Abstract
A relative decrease in β-cell mass is key in the pathogenesis of type 1 diabetes, type 2 diabetes, and in the failure of transplanted islet grafts. It is now clear that β-cell duplication plays a dominant role in the regulation of adult β-cell mass. Therefore, knowledge of the endogenous regulators of β-cell replication is critical for understanding the physiological control of β-cell mass and for harnessing this process therapeutically. We have shown that concentrations of insulin known to exist in vivo act directly on β-cells to promote survival. Whether insulin stimulates adult β-cell proliferation remains unclear. We tested this hypothesis using dispersed primary mouse islet cells double labeled with 5-bromo-2-deoxyuridine and insulin antisera. Treating cells with 200-pm insulin significantly increased proliferation from a baseline rate of 0.15% per day. Elevating glucose from 5–15 mm did not significantly increase β-cell replication. β-Cell proliferation was inhibited by somatostatin as well as inhibitors of insulin signaling. Interestingly, inhibiting Raf-1 kinase blocked proliferation stimulated by low, but not high (superphysiological), insulin doses. Insulin-stimulated mouse insulinoma cell proliferation was dependent on both phosphatidylinositol 3-kinase/Akt and Raf-1/MAPK kinase pathways. Overexpression of Raf-1 was sufficient to increase proliferation in the absence of insulin, whereas a dominant-negative Raf-1 reduced proliferation in the presence of 200-pm insulin. Together, these results demonstrate for the first time that insulin, at levels that have been measured in vivo, can directly stimulate β-cell proliferation and that Raf-1 kinase is involved in this process. These findings have significant implications for the understanding of the regulation of β-cell mass in both the hyperinsulinemic and insulin-deficient states that occur in the various forms of diabetes.
DIABETES DEVELOPS WHEN the mass of pancreatic β-cells is decreased to a level in which sufficient insulin is no longer available to meet the metabolic demands of the body. A loss of β-cell graft mass is also a key limiting factor for successful islet transplantation therapy (1). β-Cell mass is maintained through a balance of apoptosis and proliferation (2). Over the past few years, it has become better appreciated that β-cell proliferation plays a role in the control of adult β-cell mass (3,4,5). As such, there is increased interest in identifying endogenous regulators of β-cell expansion. We have recently shown that low doses of insulin protect β-cells from apoptosis (6). Insulin promotes β-cell survival in part by activating pancreatic and duodenal homeobox gene 1, a homeobox gene that is essential for pancreas development and has been linked to β-cell proliferation in the adult (7). Mice lacking insulin receptors on their pancreatic β-cells have reduced β-cell mass (8,9), although this aspect of their phenotype was not initially appreciated (8). In contrast, β-cell specific IGF-I receptor knockout mice do not have reduced β-cell mass (10). Compound knockouts of β-cell IGF-I receptor and insulin receptor demonstrated that, between these two growth factors, insulin plays the dominant role in regulating β-cell mass in adult mice (11).
More recently, it was demonstrated that high-fat fed mice lacking insulin receptors on their pancreatic β-cells failed to increase β-cell mass and had less β-cell proliferation than their wild-type counterparts (12). This suggested that insulin receptors play an essential role in the β-cell compensation to obesity. However, this study did not distinguish between the short-term and chronic (possibly indirect) effects of blocking insulin signaling because insulin receptors had been knocked out before birth in these mice. Moreover, this “all or none” experiment did not provide information on the effects of graded insulin signaling or whether higher concentrations of insulin are more effective than lower concentrations. Although it has been shown that superphysiological doses of insulin can stimulate proliferation in transformed β-cells (13), direct effects of insulin have not been reported at low doses or in primary islet cells.
Several other findings point to the importance of “insulin signaling” in regulating β-cell mass. For example, insulin receptor substrate 2 (Irs-2) knockout mice develop type 2 diabetes due to a defect in β-cell mass (14). The insulin receptor and Irs-2 mRNA levels are decreased in islets from patients with type 2 diabetes (15). Although overexpression of Akt in the β-cell increased islet mass (16), reduced β-cell Akt activity did not affect β-cell mass (17,18). These results imply that Akt activity is sufficient, but not necessary, to increase β-cell mass and indicate that “physiological” β-cell proliferation may involve alternative pathways. Other factors, such as glucagon-like peptide-1 and gastric inhibitory peptide, have stimulated the growth of β-cells through both Akt- and MAPK kinase (Mek)-dependent pathways (18). Of these two pathways, the Raf/Mek/Erk pathway has received the least attention in primary β-cells. Although the overexpression of a Raf-1 kinase inhibitory protein decreased proliferation in a hamster β-cell line (19), the role of Raf-1 kinase in primary β-cell proliferation has not been specifically addressed.
In the current study, we tested the direct effects of multiple doses of insulin on primary β-cell proliferation. We determined the basal proliferation rate of low-density cultures of primary mouse β-cells and demonstrated that β-cell proliferation in the presence of both low and high glucose concentrations likely involves insulin signaling. We found that both low and high doses of insulin can stimulate β-cell division but that only low doses of insulin require Raf-1 kinase in primary cells. Together, these results support the concept that insulin is a critical regulator of β-cell proliferation and establish the mechanism of this effect. Our findings have significance for the understanding of β-cell mass alterations in type 1 diabetes, type 2 diabetes, and during graft failure in islet transplantation.
Materials and Methods
Reagents
Hydroxy-2-naphthalenylmethylphosphonic acid (HNMPA-AM), LY294002, Raf-1 Kinase Inhibitor, and Akt Inhibitor VIII were purchased from Calbiochem (La Jolla, CA). The Mek inhibitor U0126 was purchased from Cell Signaling Technology (Beverly, MA). Somatostatin, predissolved recombinant human insulin, and all other reagents were from Sigma-Aldrich (St. Louis, MO).
Pancreatic islet isolation, dispersion, and cell culture
All studies were performed in accordance with the guidelines of the University of British Columbia Animal Care Committee. Islets were isolated from 6- to 10-wk-old male C57BL/6J mice (Jax, Bar Harbor, MA) using collagenase and filtration as described previously (20). After isolation, islets were cultured overnight in 35 × 10 mm Nunc suspension dishes (Nalge, Rochester, NY) at 37 C and 5% CO2 in RPMI 1640 media. Media were supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin (Invitrogen, Burlington, Ontario, Canada). Fetal bovine serum (10%; Invitrogen) was added when indicated. The next day the islets were dispersed into single cells by four consecutive washes with Ca2+/Mg2+-free MEM (Mediatech, Herndon, VA), followed by gentle repetitive pipetting in the presence of a 0.05% trypsin-EDTA solution (Invitrogen) diluted 1:5 in Ca2+/Mg2+-free MEM (21,22). Cells were washed with Ca2+/Mg2+-free MEM, and then transferred to glass coverslips with RPMI 1640 media and allowed to adhere overnight. For insulin treatments, cells were washed three times with serum free media before being cultured in 5 mm glucose RPMI 1640 media. 5-bromo-2-deoxyuridine (BrdU) (Roche, Laval, Quebec, Canada) was added to each primary cell culture for 3 d to label recently synthesized DNA in dividing cells or their daughter cells. The number of BrdU-positive cells was divided by three to give the percentage of proliferation per day.
Mouse insulinoma (MIN6) cells were maintained and cultured as described (23) in DMEM containing 25 mm glucose, 10% fetal bovine serum, and 100 IU/ml penicillin/100 μg/ml streptomycin (Invitrogen). MIN6 cells were seeded onto coverslips and allowed to adhere overnight. Cells were treated at approximately 75% confluence as indicated for 6 h, and BrdU was added 2 h before cells were fixed. To directly compare replication rates between primary and transformed cells, we multiplied the number of dividing cells by 12 to give a projection of the proliferation rate per day.
In some experiments, MIN6 cells were transfected with full-length or truncated, dominant-negative Raf-1-green fluorescent protein (GFP) fusion proteins under the control of the cytomegalovirus promoter (kindly provided by Dr. Tamas Balla, National Institutes of Health, Bethesda, MD) (24). We have established that the mutant Raf-1 proteins decrease phosphorylated Erk in MIN6 cells, as expected (25). Enhanced green fluorescence protein (EGFP) cDNA, provided by Dr. Chris Proud (University of British Columbia), was transfected as a control. Twenty-four hours after transfection, MIN6 cells were treated with appropriate media (10% serum, serum free, 200 pm or 200 nm insulin) for 6 h. BrdU was added 2 h before fixing the cells for staining. GFP-positive cells were studied 30 h after transfection.
Propidium iodide incorporation cell death assays were performed as described (25) on an incubated KinetiScan High-Content Imaging instrument (Cellomics, Inc., Thermo Fisher Scientific Inc., Waltham, MA), made available by Dr. T. M. Underhill (University of British Columbia).
Immunofluorescence labeling of proliferating cells
Immunofluorescence analysis of dispersed islet cells was performed essentially as described (21,26) using a Zeiss inverted microscope (Carl Zeiss, Inc., Thornwood, NY) equipped with a ×10 objective. Primary antibodies to BrdU and guinea pig insulin (LINCO Research, Inc., St. Charles, MO) were incubated separately. The secondary antibodies used were fluorescein isothiocyanate-conjugated goat antimouse, Texas Red Red-conjugated goat antimouse, and Texas Red-conjugated goat antiguinea pig (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA) was used as a nuclear counterstain. Images were analyzed and quantified using SlideBook software (Intelligent Imaging Innovations, Boulder, CO). Each proliferating cell was manually checked to ensure robust insulin costaining.
Immunoblot
Western blots were performed as described previously (25) using anti-phospho-Raf-1 (serine 338), anti-total Raf-1, and glyceraldehyde-3-phosphate dehydrogenase as a loading control. All antibodies were from Cell Signaling Technology, Inc. (Danvers, MA). Band intensities were quantified using Photoshop (Adobe Systems, Inc., San Jose, CA).
Insulin secretion
All experiments were performed in 5 mm glucose unless otherwise stated to reduce endogenous insulin secretion. To assess the relative levels of endogenous and exogenous insulin in our cultures, samples were taken from the media of dispersed cell cultures every 24 h starting at d 0. Insulin was measured using RIA (Rat Insulin RIA Kit; LINCO Research).
Statistics
At least three independent mouse islet isolations or MIN6 cell cultures were used for each experiment. Results are presented as means ± sem. Data were analyzed using ANOVA or the Student’s t test, where appropriate. The least significant difference post hoc test was used for ANOVA statistics. Statistical significance was considered achieved when P values were less than 0.05.
Results
Measurement of the β-cell proliferation rate in vitro
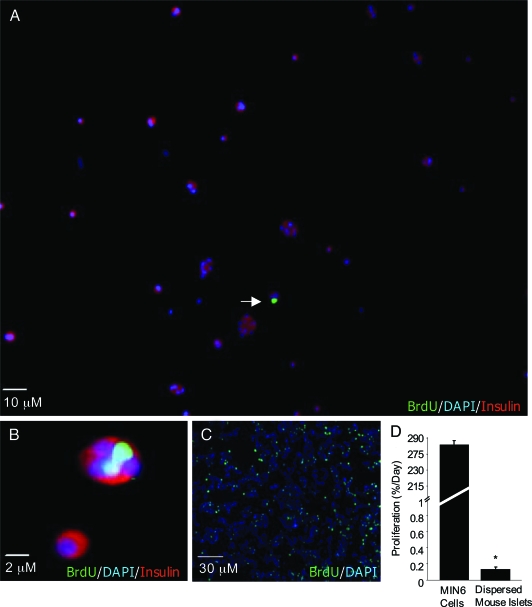
The proliferation of primary β-cells is notoriously difficult to estimate. To establish a baseline proliferation rate, dispersed C57BL/6 mouse β-cells were triple labeled with insulin, BrdU, and DAPI (Fig. 1, A and B). Under these baseline conditions, primary mouse β-cells replicated at a very slow rate of 0.155 ± 0.034% per day. By contrast, the proliferation rate of the MIN6 β-cell line was estimated to be 283.1 ± 33.90% per day (Fig. 1, C and D). Thus, although it is extremely slow, the replication of primary mouse β-cells can be reliably measured in our cultures using BrdU and insulin staining. Efforts to perform similar analysis on human islet cells were hampered by the low relative β-cell number in these preparations and the overgrowth of rapidly dividing fibroblast-like cells capable of taking up stainable insulin (1).
Figure 1.
Analysis of proliferation of primary mouse islets and MIN6 cells. A, Analysis of proliferation in cultures of dispersed primary mouse islet cells under basal (serum-containing) conditions. BrdU was added to cultures for 3 d and is identified with green staining (arrow). Cells were costained with insulin (red) and DAPI to identify nuclei (blue). B, Magnified image of β-cell proliferation. C, MIN6 cells were treated with serum for 6 h. BrdU (green) was added for 2 h. Cells were stained with DAPI (blue). D, Quantification of proliferating MIN6 cells (n = 6), and proliferating primary β-cells as a fraction of total mouse islet cells (n = 15). Due to the different durations of BrdU exposure between primary and transformed cells (3 d vs. 2 h), we have expressed both values in terms of estimated 24-h proliferation. *, P < 0.05 MIN6 cells vs. primary mouse islets.
Insulin stimulates primary β-cell proliferation
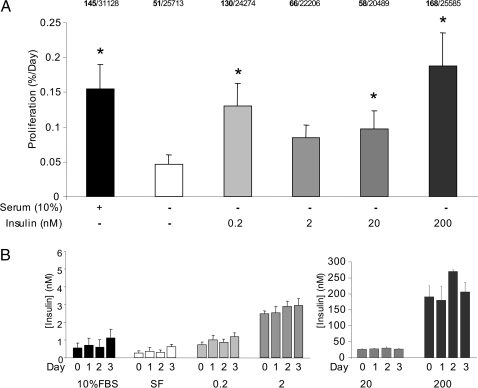
High levels of insulin are mitogenic to many cell types, but the effects of picomolar insulin doses on primary β-cell replication are unclear. We have previously shown that low doses of insulin can initiate specific signaling cascades in primary β-cells (6,27), and multiple studies have implicated insulin receptor signaling in the control of β-cell mass (8,9,11,12,23). To assess the direct effects of insulin on β-cell proliferation, dispersed islet cells were treated with a range of insulin doses for 3 d, and β-cell proliferation was quantified using BrdU incorporation over the same time period. Only cells clearly costained with insulin and BrdU were considered in the analysis of β-cell proliferation (e.g. Fig. 1, A and B). As expected, there was a significant increase in β-cell proliferation in serum-containing cultures, compared with serum-free controls (Fig. 2A). More notably, β-cells treated with the 200-pm dose of insulin had a significantly higher rate of replication compared with control cultures. β-Cells treated with 2 nm insulin had a modest rate of replication that did not achieve statistical significance despite a high number of replicate experiments (n = 15 independent cultures). Proliferation was significantly increased in β-cells treated with 20 or 200 nm insulin (Fig. 2A), a dose that likely reflects activation of IGF-I receptors (28). These results suggest that β-cell replication can be stimulated with the low levels of insulin observed in vivo.
Figure 2.
Exogenous insulin increases proliferation of primary mouse β-cells. A, Dispersed primary mouse islets treated with various doses of insulin as indicated for 3 d. Serum containing media were used as a positive control. Proliferation was quantified using immunofluorescent BrdU staining as in Fig. 1 (n = 14–16 independent culture mice). The total number of BrdU positive β-cells (bold) and the total number of dispersed islet cells counted are listed for each group. *, P < 0.05 vs. serum-free (SF) control. B, Media were sampled every 24 h starting at d 0 from culture media to assess insulin secretion by RIA (n = 9–13). FBS, Fetal bovine serum.
In these experiments, care was taken to reduce the contribution of endogenous insulin in the media (see Materials and Methods). To determine the relative levels of endogenous and exogenous insulin in our culture medium, insulin was measured at the start of the experiment and every day thereafter. The first measurement should estimate only the exogenous insulin applied because these cultures had been washed thoroughly, whereas the subsequent measurements reveal insulin accumulation in the media over the culture period. Under these culture conditions, our results show that the majority of the ambient insulin is exogenous and that there is relatively little contribution from insulin release, even at very low insulin doses (200 pm and 2 nm) (Fig. 2B). Similarly, exogenous insulin release was not observed at 20 or 200 nm. The ability of picomolar insulin to alter β-cell function is consistent with our previous experiments (6,27) and illustrates our control over the insulin levels in our cultures over time.
Role of autocrine insulin signaling in β-cell proliferation
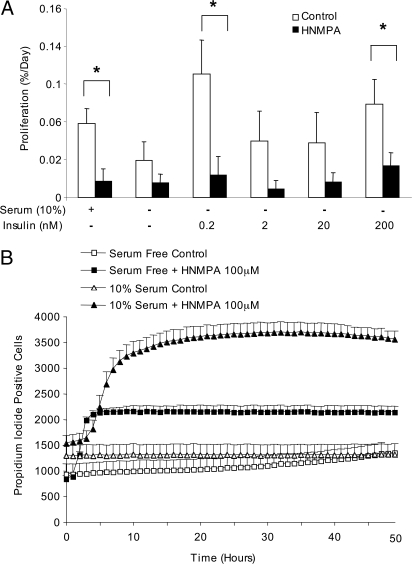
The results described previously demonstrate that exogenous insulin, when applied at specific doses, can induce proliferation in primary β-cells. However, it remained unclear whether endogenous insulin, acting in an autocrine or paracrine manner, contributes to the basal proliferation rate in β-cells. To address this question, we used HNMPA-AM, a drug known to inhibit insulin receptor tyrosine autophosphorylation. The results of this experiment suggested that insulin receptors may be required for a proportion of the β-cell proliferation seen under basal conditions (Fig. 3A). Moreover, the ability of insulin to stimulate β-cell proliferation was lost in HNMPA-AM-treated cells, confirming a receptor-mediated mechanism. Together, these observations further suggest an important role for insulin signaling in β-cell proliferation. We also observed a time- and dose-dependent increase in islet cell death with HNMPA-AM (Fig. 3B), supporting our previous findings that insulin signaling also plays antiapoptotic roles in β-cells (6,25).
Figure 3.
Insulin receptor inhibition decreases β-cell proliferation. A, Dispersed mouse islets were cultured with or without insulin as shown in the absence (white bars) or presence of 100 μm HNMPA-AM (black bars) for 3 d. *, P < 0.05 vs. control (n = 4–5). B, Cell death was measured as the incorporation of propidium iodide into cells from large populations of dispersed mouse islets (∼7500 cells total) imaged over time (n = 6).
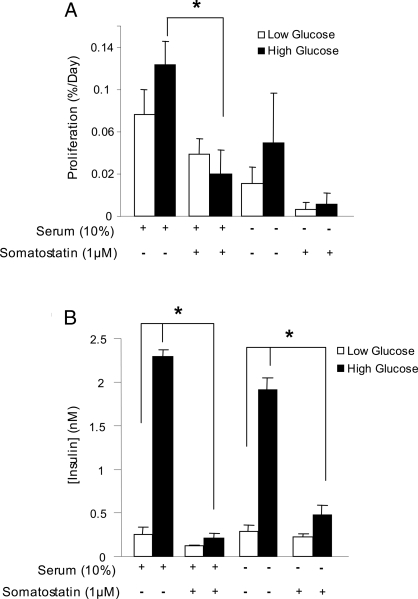
It has been suggested by others that glucose itself can increase β-cell proliferation (29), although recent studies have indicated a dominant role for insulin receptor signaling in the effects of high glucose on β-cell function (23). Our in vitro system, in which glucose and insulin levels are better controlled compared with the in vivo situation, provided an ideal model to test this hypothesis. Dispersed mouse islet cells were cultured in 5 mm glucose or 15 mm glucose, in both serum containing and serum-free conditions. β-Cell proliferation was not significantly increased with high glucose (Fig. 4A). To test whether the trends seen with glucose could be attributed to autocrine/paracrine insulin signaling, 1 μm somatostatin was added to these cultures to effectively block endogenous insulin release (Fig. 4B). Somatostatin caused a significant reduction in β-cell proliferation in cells incubated in 15 mm glucose and serum. Therefore, any effects of glucose could be explained by insulin release and autocrine/paracrine insulin signaling. Together with the findings using HNMPA-AM, these results are consistent with a role for basal insulin secretion and paracrine insulin action in the control of β-cell proliferation.
Figure 4.
Effects of elevated glucose on β-cell replication and insulin secretion. A, Dispersed primary mouse islets treated with 5 mm glucose (white bars) or 15 mm glucose (black bars) for 3 d with or without 1 μm somatostatin. *, P < 0.05 vs. serum control (n = 4). B, Insulin levels were assayed at 24 h (n = 3).
Mechanisms of insulin-stimulated proliferation of primary β-cells
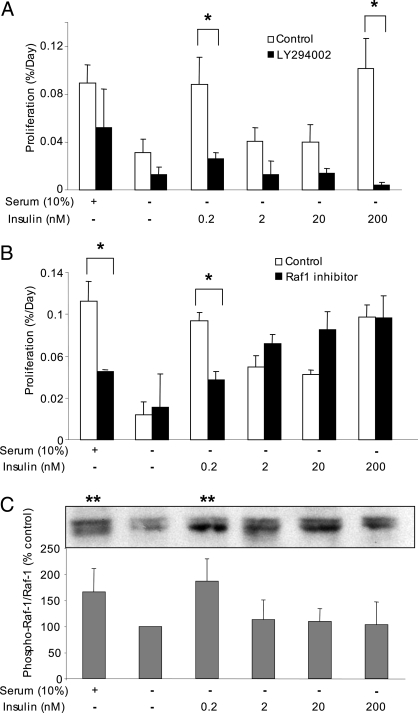
Previous investigations have focused on specific downstream targets of the insulin receptor in the regulation of β-cell mass, most notably Irs-2 (14) and Akt (16,17,30,31). However, the role of these proteins in nonsuperphysiological insulin signaling in primary β-cells remains poorly understood. The mechanism of insulin-stimulated β-cell proliferation was investigated using LY294002, an inhibitor of the phosphatidylinositol 3 (PI3)-kinase-dependent signaling cascade. We found that blocking PI3-kinase significantly prevented β-cell proliferation in the presence of 200 pm and 200 nm insulin (Fig. 5A). Insulin activates multiple signaling cascades (32). Although the PI3-kinase/Akt-dependent signaling pathway has been well studied in β-cells, insulin may also trigger its mitogenic effects via Raf-1 and Erk. A highly selective small molecule Raf-1 kinase inhibitor (25,33) was used to address further the mechanism of insulin-stimulated β-cell proliferation. This inhibitor decreased proliferation of primary β-cells at 200 pm insulin, without affecting basal proliferation in serum-free conditions (Fig. 5B). Remarkably, Raf-1 kinase inhibitor did not lower β-cell replication induced by high concentrations of insulin. In preliminary experiments the activation of Raf-1 at serine 338 was also maximal at 200 pm insulin (Fig. 5C). Together, these results demonstrate that both PI3-kinase and Raf-1 kinase pathways are important for the proliferative effects of insulin on primary mouse β-cells, although Raf-1 appears to be specifically involved in mediating insulin signals at concentrations of the hormone expected to activate the insulin receptor, but not the IGF-I receptor (34).
Figure 5.
Roles of PI3-kinase and Raf-1 in insulin-stimulated β-cell proliferation. A, Dispersed mouse islets were cultured with or without insulin as indicated and in the presence (white bars) or absence (black bars) of 50 μm of the PI3-kinase inhibitor LY294002 for 3 d (n = 3). B, Dispersed mouse islets were cultured as in A in the presence (black bars) or absence (white bars) of Raf-1 kinase inhibitor (5 μm). *, P < 0.05 vs. control (n = 4). C, Mouse islets were treated as indicated for 48 h, and phosphorylation of Raf-1 on serine 338 was examined and normalized to total Raf-1/glyceraldehyde-3-phosphate dehydrogenase. **, P < 0.05 vs. serum-free control (n = 4).
Effects of insulin in transformed β-cells
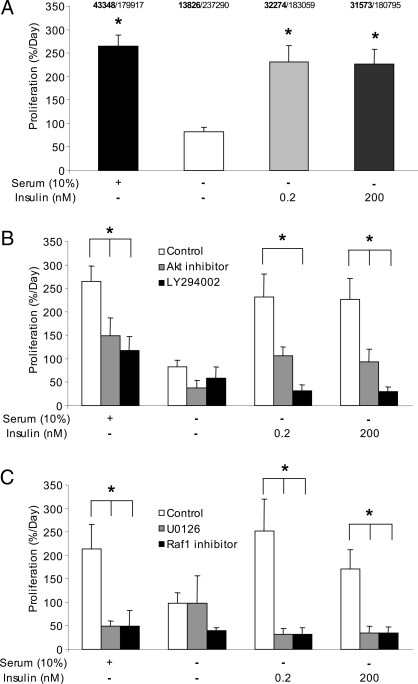
Although MIN6 cells proliferate at a very fast rate compared with primary β-cells (Fig. 1D), we examined whether the effect of insulin would be similar between these two models. The replication of MIN6 cells in the presence of insulin for 6 h was determined by the incorporation of BrdU present in the media for 2 h. As with the primary β-cells, both low and high doses of insulin significantly increased MIN6 cell proliferation (Fig. 6A). In the MIN6 cell model, the effects of insulin could be blocked using inhibitors of PI3-kinase or Akt (Fig. 6B). Inhibiting Raf-1 kinase or Erk reduced proliferation in response to levels of insulin observed in vivo. Unlike the case in primary cells, Raf-1 kinase and Erk inhibitors also reduced proliferation induced with the superphysiological dose of insulin (Fig. 6C). Substantial proliferation in serum-free media was still observed in the presence of these inhibitors, indicating that these transformed cells are relatively freed from the requirement of growth factor support for basal growth (Fig. 6, B and C). Together, these results indicate that insulin can also regulate the proliferation of transformed β-cells.
Figure 6.
Effects of insulin on MIN6 cell proliferation. A, MIN6 cells were cultured for 6 h, and percent proliferation was quantified using BrdU immunofluorescence. *, P < 0.05 vs. serum free control (n = 6). The total number of BrdU positive β-cells (bold) and the total number of β-cells counted are listed for each group. B, MIN6 cells were cultured as in A or treated with an Akt inhibitor (100 nm) or LY294002 (50 μm) (n = 3). C, MIN6 cells were cultured as in A or treated with U0126 (10 μm) or the Raf-1 inhibitor (5 μm). **, P < 0.05 vs. no drug control (n = 3).
Direct effects of Raf-1 on β-cell proliferation
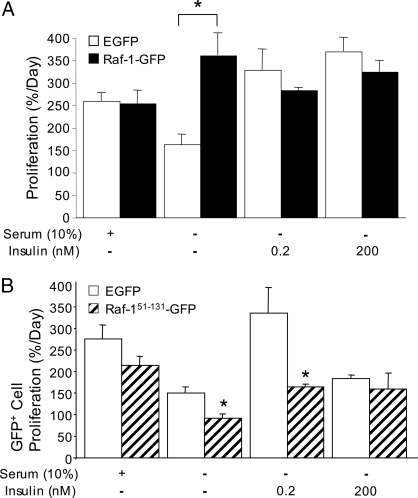
To elucidate further the role of Raf-1 in β-cell proliferation, we transiently overexpressed fusion proteins containing full-length Raf-1 and dominant-negative truncation mutants in MIN6 cells (25). Compared with EGFP-expressing control cells, MIN6 cells from cultures overexpressing Raf-1 had a significant increase in proliferation in serum-free conditions (Fig. 7A). Notably, this was sufficient to increase proliferation to the same level as insulin. No increase in proliferation was observed in cells cultured in insulin or serum, indicating that the effects of Raf-1 expression are not additive to those of insulin. Conversely, expression of truncated dominant-negative Raf-1-GFP fusion protein had inhibitory effects on the total number of cells in each culture (data not shown) and on the proliferation of GFP-positive cells in the presence of 200 pm insulin (Fig. 7B). Therefore, the effects of the mutant Raf-1 protein lacking the kinase domain are similar to the effects of the chemical Raf-1 kinase inhibitor. Together, these results strongly implicate Raf-1 in the control of β-cell proliferation.
Figure 7.
Effects of wild-type and mutant Raf-1 on β-cell proliferation. A, MIN6 cells transfected with EGFP (control) or Raf-1-GFP were treated with insulin or 10% serum as in Fig. 6. Cells were cultured for 6 h, and percent proliferation was quantified from all cells using BrdU immunofluorescence in the red channel (n = 3). B, Proliferation in MIN6 cells transfected with dominant-negative Raf-1 fusion protein (i.e. only GFP-positive cells), treated as indicated (n = 3). *, P < 0.05 vs. EGFP control.
Discussion
Pancreatic β-cell duplication is now recognized as a major regulator of adult β-cell mass (3,4,5), and new attention is being focused on endogenous regulators of β-cell growth. The present study was undertaken to test the hypothesis that insulin can stimulate the proliferation of primary β-cells. Our study produced two major findings. First, β-cell replication could be stimulated by both low and high doses of insulin, whereas elevating glucose was less effective. Second, both Raf-1 and PI3-kinase pathways regulate β-cell proliferation, with Raf-1 specifically mediating signal transduction of low insulin in primary cells. Together, our findings illustrate that insulin is a direct regulator of β-cell proliferation and establish the molecular mechanism for this effect.
Several studies have sought to estimate the proliferation rate of pancreatic β-cells. Early reports estimated an in vivo proliferation rate of roughly 1 and 6% per day, in rats of 3 and 1 month of age, respectively (35). Higher proliferation rates were obtained with monolayer cultures of neonatal rat β-cells (18). Using a mathematical model, replication rates as high as 10% per day were determined for adolescent rats (36). More recently, β-cell replication has been estimated to be between 0.07 and 2% per day, depending on the age of the mice exposed to BrdU via their drinking water (4,5). In vitro, we found the replication rate of primary β-cells to range between 0.06 and 0.15% of total islet cells per day, depending on the preparation. Given that our preparations are composed of approximately 75% β-cells, the rate of dividing β-cells expressed as a percentage of total β-cells would be approximately 0.2% per day. Our results are in the same range as the newer studies but are lower than values in earlier reports and on monolayers of neonatal cells. It is notable that some earlier experiments did not fully distinguish between BrdU labeling of noninsulin-positive cells and β-cells (35). Distinguishing β-cell nuclei in tightly packed monolayers can also be difficult. By comparison, our highly dispersed in vitro culture system, which was essential for testing the effects of exogenous insulin, permitted us to verify that every proliferating single β-cell was indeed colabeled with both BrdU and insulin. The highly dispersed nature of our cultures may have contributed to the lower proliferation rates we observed. Although it is also possible that culturing cells with BrdU for 3 d might have independent effects on the rate of β-cell proliferation, lifelong exposure to this chemical does not appear to adversely effect β-cell mass or glucose homeostasis (4,5).
In the present study, we demonstrated for the first time that insulin stimulates primary β-cell division. Many other exogenous and endogenous growth factors have increased β-cell proliferation in vivo and in vitro, including prolactin, placental lactogen, hepatocyte growth factor, PTH-related protein, glucagon-like peptide-1/exendin-4, and IGF-I (18,37,38,39,40,41,42,43,44,45). However, of the known β-cell growth factors, insulin appears to be unique, as the only factor secreted in substantial amounts from within the islet. We hypothesize that autocrine insulin signaling may be one of the more important endogenous regulators of β-cell proliferation in physiological conditions. Understanding the mechanisms by which insulin stimulates β-cell proliferation could potentially lead to new strategies to correct deficient β-cell mass in diabetes. Recent analyses of type 1 diabetic pancreas sections have revealed that β-cells continue to proliferate after years of autoimmune attack, presumably in an attempt to counter β-cell destruction (46).
Recent genome-wide analyses of single nucleotide polymorphisms underlying genetic susceptibility to type 2 diabetes identified cell cycle regulators known to participate in β-cell replication (47). In obesity and pre-type 2 diabetes, the pancreas attempts to compensate for insulin resistance by increasing its β-cell mass (48). Our results are consistent with a model in which modest hyperinsulinemia could drive this response, a hypothesis that is supported by in vivo studies. For example, overexpression of an insulin transgene led to increased β-cell mitogenesis (49). Another example is the recent finding that mice lacking insulin receptors in their β-cells have an age-dependent decrease in β-cell mass and impaired proliferation in response to insulin resistance (8,9). On the other hand, a complete lack of insulin genes in mice led to islet hyperproliferation (50,51), suggesting that either the effects of insulin on proliferation are nonlinear or that substantial overcompensation occurred through alternative growth factor pathways. Although these studies highlighted the importance of insulin signaling in the control of β-cell mass, they could not address the possibility of direct acute effects of insulin on β-cell proliferation or determine the relative effectiveness of specific insulin concentrations. Our study complements these in vivo models and demonstrates that insulin can rapidly (within days) stimulate β-cell proliferation in the absence of other putative stimuli, such as hyperglycemia. In contrast to previous reports (29,52,53,54), we did not observe significant stimulatory effects of glucose on β-cell proliferation that could not be fully accounted for by autocrine insulin signaling. In a previous study, we noted that approximately 80% of gene expression changes induced by glucose in MIN6 cells were no longer seen when insulin receptors were knocked down by 80% using RNA interference (23), illustrating the importance of autocrine insulin signaling in glucose-stimulated events. These stable insulin receptor knockdown cell lines also exhibited fewer G1 to S phase transitions and significant changes in cell cycle molecules, including cyclin D2, p21, and p18 (23). Efforts to characterize the expression of cell cycle regulatory proteins (cyclin D2, p21, p16, and phosphorylated retinoblastoma) and mRNAs (cyclin D1, cyclin D2, and p21) in the extremely small population of dividing primary β-cells were unsuccessful (data not shown).
In the present study, we examined the effectiveness of a wide range of insulin doses. Insulin is secreted in a pulsatile manner with basal portal vein concentrations of 200-1000 pm and basal systemic concentrations of 40–100 pm in humans (55). Therefore, our results imply that insulin stimulates significant β-cell proliferation at concentrations expected in the portal circulation or during periods of elevated insulin release. One might speculate that insulin levels surrounding β-cells may be higher than those observed in the portal circulation. Unfortunately, efforts to measure or estimate the local insulin concentrations within islets are complicated by several factors. First, insulin is released from β-cells as an insoluble microcrystal that only becomes a soluble monomer capable of binding to its receptor after exposure to the bloodstream pH (56). Second, within the complex islet microvasculature, β-cells are exposed first to the arterial blood supply (i.e. 40–100 pm insulin). These factors suggest to us that β-cells may be exposed to insulin at levels that are similar to or only slightly higher than those measured in the circulation. Together, with our previous studies (6,21,22,57) and those of other groups (58), our current results suggest a “sweet spot” in the dose response profile of autocrine insulin signaling at approximately 200 pm. Importantly, both the mitogenic and antiapoptotic effects of insulin on the β-cell are self-limiting, with reduced effectiveness at 2–20 nm (6,21,22,57). We speculate that the effects of 200 nm insulin are due to cross-activation of IGF-I receptors (34).
Our results suggest that insulin-stimulated β-cell replication is mediated by a combination of two signaling pathways, the PI3-kinase/Akt pathway and the Raf-1/Erk pathway. Although evidence from overexpression studies led many investigators to focus on Akt in the control of β-cell mass (16,31), it is notable that mice lacking approximately 80% of islet Akt activity have normal β-cell mass, even in response to high-fat feeding (17). We have previously reported that Akt mediates only part of the antiapoptotic effects of insulin and that 200 pm insulin did not stimulate Akt serine 473 phosphorylation in primary islets (6). In contrast, we have found that Raf-1 is required specifically for the proliferative effects of low insulin doses. Our results with Raf-1 mutants lacking the kinase domain support the conclusions drawn from studies using the Raf-1 kinase inhibitor. We also demonstrated that Raf-1 overexpression was sufficient to increase MIN6 cell proliferation and that this effect was not additive to that of insulin. Previously, it had been shown in HIT-T15 cells that overexpression of a Raf-1 kinase inhibitory protein decreased β-cell proliferation (19). Our results are the first to implicate the Raf/Mek/Erk pathway in insulin-stimulated β-cell proliferation. Therefore, further studies on the role of Raf-1 in the control of β-cell mass should be pursued.
A loss of β-cell mass is a hallmark of type 1 diabetes, and increasing evidence points to a relative β-cell deficiency in type 2 diabetes compared with age- and weight-matched controls (59). Furthermore, inadequate β-cell mass is a also major limiting factor of islet transplantation (1). It is now clear that β-cell proliferation is critical for maintaining a stable and adaptable β-cell mass (3,5,12,60,61,62,63). Our findings suggest that both physiological and superphysiological doses of insulin can directly stimulate β-cell proliferation via Raf-1 and PI3-kinase. Understanding the endogenous regulators of β-cell proliferation has the potential to improve the ability of increasing β-cell mass in diabetes and islet transplantation. The downstream targets and cell cycle molecules involved in increasing β-cell proliferation with physiological doses of insulin warrant further investigation.
Acknowledgments
We thank Xiaoke Hu for technical assistance with RIAs.
Footnotes
Research was supported by operating grants to J.D.J. from the Canadian Institutes for Health Research. J.D.J. is also supported by a Career Development Award from the Juvenile Diabetes Research Foundation, and salary awards from the Michael Smith Foundation for Health Research, the Canadian Diabetes Association, and the Canadian Institutes for Health Research. E.U.A. is a Cordula and Gunter Paetzold Fellow and a National Institutes of Health National Research Service Award recipient (F31DK079346).
Disclosure Statement: The authors have nothing to disclose.
First Published Online January 17, 2008
Abbreviations: BrdU, 5-Bromo-2-deoxyuridine; DAPI, 4′,6-diamidino-2-phenylindole; EGFP, enhanced GFP; GFP, green fluorescent protein; HNMPA-AM, hydroxy-2-naphthalenylmethylphosphonic acid; Irs-2, insulin receptor substrate 2; Mek, MAPK kinase; MIN6, mouse insulinoma; PI3, phosphatidylinositol 3.
References
- Robertson RP 2004 Islet transplantation as a treatment for diabetes—a work in progress. N Engl J Med 350:694–705 [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S 2000 Life and death of the pancreatic β cells. Trends Endocrinol Metab 11:375–378 [DOI] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA 2004 Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429:41–46 [DOI] [PubMed] [Google Scholar]
- Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA 2005 Very slow turnover of β-cells in aged adult mice. Diabetes 54:2557–2567 [DOI] [PubMed] [Google Scholar]
- Teta M, Rankin MM, Long SY, Stein GM, Kushner JA 2007 Growth and regeneration of adult β cells does not involve specialized progenitors. Dev Cell 12:817–826 [DOI] [PubMed] [Google Scholar]
- Johnson JD, Bernal-Mizrachi E, Alejandro EU, Han Z, Kalynyak TB, Li H, Beith JL, Gross J, Warnock GL, Townsend RR, Permutt MA, Polonsky KS 2006 Insulin protects islets from apoptosis via Pdx1 and specific changes in the human islet proteome. Proc Natl Acad Sci USA 103:19575–19580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni RN, Jhala US, Winnay JN, Krajewski S, Montminy M, Kahn CR 2004 PDX-1 haploinsufficiency limits the compensatory islet hyperplasia that occurs in response to insulin resistance. J Clin Invest 114:828–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni RN, Bruning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR 1999 Tissue-specific knockout of the insulin receptor in pancreatic β cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 96:329–339 [DOI] [PubMed] [Google Scholar]
- Otani K, Kulkarni RN, Baldwin AC, Krutzfeldt J, Ueki K, Stoffel M, Kahn CR, Polonsky KS 2004 Reduced β-cell mass and altered glucose sensing impair insulin-secretory function in βIRKO mice. Am J Physiol Endocrinol Metab 286:E41–E49 [DOI] [PubMed] [Google Scholar]
- Kulkarni RN, Holzenberger M, Shih DQ, Ozcan U, Stoffel M, Magnuson MA, Kahn CR 2002 β-cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter β-cell mass. Nat Genet 31:111–115 [DOI] [PubMed] [Google Scholar]
- Ueki K, Okada T, Hu J, Liew CW, Assmann A, Dahlgren GM, Peters JL, Shackman JG, Zhang M, Artner I, Satin LS, Stein R, Holzenberger M, Kennedy RT, Kahn CR, Kulkarni RN 2006 Total insulin and IGF-I resistance in pancreatic β cells causes overt diabetes. Nat Genet 38:583–588 [DOI] [PubMed] [Google Scholar]
- Okada T, Liew CW, Hu J, Hinault C, Michael MD, Krutzfeldt J, Yin C, Holzenberger M, Stoffel M, Kulkarni RN 2007 Insulin receptors in β-cells are critical for islet compensatory growth response to insulin resistance. Proc Natl Acad Sci USA 104:8977–8982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D, Jones PM, Persaud SJ 2006 Autocrine anti-apoptotic and proliferative effects of insulin in pancreatic β-cells. FEBS Lett 580:6977–6980 [DOI] [PubMed] [Google Scholar]
- Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, Bonner-Weir S, White MF 1998 Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391:900–904 [DOI] [PubMed] [Google Scholar]
- Gunton JE, Kulkarni RN, Yim S, Okada T, Hawthorne WJ, Tseng YH, Roberson RS, Ricordi C, O’Connell PJ, Gonzalez FJ, Kahn CR 2005 Loss of ARNT/HIF1β mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell 122:337–349 [DOI] [PubMed] [Google Scholar]
- Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA 2001 Islet β cell expression of constitutively active Akt1/PKB β induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J Clin Invest 108:1631–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal-Mizrachi E, Fatrai S, Johnson JD, Ohsugi M, Otani K, Han Z, Polonsky KS, Permutt MA 2004 Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet β cells. J Clin Invest 114:928–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichsen BN, Neubauer N, Lee YC, Gram VK, Blume N, Petersen JS, Nielsen JH, Moldrup A 2006 Stimulation of pancreatic β-cell replication by incretins involves transcriptional induction of cyclin D1 via multiple signalling pathways. J Endocrinol 188:481–492 [DOI] [PubMed] [Google Scholar]
- Zhang L, Fu Z, Binkley C, Giordano T, Burant CF, Logsdon CD, Simeone DM 2004 Raf kinase inhibitory protein inhibits β-cell proliferation. Surgery 136:708–715 [DOI] [PubMed] [Google Scholar]
- Salvalaggio PR, Deng S, Ariyan CE, Millet I, Zawalich WS, Basadonna GP, Rothstein DM 2002 Islet filtration: a simple and rapid new purification procedure that avoids ficoll and improves islet mass and function. Transplantation 74:877–879 [DOI] [PubMed] [Google Scholar]
- Johnson JD, Han Z, Otani K, Ye H, Zhang Y, Wu H, Horikawa Y, Misler S, Bell GI, Polonsky KS 2004 RyR2 and calpain-10 delineate a novel apoptosis pathway in pancreatic islets. J Biol Chem 279:24794–24802 [DOI] [PubMed] [Google Scholar]
- Johnson JD, Misler S 2002 Nicotinic acid-adenine dinucleotide phosphate-sensitive calcium stores initiate insulin signaling in human β cells. Proc Natl Acad Sci USA 99:14566–14571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsugi M, Cras-Meneur C, Zhou Y, Bernal-Mizrachi E, Johnson JD, Luciani DS, Polonsky KS, Permutt MA 2005 Reduced expression of the insulin receptor in mouse insulinoma (MIN6) cells reveals multiple roles of insulin signaling in gene expression, proliferation, insulin content, and secretion. J Biol Chem 280:4992–5003 [DOI] [PubMed] [Google Scholar]
- Bondeva T, Balla A, Varnai P, Balla T 2002 Structural determinants of Ras-Raf interaction analyzed in live cells. Mol Biol Cell 13:2323–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alejandro EU, Johnson JD 2008 Inhibition of raf-1 alters multiple downstream pathways to induce pancreatic β-cell apoptosis. J Biol Chem 283:2407–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Ahmed NT, Luciani DS, Han Z, Tran H, Fujita J, Misler S, Edlund H, Polonsky KS 2003 Increased islet apoptosis in Pdx1+/− mice. J Clin Invest 111:1147–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciani DS, Johnson JD 2005 Acute effects of insulin on β-cells from transplantable human islets. Mol Cell Endocrinol 241:88–98 [DOI] [PubMed] [Google Scholar]
- White MF 2006 Regulating insulin signaling and β-cell function through IRS proteins. Can J Physiol Pharmacol 84:725–737 [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S, Deery D, Leahy JL, Weir GC 1989 Compensatory growth of pancreatic β-cells in adult rats after short-term glucose infusion. Diabetes 38:49–53 [DOI] [PubMed] [Google Scholar]
- Elghazi L, Balcazar N, Bernal-Mizrachi E 2006 Emerging role of protein kinase B/Akt signaling in pancreatic β-cell mass and function. Int J Biochem Cell Biol 38:157–163 [DOI] [PubMed] [Google Scholar]
- Fatrai S, Elghazi L, Balcazar N, Cras-Meneur C, Krits I, Kiyokawa H, Bernal-Mizrachi E 2006 Akt induces β-cell proliferation by regulating cyclin D1, cyclin D2, and p21 levels and cyclin-dependent kinase-4 activity. Diabetes 55:318–325 [DOI] [PubMed] [Google Scholar]
- Taniguchi CM, Emanuelli B, Kahn CR 2006 Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 7:85–96 [DOI] [PubMed] [Google Scholar]
- Lackey K, Cory M, Davis R, Frye SV, Harris PA, Hunter RN, Jung DK, McDonald OB, McNutt RW, Peel MR, Rutkowske RD, Veal JM, Wood ER 2000 The discovery of potent cRaf1 kinase inhibitors. Bioorg Med Chem Lett 10:223–226 [DOI] [PubMed] [Google Scholar]
- De Meyts P, Whittaker J 2002 Structural biology of insulin and IGF1 receptors: implications for drug design. Nat Rev Drug Discov 1:769–783 [DOI] [PubMed] [Google Scholar]
- Montanya E, Nacher V, Biarnes M, Soler J 2000 Linear correlation between β-cell mass and body weight throughout the lifespan in Lewis rats: role of β-cell hyperplasia and hypertrophy. Diabetes 49:1341–1346 [DOI] [PubMed] [Google Scholar]
- Finegood DT, Scaglia L, Bonner-Weir S 1995 Dynamics of β-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes 44:249–256 [DOI] [PubMed] [Google Scholar]
- Buteau J, Foisy S, Rhodes CJ, Carpenter L, Biden TJ, Prentki M 2001 Protein kinase Czeta activation mediates glucagon-like peptide-1-induced pancreatic β-cell proliferation. Diabetes 50:2237–2243 [DOI] [PubMed] [Google Scholar]
- Freemark M, Avril I, Fleenor D, Driscoll P, Petro A, Opara E, Kendall W, Oden J, Bridges S, Binart N, Breant B, Kelly PA 2002 Targeted deletion of the PRL receptor: effects on islet development, insulin production, and glucose tolerance. Endocrinology 143:1378–1385 [DOI] [PubMed] [Google Scholar]
- Fujinaka Y, Sipula D, Garcia-Ocana A, Vasavada RC 2004 Characterization of mice doubly transgenic for parathyroid hormone-related protein and murine placental lactogen: a novel role for placental lactogen in pancreatic β-cell survival. Diabetes 53:3120–3130 [DOI] [PubMed] [Google Scholar]
- Garcia-Ocana A, Vasavada RC, Cebrian A, Reddy V, Takane KK, Lopez-Talavera JC, Stewart AF 2001 Transgenic overexpression of hepatocyte growth factor in the β-cell markedly improves islet function and islet transplant outcomes in mice. Diabetes 50:2752–2762 [DOI] [PubMed] [Google Scholar]
- Parsons JA, Brelje TC, Sorenson RL 1992 Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology 130:1459–1466 [DOI] [PubMed] [Google Scholar]
- Stoffers DA, Kieffer TJ, Hussain MA, Drucker DJ, Bonner-Weir S, Habener JF, Egan JM 2000 Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes 49:741–748 [DOI] [PubMed] [Google Scholar]
- Swenne I, Hill DJ, Strain AJ, Milner RD 1987 Growth hormone regulation of somatomedin C/insulin-like growth factor I production and DNA replication in fetal rat islets in tissue culture. Diabetes 36:288–294 [DOI] [PubMed] [Google Scholar]
- Villanueva-Penacarrillo ML, Cancelas J, de Miguel F, Redondo A, Valin A, Valverde I, Esbrit P 1999 Parathyroid hormone-related peptide stimulates DNA synthesis and insulin secretion in pancreatic islets. J Endocrinol 163:403–408 [DOI] [PubMed] [Google Scholar]
- Vasavada RC, Garcia-Ocana A, Zawalich WS, Sorenson RL, Dann P, Syed M, Ogren L, Talamantes F, Stewart AF 2000 Targeted expression of placental lactogen in the β cells of transgenic mice results in β cell proliferation, islet mass augmentation, and hypoglycemia. J Biol Chem 275:15399–15406 [DOI] [PubMed] [Google Scholar]
- Meier JJ, Lin JC, Butler AE, Galasso R, Martinez DS, Butler PC 2006 Direct evidence of attempted β cell regeneration in an 89-year-old patient with recent-onset type 1 diabetes. Diabetologia 49:1838–1844 [DOI] [PubMed] [Google Scholar]
- Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, Hughes TE, Groop L, Altshuler D, Almgren P, Florez JC, Meyer J, Ardlie K, Bengtsson Bostrom K, Isomaa B, Lettre G, Lindblad U, Lyon HN, Melander O, Newton-Cheh C, Nilsson P, Orho-Melander M, Rastam L, Speliotes EK, Taskinen MR, Tuomi T, Guiducci C, Berglund A, Carlson J, Gianniny L, Hackett R, Hall L, Holmkvist J, Laurila E, Sjogren M, Sterner M, Surti A, Svensson M, Svensson M, Tewhey R, Blumenstiel B, Parkin M, Defelice M, Barry R, Brodeur W, Camarata J, Chia N, Fava M, Gibbons J, Handsaker B, Healy C, Nguyen K, Gates C, Sougnez C, Gage D, Nizzari M, Gabriel SB, Chirn GW, Ma Q, Parikh H, Richardson D, Ricke D, Purcell S 2007 Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316:1331–1336 [DOI] [PubMed] [Google Scholar]
- Bell GI, Polonsky KS 2001 Diabetes mellitus and genetically programmed defects in β-cell function. Nature 414:788–791 [DOI] [PubMed] [Google Scholar]
- Vincent MT, Carroll RJ, Hammer RE, Chan SJ, Guz Y, Steiner DF, Teitelman G 1995 A transgene coding for a human insulin analog has a mitogenic effect on murine embryonic β cells. Proc Natl Acad Sci USA 92:6239–6243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvillie B, Cordonnier N, Deltour L, Dandoy-Dron F, Itier JM, Monthioux E, Jami J, Joshi RL, Bucchini D 1997 Phenotypic alterations in insulin-deficient mutant mice. Proc Natl Acad Sci USA 94:5137–5140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvillie B, Currie C, Chrones T, Bucchini D, Jami J, Joshi RL, Hill DJ 2002 Increased islet cell proliferation, decreased apoptosis, and greater vascularization leading to β-cell hyperplasia in mutant mice lacking insulin. Endocrinology 143:1530–1537 [DOI] [PubMed] [Google Scholar]
- Bernard C, Berthault MF, Saulnier C, Ktorza A 1999 Neogenesis vs. apoptosis As main components of pancreatic β cell mass changes in glucose-infused normal and mildly diabetic adult rats. FASEB J 13:1195–1205 [DOI] [PubMed] [Google Scholar]
- Bernard C, Thibault C, Berthault MF, Magnan C, Saulnier C, Portha B, Pralong WF, Penicaud L, Ktorza A 1998 Pancreatic β-cell regeneration after 48-h glucose infusion in mildly diabetic rats is not correlated with functional improvement. Diabetes 47:1058–1065 [DOI] [PubMed] [Google Scholar]
- Hoorens A, Van de Casteele M, Kloppel G, Pipeleers D 1996 Glucose promotes survival of rat pancreatic β cells by activating synthesis of proteins which suppress a constitutive apoptotic program. J Clin Invest 98:1568–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SH, McIntyre SS, Shah H, Veldhuis JD, Hayes PC, Butler PC 2000 Direct measurement of pulsatile insulin secretion from the portal vein in human subjects. J Clin Endocrinol Metab 85:4491–4499 [DOI] [PubMed] [Google Scholar]
- Dodson G, Steiner D 1998 The role of assembly in insulin’s biosynthesis. Curr Opin Struct Biol 8:189–194 [DOI] [PubMed] [Google Scholar]
- Johnson JD, Ford EL, Bernal-Mizrachi E, Kusser KL, Luciani DS, Han Z, Tran H, Randall TD, Lund FE, Polonsky KS 2006 Suppressed insulin signaling and increased apoptosis in CD38-null islets. Diabetes 55:2737–2746 [DOI] [PubMed] [Google Scholar]
- Jimenez-Feltstrom J, Lundquist I, Obermuller S, Salehi A 2004 Insulin feedback actions: complex effects involving isoforms of islet nitric oxide synthase. Regul Pept 122:109–118 [DOI] [PubMed] [Google Scholar]
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC 2003 β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52:102–110 [DOI] [PubMed] [Google Scholar]
- Georgia S, Bhushan A 2004 β Cell replication is the primary mechanism for maintaining postnatal β cell mass. J Clin Invest 114:963–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner JA 2006 β-Cell growth: an unusual paradigm of organogenesis that is cyclin D2/Cdk4 dependent. Cell Cycle 5:234–237 [DOI] [PubMed] [Google Scholar]
- Kushner JA, Ciemerych MA, Sicinska E, Wartschow LM, Teta M, Long SY, Sicinski P, White MF 2005 Cyclins D2 and D1 are essential for postnatal pancreatic β-cell growth. Mol Cell Biol 25:3752–3762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzo N, Mora C, Fabregat ME, Martin J, Usac EF, Franco C, Barbacid M, Gomis R 2004 Pancreatic islets from cyclin-dependent kinase 4/R24C (Cdk4) knockin mice have significantly increased beta cell mass and are physiologically functional, indicating that Cdk4 is a potential target for pancreatic β cell mass regeneration in type 1 diabetes. Diabetologia 47:686–694 [DOI] [PubMed] [Google Scholar]