolated weakly phospho-ERK1/2 immunoreactive neurons observed (Fig. 3, C and D).
Figure 3.
Effect of the MAPK inhibitor, PD98059, on LPS-induced ERK1/2 activation in the PVN. Veh (15% DMSO)-treated animals (A), LPS-treated animals (B), and PD98059-treated animals before LPS administration (C). Note attenuated LPS response in the PVN in C. D, Semiquantitative image analysis of the phospho-ERK1/2 immunoreactivity (optical density) in the PVN. *, P < 0.001 compared with Veh and PD98059/LPS groups. Scale bar, 200 μm. III, Third ventricle.
Effect of MAPK inhibitor, PD98059, on LPS-induced c-fos activation in the PVN and brainstem
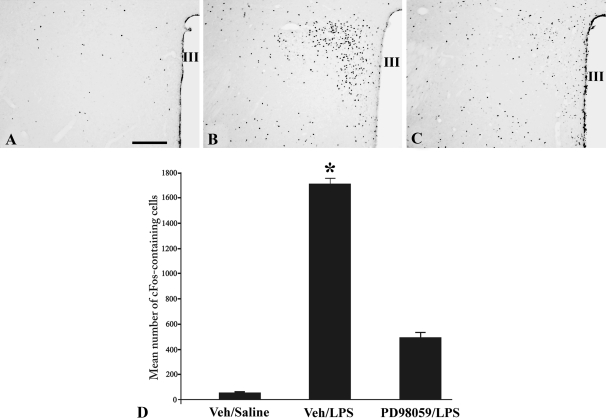
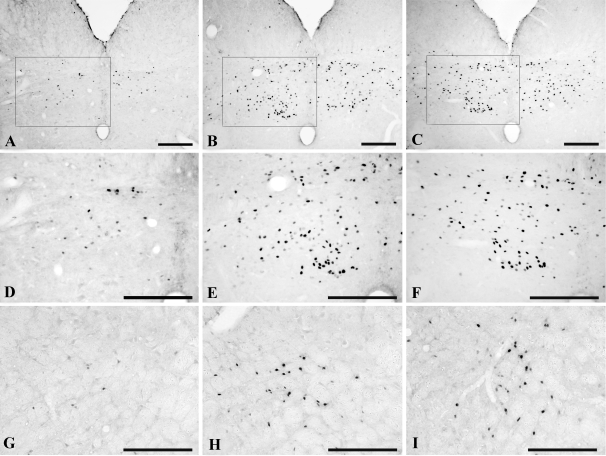
Only isolated c-fos immunoreactive nuclei were observed in the PVN of icv Veh (15% DMSO)-treated animals injected with ip saline (Fig. 4, A and D), but a dramatic increase was apparent in Veh-treated animals injected with LPS (Fig. 4, B and D). Compared with Veh/LPS-treated animals, the icv administration of PD98059 before ip LPS resulted in a marked and significant reduction (P < 0.001) in c-fos immunoreactive nuclei in the PVN (Fig. 4, C and D). Quantitative analysis revealed a 72% reduction in c-fos cells in the PVN of PD98059/LPS-treated animals compared with Veh/LPS-treated animals (Veh/saline 47 ± 8.3; Veh/LPS 1718 ± 36.6; PD98059/LPS 487 ± 43.3). In the A2-C1/C2 regions of the lower brainstem, only rare cells contained c-fos immunoreactivity in the control ip saline-treated animals (Fig. 5, A, D, and G), but there was a dramatic increase after LPS (Fig. 5, B, E, and H). However, in contrast to the PVN, the MAPK inhibitor had no effect on LPS-induced c-fos expression in these regions, which showed equally intense c-fos immunostaining as the icv Veh/ip LPS-treated group (Fig. 5, C, F, and I).
Figure 4.
Effect of MAPK inhibitor, PD98059, on LPS-induced c-fos expression in the PVN. Veh (15% DMSO)-treated animals (A), LPS-treated animals (B), and PD98059-treated animals before LPS administration (C). Note profound reduction in LPS-induced c-fos immunoreactivity in the PVN in C. D, Semiquantitative image analysis of the number of c-fos immunoreactive cells in the PVN. *, P < 0.001 compared with Veh/Saline and PD98059/LPS groups. Scale bar, 200 μm. III, Third ventricle.
Figure 5.
Effect of MAPK inhibitor, PD98059, on LPS-induced c-fos expression in the A2 (A–F) and C1 (G–I) cell groups in the lower brainstem. Veh (15% DMSO)-treated animals (A, D, and G), LPS-treated animals (B, E, and H), and PD98059-treated animals before LPS administration (C, F, and I). D–F, High-magnification photomicrographs of the area marked with a rectangle in A–C, respectively. MAPK inhibition has no effect on LPS-induced c-fos expression in the C1 and A2 regions. Scale bar, 200 μm.
Effect of MAPK inhibitor, PD98059, on LPS-induced CRH mRNA expression in the PVN
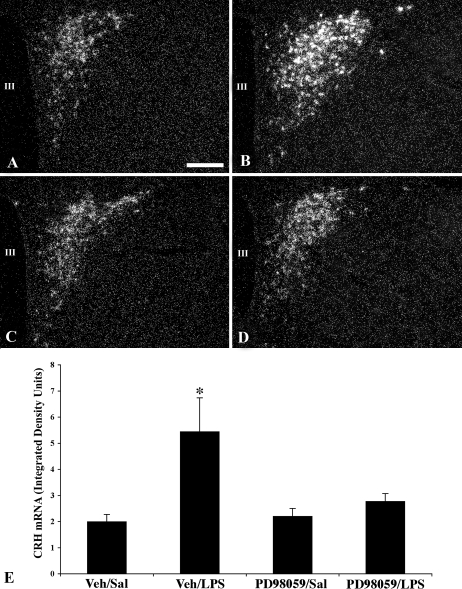
Weak CRH mRNA signal was observed in the PVN in icv Veh (15% DMSO)-treated animals after ip saline injection (Fig. 6, A and E). However, a dramatic increase in CRH mRNA expression was apparent in the PVN after LPS administration (density values Veh/saline 1.99 ± 0.28 vs. Veh/LPS 5.44 ± 1.38; P < 0.05) (Fig. 6, B and E). icv administration of the MAPK inhibitor, PD98059, had no effect on CRH mRNA expression in the PVN of saline injected control animals compared with animals receiving Veh icv (density value PD98059/saline 2.2 ± 0.3; P > 0.05) (Fig. 6, C and E). However, compared with Veh/LPS-treated animals, PD98059 significantly diminished LPS-induced CRH mRNA expression in the PVN (density value PD98059/LPS 2.7 ± 0.3; P < 0.05) (Fig. 6, D and E).
Figure 6.
Dark-field photomicrographs showing CRH mRNA signal in the PVN in icv Veh (15% DMSO)-treated animals receiving an ip injection of saline (Sal) (A) or LPS (B). The icv administration of the MAPK inhibitor, PD98059, has no effect on CRH mRNA expression in saline-treated control animals (C) but diminishes the CRH mRNA response after LPS administration (D). E, Semiquantitative image analysis of integrated density units representing CRH mRNA signal in the PVN. *, P < 0.05 compared with Veh/saline, PD98059/saline, and PD98059/LPS groups. Scale bar, 200 μm. III, Third ventricle.
Discussion
These studies demonstrate a strong association between endotoxin-induced increase in CRH expression and the phosphorylation of ERK 1/2 in CRH neurons in the PVN, supporting the hypothesis that during bacterial infection, the MAPK signaling pathway has an important role in the activation of the HPA axis by inducing CRH gene expression in hypophysiotropic neurons. Although only a few weakly positive phospho-ERK1/2-immunoreactive neurons were present in the PVN in normal control animals, a single ip administered dose of LPS induced a dramatic early increase in phospho-ERK1/2 immunoreactivity in the PVN that persisted for at least 7 h thereafter. Within the PVN, the increase in ERK1/2 phosphorylation occurred primarily in CRH neurons, with all visible hypophysiotropic CRH neurons showing evidence of double labeling. However, the importance of the MAPK signaling pathway in inducing CRH gene activation after endotoxin administration was established by the demonstration that inhibition of MAPK by the icv administration of PD98059 before LPS administration, not only led to a significant reduction in CRH gene expression in the PVN, essentially to control levels, but also in a marked reduction in c-fos activation selectively in the PVN.
The importance of MAPK in mediating the activating effects of hypoglycemia on CRH gene expression in the PVN has recently been demonstrated by Khan et al. (14). After the systemic administration of insulin or 2-deoxyglucose, a rapid increase in ERK1/2 phosphorylation was observed in the PVN that coincided with elevation in CRH heteronuclear mRNA and circulating levels of ACTH and corticosterone. Because microinjection of norepinephrine directly into the PVN or the addition of norepinephrine to hypothalamic slices containing the PVN replicates the effects of hypoglycemia to phosphorylate ERK1/2 in CRH neurons, Khan et al. (14) have proposed that norepinephrine may have an essential role in mediating the MAPK signaling cascade in these neurons in response to glucoprivation.
The ascending brainstem catecholamine pathways (A2/C1-C2) that contribute to massive projections to PVN CRH neurons (32,33,34,35,36) have also been implicated in the activating effects of endotoxin on hypophysiotropic CRH neurons (6,12,29,37). Transection of these pathways disrupts LPS or IL-1-induced activation and CRH gene expression in PVN neurons (6,29,38), suggesting that like hypoglycemia, the mechanism for MAPK activation in hypophysiotropic CRH neurons after LPS may also be mediated by norepinephrine. However, after high-dose LPS administration, transection of the ascending catecholamine pathways only partly reduces activation of CRH neurons in the PVN (6), indicating that mechanisms other than catecholamines may also contribute to increased CRH gene expression by endotoxin. Although it is possible that the icv administration of PD98059 could also have acted as an inhibitor of MAPK at sites other than CRH neurons in the PVN, including the brainstem, no effect of the inhibitor was observed on LPS-induced c-fos activation in the catecholamine A2-C1/C2 regions in the brainstem. These observations make it less likely that the MAPK inhibitor acted downstream of hypophysiotropic CRH neurons in the PVN rather than through direct effects in the PVN.
A potential candidate that could mediate the response of LPS on the activation of hypophysiotropic CRH neurons is the prostaglandins. The icv administration of prostaglandin E2 (PGE2) elevates plasma levels of ACTH and corticosterone (39), and increases c-fos in CRH neurons in the PVN (40). Furthermore, pretreatment with indomethacin, an inhibitor of prostaglandin synthesis, inhibits the HPA axis (7,41,42,43), and mice with targeted deletion of the prostanoid receptor, EP1, show impaired c-fos expression in the PVN after endotoxin (44). Although the action of PGE2 is believed to be primarily on catecholamine-producing neurons in the brainstem, activating CRH neurons in the PVN indirectly via catecholamine ascending pathways (7,45), iv treatment with IL-1β stimulates production of PGE2 within the PVN (46), and EP1 has been identified at synapses on CRH neurons in the PVN (44). Thus, in response to LPS, PGE2 may also be produced locally within the PVN and trigger CRH activation through direct actions. Along these lines, it is of interest that prostaglandin induces ERK activation in primary pulmonary microvascular endothelial cells (47) and activates MAPK in cardiac myocytes (48,49).
In addition to the association between the phospho-ERK1/2 and CRH activation in PVN neurons after endotoxin or hypoglycemic stress, the MAPK signaling pathway may also have a role in the activation of hypophysiotropic CRH neurons by immobilization stress and in association with melanocortin signaling. Restraint stress is associated with an increase in c-fos expression in CRH neurons (50), and markedly increases the number and intensity of phospho-ERK1/2 immunoreactivity in the PVN in a distribution characteristic for CRH neurons (51). The mechanism for restraint stress-induced activation of the HPA axis remains unclear but does not appear to involve the ascending catecholamine system. Lesions that deplete the PVN of catecholamines do not significantly reduce stress-induced c-fos activation in this nucleus (6), suggesting that other mechanisms are operable. Signaling through the melanocortin type 4 receptor also activates MAPK both in vitro in COS-1 cells and in vivo in the PVN (52). Although the literature on the central regulation of the HPA axis by melanocortin signaling remains controversial, α-MSH or agonists of the melanocortin 4 receptor stimulate ACTH secretion (53,54), and the icv administration of α-MSH to fasting rats restores suppressed levels of CRH mRNA in the PVN to normal fed levels (55). Because α-MSH-producing neurons in the hypothalamic arcuate nucleus establish monosynaptic projections with CRH neurons in the PVN (56) and are activated by LPS (8,9,10,11), α-MSH may contribute to LPS-induced activation of hypophysiotropic CRH through the MAPK signaling pathway.
Phosphorylation of CREB is believed to have an important role in the transcriptional regulation of the CRH gene by binding to a functional CRE in its promoter region (22,57,58). The importance of this regulatory mechanism for hypophysiotropic CRH neurons was suggested by Legradi et al. (59) and Kovacs and Sawchenko (21) in immunocytochemical studies by showing that CREB is phosphorylated in the nucleus of CRH neurons in the PVN within minutes of a stress response. Because MAPKs can be activated by increases in cAMP (20,60), and ERK phosphorylation is one of several factors that can result in CREB phosphorylation (20,61), it is feasible to consider the possibility that activation of MAPK is a proximal step in the signaling cascade that ultimately leads to the phosphorylation of CREB in the nucleus of CRH neurons and activation of the CRH gene. Indeed, activation of the CRH gene in JEG3 human choriocarcinoma cells by the MAPK signaling pathway can be abolished by mutating the CRE in the CRH promoter (62).
We conclude that LPS rapidly increases the phosphorylation of ERK1/2 in CRH-containing neurons in the PVN and substantially contributes to the increase in CRH mRNA gene expression in these neurons. We further propose that the MAPK signaling pathway may be a common final mechanism in hypophysiotropic CRH neurons that links a variety of different stimuli that activate the HPA axis to an increase in CRH gene expression.
Footnotes
This work was supported by National Institutes of Health Grant DK-37021.
Disclosure Statement: The authors have nothing to disclose.
First Published Online January 10, 2008
Abbreviations: ABC, Avidin-biotin-peroxidase complex; BW, body weight; CRE, cAMP response element; CREB, cAMP response element binding protein; DMSO, dimethyl sulfoxide; HPA, hypothalamic-pituitary-adrenal axis; icv, intracerebroventricular(ly); LPS, lipopolysaccharide; PGE2, prostaglandin E2; PVN, paraventricular nucleus; Veh, vehicle.
References
- Akil HCS, Cullinan WE, Lechan RM, Toni R, Watson SJ, Moore RY 1999 Neuroendocrine systems I: overview-thyroid and adrenal axes. In: Fundamental neuroscience. Zigmond H, Bloom F, Landis S, Roberts J, Squire L, eds. San Diego: Academic Press; 1127–1150 [Google Scholar]
- Whitnall MH 1989 Stress selectively activates the vasopressin-containing subset of corticotropin-releasing hormone neurons. Neuroendocrinology 50:702–707 [DOI] [PubMed] [Google Scholar]
- Whitnall MH 1993 Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol 40:573–629 [DOI] [PubMed] [Google Scholar]
- Beishuizen A, Thijs LG 2003 Endotoxin and the hypothalamo-pituitary-adrenal (HPA) axis. J Endotoxin Res 9:3–24 [DOI] [PubMed] [Google Scholar]
- Khan AM, Watts AG 2004 Intravenous 2-deoxy-D-glucose injection rapidly elevates levels of the phosphorylated forms of p44/42 mitogen-activated protein kinases (extracellularly regulated kinases 1/2) in rat hypothalamic parvicellular paraventricular neurons. Endocrinology 145:351–359 [DOI] [PubMed] [Google Scholar]
- Schiltz JC, Sawchenko PE 2007 Specificity and generality of the involvement of catecholaminergic afferents in hypothalamic responses to immune insults. J Comp Neurol 502:455–467 [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Li HY, Ericsson A 2000 Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Prog Brain Res 122:61–78 [DOI] [PubMed] [Google Scholar]
- Loum-Ribot E, Lafon P, Chaigniau M, Tramu G, Corio M 2006 Glucocorticoids down-regulate lipopolysaccharide-induced de novo production of neurotensin mRNA in the rat hypothalamic, paraventricular, corticotrophin-releasing hormone neurons. Neuroimmunomodulation 13:170–178 [DOI] [PubMed] [Google Scholar]
- Rivest S, Laflamme N 1995 Neuronal activity and neuropeptide gene transcription in the brains of immune-challenged rats. J Neuroendocrinol 7:501–525 [DOI] [PubMed] [Google Scholar]
- Kakucska I, Qi Y, Clark BD, Lechan RM 1993 Endotoxin-induced corticotropin-releasing hormone gene expression in the hypothalamic paraventricular nucleus is mediated centrally by interleukin-1. Endocrinology 133:815–821 [DOI] [PubMed] [Google Scholar]
- Grinevich V, Harbuz M, Ma XM, Jessop D, Tilders FJ, Lightman SL, Aguilera G 2002 Hypothalamic pituitary adrenal axis and immune responses to endotoxin in rats with chronic adjuvant-induced arthritis. Exp Neurol 178:112–123 [DOI] [PubMed] [Google Scholar]
- Takemura T, Makino S, Takao T, Asaba K, Suemaru S, Hashimoto K 1997 Hypothalamic-pituitary-adrenocortical responses to single vs. repeated endotoxin lipopolysaccharide administration in the rat. Brain Res 767:181–191 [DOI] [PubMed] [Google Scholar]
- Gadek-Michalska A, Bugajski J 2004 Role of prostaglandins and nitric oxide in the lipopolysaccharide-induced ACTH and corticosterone response. J Physiol Pharmacol 55:663–675 [PubMed] [Google Scholar]
- Khan AM, Ponzio TA, Sanchez-Watts G, Stanley BG, Hatton GI, Watts AG 2007 Catecholaminergic control of mitogen-activated protein kinase signaling in paraventricular neuroendocrine neurons in vivo and in vitro: a proposed role during glycemic challenges. J Neurosci 27:7344–7360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal SS, York RD, Stork PJ 1999 Extracellular-signal-regulated kinase signalling in neurons. Curr Opin Neurobiol 9:544–553 [DOI] [PubMed] [Google Scholar]
- Schulz S, Hollt V 1998 Opioid withdrawal activates MAP kinase in locus coeruleus neurons in morphine-dependent rats in vivo. Eur J Neurosci 10:1196–201 [DOI] [PubMed] [Google Scholar]
- Gille H, Kortenjann M, Thomae O, Moomaw C, Slaughter C, Cobb MH, Shaw PE 1995 ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J 14:951–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki K, Sagata N 1995 The Mos/MAP kinase pathway stabilizes c-Fos by phosphorylation and augments its transforming activity in NIH 3T3 cells. EMBO J 14:5048–5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipskind RA, Baccarini M, Nordheim A 1994 Transient activation of RAF-1, MEK, and ERK2 coincides kinetically with ternary complex factor phosphorylation and immediate-early gene promoter activity in vivo. Mol Cell Biol 14:6219–6231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgambato V, Pages C, Rogard M, Besson MJ, Caboche J 1998 Extracellular signal-regulated kinase (ERK) controls immediate early gene induction on corticostriatal stimulation. J Neurosci 18:8814–8825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs KJ, Sawchenko PE 1996 Sequence of stress-induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons. J Neurosci 16:262–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiola-Diaz HM, Boswell C, Seasholtz AF 1994 The cAMP-responsive element in the corticotropin-releasing hormone gene mediates transcriptional regulation by depolarization. J Biol Chem 269:14784–14791 [PubMed] [Google Scholar]
- Wolfl S, Martinez C, Majzoub JA 1999 Inducible binding of cyclic adenosine 3′,5′-monophosphate (cAMP)-responsive element binding protein (CREB) to a cAMP-responsive promoter in vivo. Mol Endocrinol 13:659–669 [DOI] [PubMed] [Google Scholar]
- Cherian S, Thoresen M, Silver IA, Whitelaw A, Love S 2004 Transforming growth factor-βs in a rat model of neonatal posthaemorrhagic hydrocephalus. Neuropathol Appl Neurobiol 30:585–600 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Flores O, Shu J, Camacho-Arroyo I, Etgen AM 2004 Regulation of lordosis by cyclic 3′,5′-guanosine monophosphate, progesterone, and its 5α-reduced metabolites involves mitogen-activated protein kinase. Endocrinology 145:5560–5567 [DOI] [PubMed] [Google Scholar]
- Rahmouni K, Morgan DA, Morgan GM, Liu X, Sigmund CD, Mark AL, Haynes WG 2004 Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J Clin Invest 114:652–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugues S, Deschaux O, Garcia R 2004 Postextinction infusion of a mitogen-activated protein kinase inhibitor into the medial prefrontal cortex impairs memory of the extinction of conditioned fear. Learn Mem 11:540–543 [DOI] [PubMed] [Google Scholar]
- Ohnishi M, Katsuki H, Fujimoto S, Takagi M, Kume T, Akaike A 2007 Involvement of thrombin and mitogen-activated protein kinase pathways in hemorrhagic brain injury. Exp Neurol 206:43–52 [DOI] [PubMed] [Google Scholar]
- Fekete C, Singru PS, Sarkar S, Rand WM, Lechan RM 2005 Ascending brainstem pathways are not involved in lipopolysaccharide-induced suppression of thyrotropin-releasing hormone gene expression in the hypothalamic paraventricular nucleus. Endocrinology 146:1357–1363 [DOI] [PubMed] [Google Scholar]
- Thompson RC, Seasholtz AF, Herbert E 1987 Rat corticotropin-releasing hormone gene: sequence and tissue-specific expression. Mol Endocrinol 1:363–370 [DOI] [PubMed] [Google Scholar]
- Kakucska I, Qi Y, Lechan RM 1995 Changes in adrenal status affect hypothalamic thyrotropin-releasing hormone gene expression in parallel with corticotropin-releasing hormone. Endocrinology 136:2795–2802 [DOI] [PubMed] [Google Scholar]
- Cunningham Jr ET, Sawchenko PE 1988 Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol 274:60–76 [DOI] [PubMed] [Google Scholar]
- Cunningham Jr ET, Bohn MC, Sawchenko PE 1990 Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol 292:651–667 [DOI] [PubMed] [Google Scholar]
- Fuzesi T, Wittmann G, Liposits Z, Lechan RM, Fekete C 2007 Contribution of noradrenergic and adrenergic cell groups of the brainstem and agouti-related protein-synthesizing neurons of the arcuate nucleus to neuropeptide-y innervation of corticotropin-releasing hormone neurons in hypothalamic paraventricular nucleus of the rat. Endocrinology 148:5442–5450 [DOI] [PubMed] [Google Scholar]
- Liposits Z, Phelix C, Paull WK 1986 Adrenergic innervation of corticotropin releasing factor (CRF)-synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. A combined light and electron microscopic immunocytochemical study. Histochemistry 84:201–205 [DOI] [PubMed] [Google Scholar]
- Liposits Z, Paull WK 1989 Association of dopaminergic fibers with corticotropin releasing hormone (CRH)-synthesizing neurons in the paraventricular nucleus of the rat hypothalamus. Histochemistry 93:119–127 [DOI] [PubMed] [Google Scholar]
- Ericsson A, Kovacs KJ, Sawchenko PE 1994 A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci 14:897–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko PE 1988 Effects of catecholamine-depleting medullary knife cuts on corticotropin-releasing factor and vasopressin immunoreactivity in the hypothalamus of normal and steroid-manipulated rats. Neuroendocrinology 48:459–470 [DOI] [PubMed] [Google Scholar]
- Rassnick S, Zhou D, Rabin BS 1995 Central administration of prostaglandin E2 suppresses in vitro cellular immune responses. Am J Physiol 269(1 Pt 2):R92–R97 [DOI] [PubMed] [Google Scholar]
- Lacroix S, Vallieres L, Rivest S 1996 C-fos mRNA pattern and corticotropin-releasing factor neuronal activity throughout the brain of rats injected centrally with a prostaglandin of E2 type. J Neuroimmunol 70:163–179 [DOI] [PubMed] [Google Scholar]
- Lacroix S, Rivest S 1997 Functional circuitry in the brain of immune-challenged rats: partial involvement of prostaglandins. J Comp Neurol 387:307–324 [DOI] [PubMed] [Google Scholar]
- Rivier C, Rivest S 1993 Mechanisms mediating the effects of cytokines on neuroendocrine functions in the rat. Ciba Found Symp 172:204–220 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Morimoto A, Sakata Y, Murakami N 1990 ACTH response induced by interleukin-1 is mediated by CRF secretion stimulated by hypothalamic PGE. Experientia 46:481–484 [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Furuyashiki T, Bito H, Ushikubi F, Tanaka Y, Kobayashi T, Muro S, Satoh N, Kayahara T, Higashi M, Mizoguchi A, Shichi H, Fukuda Y, Nakao K, Narumiya S 2003 Impaired adrenocorticotropic hormone response to bacterial endotoxin in mice deficient in prostaglandin E receptor EP1 and EP3 subtypes. Proc Natl Acad Sci USA 100:4132–4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiltz JC, Sawchenko PE 2003 Signaling the brain in systemic inflammation: the role of perivascular cells. Front Biosci 8:s1321–s1329 [DOI] [PubMed] [Google Scholar]
- Watanobe H, Takebe K 1994 Effects of intravenous administration of interleukin-1-β on the release of prostaglandin E2, corticotropin-releasing factor, and arginine vasopressin in several hypothalamic areas of freely moving rats: estimation by push-pull perfusion. Neuroendocrinology 60:8–15 [DOI] [PubMed] [Google Scholar]
- Rao R, Redha R, Macias-Perez I, Su Y, Hao C, Zent R, Breyer MD, Pozzi A 2007 Prostaglandin E2-EP4 receptor promotes endothelial cell migration via ERK activation and angiogenesis in vivo. J Biol Chem 282:16959–16968 [DOI] [PubMed] [Google Scholar]
- Qian JY, Leung A, Harding P, LaPointe MC 2006 PGE2 stimulates human brain natriuretic peptide expression via EP4 and p42/44 MAPK. Am J Physiol Heart Circ Physiol 290:H1740–H1746 [DOI] [PubMed] [Google Scholar]
- Mendez M, LaPointe MC 2005 PGE2-induced hypertrophy of cardiac myocytes involves EP4 receptor-dependent activation of p42/44 MAPK and EGFR transactivation. Am J Physiol Heart Circ Physiol 288:H2111–H2117 [DOI] [PubMed] [Google Scholar]
- Rivalland ET, Clarke IJ, Turner AI, Pompolo S, Tilbrook AJ 2007 Isolation and restraint stress results in differential activation of corticotrophin-releasing hormone and arginine vasopressin neurons in sheep. Neuroscience 145:1048–1058 [DOI] [PubMed] [Google Scholar]
- Sasaguri K, Kikuchi M, Hori N, Yuyama N, Onozuka M, Sato S 2005 Suppression of stress immobilization-induced phosphorylation of ERK 1/2 by biting in the rat hypothalamic paraventricular nucleus. Neurosci Lett 383:160–164 [DOI] [PubMed] [Google Scholar]
- Daniels D, Patten CS, Roth JD, Yee DK, Fluharty SJ 2003 Melanocortin receptor signaling through mitogen-activated protein kinase in vitro and in rat hypothalamus. Brain Res 986:1–11 [DOI] [PubMed] [Google Scholar]
- Von Frijtag JC, Croiset G, Gispen WH, Adan RA, Wiegant VM 1998 The role of central melanocortin receptors in the activation of the hypothalamus-pituitary-adrenal-axis and the induction of excessive grooming. Br J Pharmacol 123:1503–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillo WS, Small CJ, Stanley SA, Jethwa PH, Seal LJ, Murphy KG, Ghatei MA, Bloom SR 2002 Hypothalamic interactions between neuropeptide Y, agouti-related protein, cocaine- and amphetamine-regulated transcript and α-melanocyte-stimulating hormone in vitro in male rats. J Neuroendocrinol 14:725–730 [DOI] [PubMed] [Google Scholar]
- Fekete C, Legradi G, Mihaly E, Tatro JB, Rand WM, Lechan RM 2000 α-Melanocyte stimulating hormone prevents fasting-induced suppression of corticotropin-releasing hormone gene expression in the rat hypothalamic paraventricular nucleus. Neurosci Lett 289:152–156 [DOI] [PubMed] [Google Scholar]
- Liposits Z, Sievers L, Paull WK 1988 Neuropeptide-Y and ACTH-immunoreactive innervation of corticotropin releasing factor (CRF)-synthesizing neurons in the hypothalamus of the rat. An immunocytochemical analysis at the light and electron microscopic levels. Histochemistry 88:227–234 [DOI] [PubMed] [Google Scholar]
- Spengler D, Rupprecht R, Van LP, Holsboer F 1992 Identification and characterization of a 3′,5′-cyclic adenosine monophosphate-responsive element in the human corticotropin-releasing hormone gene promoter. Mol Endocrinol 6:1931–1941 [DOI] [PubMed] [Google Scholar]
- Seasholtz AF, Thompson RC, Douglass JO 1988 Identification of a cyclic adenosine monophosphate-responsive element in the rat corticotropin-releasing hormone gene. Mol Endocrinol 2:1311–1319 [DOI] [PubMed] [Google Scholar]
- Legradi G, Holzer D, Kapcala LP, Lechan RM 1997 Glucocorticoids inhibit stress-induced phosphorylation of CREB in corticotropin-releasing hormone neurons of the hypothalamic paraventricular nucleus. Neuroendocrinology 66:86–97 [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Storm DR 1999 Making new connections: role of ERK/MAP kinase signaling in neuronal plasticity. Neuron 23:11–14 [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M 2001 Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol 2:599–609 [DOI] [PubMed] [Google Scholar]
- Cheng YH, Handwerger S 2005 Mitogen-activated protein kinase activation induces corticotrophin-releasing hormone gene expression in human placenta. Life Sci 77:1263–1272 [DOI] [PubMed] [Google Scholar]