Abstract
The glucocorticoid receptor (GR) and its ligand, cortisol, play a central role in human physiology. The exact mechanisms by which GR activation regulates these processes are the subject of intensive investigation. We and others have shown that GR activation can indirectly down-regulate specific genes via serum and glucocorticoid (GC) regulated kinase-1-mediated inhibition of forkhead box O3a (FOXO3a) transcriptional activity. We previously used gene expression microarrays, together with bioinformatic analyses, to identify putative FOXO3a target genes in breast epithelial cells. In this paper we refine our analysis through the use of FOXO3a chromatin immunoprecipitation (ChIP) microarrays. ChIP microarray results reveal urokinase plasminogen activator (uPA) as a putative novel target of FOXO3a in breast epithelial and breast cancer cell lines. We further show that uPA down-regulation after GC treatment requires serum and GC regulated kinase-1-mediated inactivation of FOXO3a activity. ChIP and luciferase assays confirm that FOXO3a can both occupy and transactivate the uPA promoter. Our data suggest that inactivation of FOXO3a after GR activation is an important mechanism contributing to GC-mediated repression of uPA gene expression in breast epithelial and cancer cells.
GLUCOCORTICOIDS (GCs) ARE steroid hormones involved in diverse cell type-specific physiological processes, including glucose, protein, and fat metabolism, as well as the response to systemic stressors and inflammation (1). Interestingly, although GCs can induce apoptosis in some cell types, including benign and malignant lymphocytes (2,3), they also have an antiapoptotic, or “pro-survival” function in mammary epithelial (4), breast cancer (5), and ovarian cancer (6) cells. Some researchers have hypothesized that the dichotomy of GC action is consistent with the generally anti-inflammatory effects of GCs in response to stress (7). GCs cause apoptosis in cells that are proinflammatory (e.g. lymphocytes), whereas they promote survival in epithelial cells required to preserve organ function in the face of physiological stressors. The exact signaling pathways through which GCs protect cancer cells from apoptosis are not well understood, although they may provide insight into both chemoresistance and anti-inflammatory signaling pathways.
As an approach to dissecting GC’s influence on gene transcription networks in different cell types, our laboratory is examining gene expression data over time after GC treatment. In this way we hope to untangle direct transcriptional events after glucocorticoid receptor (GR) activation, as well as to uncover novel mechanisms of indirect GR-mediated regulation of gene expression. Studying gene expression at several time points has made it clear that there are distinctive groups of genes with varying temporal patterns of gene expression after GR activation. By clustering genes of similar patterns (8), we can predict possible regulatory pathways downstream of GR activation. For example, many genes whose expression levels change at a similar time point may be direct targets of a common GR-regulated transcription factor. By identifying those transcription factors and looking for common target genes, we are beginning to construct regulatory networks downstream of GR activation. These networks may help to uncover GR’s indirect mechanisms for regulating gene expression, including the mechanisms underlying GC-dependent down-regulation of proinflammatory genes.
In a previous paper (9), we investigated the role of the transcription factor forkhead box O3a (FOXO3a) in GR-mediated cell survival signaling. GCs induce serum and GC regulated kinase (SGK) (10), a serine threonine kinase that is subsequently phosphorylated and activated in a phosphatidylinositol 3-kinase dependent fashion. After transcriptional induction by GR, activated SGK-1 phosphorylates FOXO3a, resulting in FOXO3a nuclear exclusion and inactivation (9,11). Overexpression of FOXO3a also limits the ability of dexamethasone (Dex) to protect mammary epithelial cells from apoptosis. We have previously identified genes down-regulated at least 30% by GCs that contain putative FOXO3a binding sites in the 1000-bp upstream of the transcriptional start site (TSS) to begin to identify novel indirect targets of GR repression. More recently, the chromatin immunoprecipitation microarray (ChIP-chip) assay has emerged as a genome-wide tool for studying transcription factor-chromatin interaction. In the current study, we additionally use FOXO3a ChIP-chip results to refine our identification of FOXO3a target genes. Interestingly, one of the most significantly down-regulated genes also found using the FOXO3a ChIP-chip assay was urokinase plasminogen activator (uPA or PLAU), a very well-established down-regulated target of GC action that is not a known FOXO3a target. These results suggest a novel mechanism whereby GCs indirectly down-regulate uPA.
uPA plays an important role in several diseases, most notably cancer. uPA is a serine protease that generates plasmin, causing matrix metalloproteinase activation and degradation of the extracellular matrix. In breast cancer, uPA and the plasminogen system are thought to degrade the extracellular matrix, mediating tumor cell invasion (12). Furthermore, elevated uPA activity correlates with poor breast cancer prognosis (13). GCs suppress uPA mRNA expression and activity in breast cancer cells (14,15). Down-regulation of uPA via inhibition of activator protein-1 (AP-1) and nuclear factor κ-B (NF-κB) function (16,17) has been long postulated as the major mechanism. In this study we identify FOXO3a inactivation as an additional important mechanism for uPA repression in breast epithelial cells.
Materials and Methods
Cell culture
MCF10A-Myc cells were cultured in a 1:1 mixture of DMEM and Ham’s F12 medium (BioWhittaker, Inc., Walkersville, MD) supplemented with hydrocortisone (0.5 μg/ml), human recombinant epidermal growth factor (10 ng/ml), and insulin (5 ng/ml; Sigma-Aldrich, St. Louis, MO). MDA-MB-231, SK-BR-3, and C-28/I2 cells were cultured in DMEM supplemented with 10, 5, or 10% fetal bovine serum, respectively, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Both MCF10A-Myc mammary epithelial and MDA-MB-231 breast cancer cell lines were chosen because they express abundant endogenous GR despite the absence of estrogen, progesterone, and Her-2/neu receptors.
Retroviruses were made by transient transfection of retroviral vectors into amphotropic Phoenix cells (a gift of Dr. Gary Nolan, Stanford University, Palo Alto, CA) using Polyfect Transfection Reagent (QIAGEN, Inc., Valencia, CA) per manufacturer’s instructions. Retroviruses were used to create stable MCF10A-Myc or MDA-MB-231 cells expressing pLPCX alone, pLPCX- wild-type (WT)-FOXO3a, pLPCX-triple mutant (TM) FOXO3a, scrambled sequence (SS) short hairpin RNA (shRNA), or SGK shRNA; colonies were selected with puromycin as described previously (18). Fugene HD (Roche Diagnostics Corp., Roche Applied Science, Indianapolis, IN) was used following the manufacturer’s protocol with a 5:2 Fugene to DNA ratio to create C-28/I2 cells transiently expressing pLPCX alone, or pLPCX containing WT-FOXO3a, or TM-FOXO3a.
Plasmids
WT-FOXO3a-hemagglutinin (HA), TM-FOXO3a-HA were obtained from Dr. Michael E. Greenberg (Harvard Medical School, Boston, MA) and inserted into pLPCX retroviral vectors as previously described (9). SGK shRNA and SS shRNA constructs were created in the retroviral expression vector RNAi-Ready pSIREN-RetroQ (BD Biosciences, Palo Alto, CA) as described previously (9).
Drug treatment
Dex (Sigma-Aldrich) was dissolved in 100% ethanol to make stock solutions. Before treatment, cell culture dishes were washed twice with 1× PBS, placed in media without serum, and starved 24 h for MDA-MB-231 cells or C-28/I2 cells or 72 h for MCF10A-Myc and SK-BR-3 cells before drug treatment. Cells were treated for the time specified in the experiment with Dex (10−6 m) or an equal volume of ethanol.
Time course microarray data
The procedure for cRNA preparation and hybridization to high-density oligonucleotide arrays was described previously (19). The experiment was previously performed (9) on MCF10A-Myc cells starved for 72 h, followed by treatment with either vehicle (ethanol), Dex (10−6 m), or concomitant Dex/RU486 (10−7 m) for 0.5, 2, 4, or 24 h. Preparation of the microarrays (Affymetrix human genechip HG-U133A; Affymetrix, Inc., Santa Clara, CA) was performed at the University of Chicago Microarray Core Facility. The genechip used contains 22,215 probe sets representing approximately 16,000 human genes. The experiment was performed three independent times. Fold-change values (Dex vs. vehicle) were determined after analysis using Robust Multiarray Average algorithm (40).
ChIP-chip analysis
Ethanol and Dex treated immunoprecipitated chromatin and cellular input DNA were produced as in the conventional chromatin immunoprecipitation (ChIP) assay. These were then amplified, fragmented, and labeled according to the Affymetrix ChIP Protocol. These samples were then hybridized to the Affymetrix GeneChip Human Promoter 1.0R Array (P/N 900775) and scanned at the University of Chicago Functional Genomics Core Facility.
Probe signals were analyzed using the software Model-based Analysis of Tiling-array (MAT) (20) to detect enriched regions of transcription factor binding based on National Center for Biotechnology Information’s build 36 of the human genome. Data were normalized using the corresponding input sample. A window size of 400 bp was set based on observed DNA shearing. A threshold of P < 10−3 was used to determine which regions were occupied by FOXO3a, and the resulting browser extensible display (“.bed”) file was analyzed by the cis-regulatory element annotation system (21) to generate a list of genes with putative FOXO3a chromatin interaction in their core promoter (−5000/+500). Only regions that appeared to be occupied by FOXO3a in the ethanol vs. the Dex samples were identified in the final list.
Quantitative real-time RT-PCR
Total RNA was extracted, using the RNeasy Mini kit (QIAGEN) from cells after treatment. cDNA was synthesized from RNA with TaqMan RT reagents (Applied Biosystems, Foster City, CA) per manufacturer’s instructions in a 7300 Real-Time PCR System (Applied Biosystems). Quantitative real-time RT-PCRs were performed as previously described (9). The following primers were used: uPA (PLAU, NM_002658), 5′-ACGCAAGGGGAGATGAAG-3′ (forward) and 5′-TCAGCAAGGCAATGTCGT-3′ (reverse); and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (NM_002046), 5′-GAGTCAACGGATTTGGTCGT-3′ (forward) and 5′-TTGATTTTGGAGGGATCTCG-3′ (reverse). GAPDH was amplified as an internal control. The samples were loaded in triplicate for each primer pair, and the results of each sample were normalized to GAPDH. Fold change was calculated as a ratio of treatment over control (vehicle). Agarose DNA gels were run, or Dissociated Curve analysis was performed to verify the absence of nonspecific products.
ChIP assay
MCF10A-Myc cells were starved for 72 h and then treated with either vehicle (ethanol) or Dex (10−6 m). With slight modifications, manufacturer’s directions in the Chromatin Immunoprecipitation Assay Kit (Upstate Biotechnology, Waltham, MA) were followed. Cells were fixed with formaldehyde for 20 min, and lysates were sonicated on ice four times for 30 sec each. Lysates were precleared for 1 h, after which 1% of the lysates were taken for input. Lysates were incubated with anti-FOXO3a antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or antirabbit IgG (Santa Cruz Biotechnology). DNA was purified with a PCR Purification Kit (QIAGEN). PCR was performed on the purified DNA and analyzed on agarose gels. Primers testing a known region of TNF-related apoptosis inducing ligand (TRAIL) were used as a positive control: forward, 5′-CCTGGGCGATAAAGTGAGAT-3′; reverse, 5′-GGCCCAGCTGTATGTTGTCT-3′.
To test the uPA binding sites, three primer pairs were designed to test the different regions of the uPA promoter. Because the resolution of a ChIP assay is limited by the size of DNA shearing, it is unlikely that the ChIP assay could distinguish further among these four binding sites. The uPA primers used were: uPA primer pair 1, forward 5′-TCTGTTTATGTTCTGGTCACGTTT-3′ and reverse 5′-CACTGTCTTCTGGAGAGACTTCTG-3′; uPA primer pair 2, forward 5′-TGGAATGGGCTATACAGAGATG-3′ and reverse 5′-TGGAGGACAAAATAAGAGGAAGA-3′; and uPA primer pair 3, forward 5′-ATCAAGCCCTGTCAAAAACG-3′ and reverse 5′-ACCAGTGGATGGAACAAAGC-3′.
Western blot analysis
MCF10A-Myc cells, MDA-MB-231 cells, or C-28/I2 cells were starved of growth factors for times previously described, and then treated with Dex (10−6 m) or vehicle (ethanol) for 24 h. Cell lysates were harvested with 2× Laemmli lysis buffer or as directed for the ChIP assay. Equal amounts of protein were resolved by SDS-PAGE, transferred to a nitrocellulose membrane, blocked by incubation in 5% skim milk in 0.1% Tween 20 in Tris-buffered saline, and probed with a 1:1000 dilution of anti-SGK-1 (22), anti-FOXO3a (Santa Cruz Biotechnology), anti-P-FOXO3a (Santa Cruz Biotechnology), anti-uPA (American Diagnostica Inc., Stamford, CT), anti-Na+/K+ ATPase (Affinity BioReagents, Inc., Golden, CO), anti-α-tubulin (Calbiochem, San Diego, CA), or anti-β-Actin (Sigma-Aldrich). After washing with 0.1% Tween 20 in Tris-buffered saline and incubation with peroxidase-conjugated goat antirabbit or antimouse secondary antibody (1:5000 dilution), the membranes were treated with enhanced chemiluminescent staining (Amersham Biosciences Inc., Piscataway, NJ) per manufacturer’s instructions before film development.
Luciferase constructs
A 1270-bp fragment of the uPA promoter (−1252/+18) was generated with Kpn-1 and Bgl-II restriction sites using standard nested PCR and inserted into the luciferase vector pGL3-basic (Promega Corp., Madison, WI). The primers used were: left outer primer (5′-CTGCAAAGGAAGGAGAAGTCAGGGCAAG-3′); right outer primer (5′-CACAGAAGTCTCTCCAGAAGACAGTGGGTCA-3′); left inner primer (5′-GGGGTACCCAGAGATGCGTGTGTGTGTG-3′); and right inner primer (5′-GAAGATCTCTGCGGTCTCCGCACTGT-3′).
The fragment of the human uPA gene promoter was then mutated to introduce an A to G mutation (mut) in the core (AAACA) FOXO3a consensus binding site (AGACA) by site-directed mutagenesis (QuikChange site-directed mutagenesis kit; Stratagene, La Jolla, CA) per manufacturer’s instructions using the primers 5′-GTTAAGAGTGTAAAAGGACAGACAGTGAAAAATAAATCTTCC-3′ (forward) and 5′-GGAAGATTTATTTTTCACTGTCTGTCCTTTTACACTCTTAAC-3′ (reverse). Underlining indicates which nucleotide was mutated. The resulting vectors (WT- and mut-uPA-pGL3) were sequenced for validation.
Luciferase assay
Thirty-five millimeter plates of SK-BR-3 cells were transfected with 0.9 μg WT-uPA-pGL3, mut-uPA-pGL3, or pGL3 vector alone together with 0.5 μg of either pLPCX, WT-FOXO3a, or TM-FOXO3a, and 0.1 μg of a pCMV-β-galactosidase-encoding vector (gift of Dr. Geoffrey Greene, University of Chicago, Chicago, IL). Forty-eight hours after transfection using PolyFect (QIAGEN) per manufacturer’s instructions, each transfection was split into multiple wells to provide triplicates for luciferase measurement. The protocol for the Luciferase Assay Kit (Promega) was followed to collect and prepare the luciferase lysates. Luminescence was measured using a Wallace luminescence counter (Beckman Coulter, Inc., Fullerton, CA), and β-galactosidase activity was measured separately at 420 nm. The relative luciferase activity in each condition was normalized to β-galactosidase activity.
Statistical analysis
For all gene expression and luciferase assays, statistical significance between two conditions was determined by an unpaired two-tailed t test. Results were considered statistically significant if the P value was less than 0.05.
Results
Identification of uPA as a FOXO3a target
We have previously demonstrated that GC treatment up-regulates SGK-1 expression and activity, resulting in FOXO3a phosphorylation and nuclear exclusion, and thereby leading to down-regulation of FOXO3a target gene expression at later time points (Fig. 1A) (9).
Figure 1.
Down-regulation of putative FOXO3a target genes by GCs. A, Model depicting GC-mediated regulation of uPA and related genes via FOXO3a. B, Diagram showing the number of genes identified as down-regulated at least 30% by 24 h GC treatment, the number of genes identified by FOXO3a ChIP-chip with a P value of less than 10−3, and the number of genes that was common to both lists. C, List of the genes that were both down-regulated by GCs and had FOXO3a promoter interaction. hrs, Hours.
In the current study, we used a ChIP-chip promoter screen to identify FOXO3a target genes after 24 h vehicle (ethanol) or Dex treatment (to exclude FOXO3a from the nucleus) in the MCF10A-Myc mammary epithelial cell line. MAT software was used to analyze the ChIP-chip data (20). MAT calculates a P-value significance for each identified binding region based on the probe signals in that region compared with other microarray regions. A P-value cutoff of 10−3 generated a list of 1037 genes having a promoter region with significant FOXO3a occupancy under ethanol (when FOXO3a is in the nucleus) but not Dex (FOXO3a excluded) conditions.
We then compared (Fig. 1B) our list of significantly down-regulated genes after GC treatment in the same MCF10A-Myc cell line (9) with the list of genes identified by ChIP-chip. Although the ChIP-chip technique provides evidence for complex formation between the immunoprecipitated transcription factor and DNA, the functional significance of this interaction is not always clear (23). By examining only those genes down-regulated after GR activation (potentially with reduced transcription secondary to SGK-1-mediated inactivation of FOXO3a), we attempted to reduce false positives and to increase physiologically relevant FOXO3a target genes. These data revealed 11 genes (Fig. 1C) as likely regulated by the GR through an indirect mechanism involving FOXO3a inactivation. These genes met several stringent criteria to be considered as putative FOXO3a targets, suggesting that they deserved further study. The known FOXO3a target TRAIL appeared on the list, whereas IGF binding protein-3, previously identified as a FOXO3a target (9), was absent. Therefore, although this list may be useful as a starting point for future investigation, it is unlikely to encompass all functional FOXO3a targets due to the stringency of the ChIP-chip assay.
Of the genes identified, uPA was one of the most highly down-regulated genes, and also contains one of the most significant P values using MAT analysis of the ChIp-chip data. Because uPA plays a well-established role in both cancer and arthritis, we chose to study it as a potential FOXO3a target.
Dex down-regulates uPA mRNA expression at 24 h
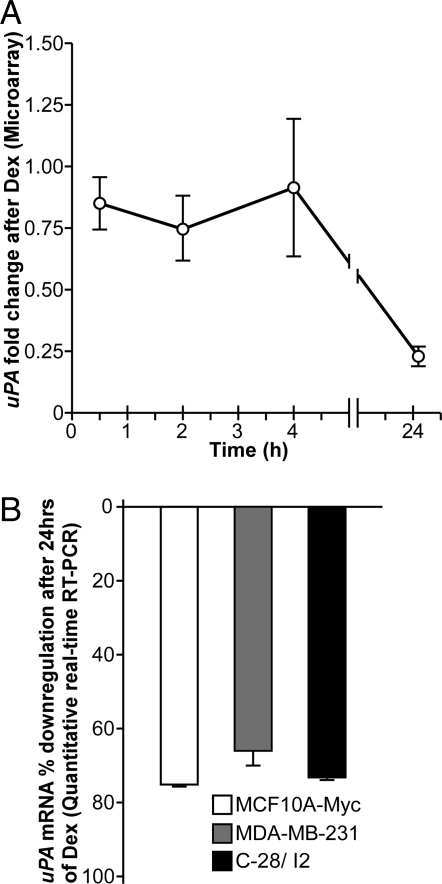
To confirm the expression microarray data previously generated (9) for uPA (Fig. 2A), semiquantitative real-time RT-PCR was used to examine uPA mRNA after treatment with Dex (10−6 m) or vehicle (ethanol) for 24 h in MCF10A-Myc cells. We also examined the MDA-MB-231 breast cancer cell line as, well as the C-28/I2 chondrocyte cell line because of the role uPA plays in both cancer and arthritis.
Figure 2.
Down-regulation of uPA mRNA in different cell lines. A, Microarray expression of uPA after Dex treatment in MCF10A-Myc cells. The fold change of gene expression is shown relative to vehicle after normalization, and data are reported as a mean ± se of three independent experiments. B, Quantitative real-time RT-PCR evaluation of uPA mRNA levels after 24 h Dex or vehicle treatment in MCF10A-Myc (white bars), MDA-MB-231 cells (gray bars), and C-28/I2 cells (black bars). The fold change of gene expression is shown relative to vehicle after normalization to GAPDH, and data are reported as a mean ± se of triplicate experiments. uPA mRNA was significantly down-regulated compared with vehicle in all three cell lines with a P < 0.05 using a two-tailed unpaired Student’s t test.
Previous studies using Northern analysis have shown that Dex (10−7 m) down-regulates uPA mRNA expression in MDA-MB-231 cells (15) and that this down-regulation is reversed by RU486 (24). uPA is also down-regulated by Dex in chondrocyte cell lines (25), but its effects have not been reported in C-28/I2 cells. Figure 2B shows that uPA is significantly down-regulated 24 h after Dex treatment in all three cell lines by real-time RT-PCR.
Conventional ChIP confirms uPA promoter occupancy by FOXO3a in breast cells
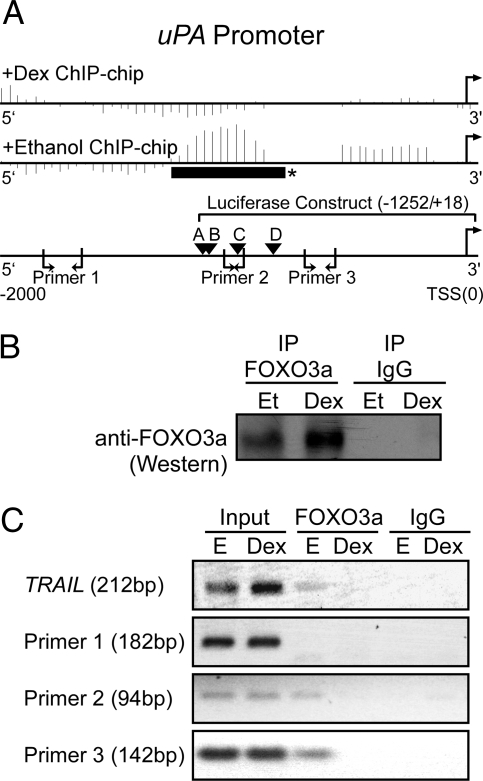
Figure 3A presents the 2000-bp promoter region upstream of the uPA TSS, with the corresponding ChIP-chip data shown above the uPA promoter. In the ChIP-chip data, the vertical lines represent a score calculated for a window around each microarray probe by the MAT software, and the solid horizontal bar with an asterisk is the region determined by MAT to be a significant FOXO3a binding site (P = 2.74 × 10−4) in the vehicle but not the Dex-treated immunoprecipitates. Three primer pairs were then chosen to examine by conventional ChIP assay the four predicted FOXO3a binding sites (A–D) found in this region (Fig. 3A).
Figure 3.
FOXO3a binding to the uPA promoter. A, Schematic of the uPA promoter (−2000/+50) showing the data from the FOXO3a ChIP-chip experiment for uPA, the location of putative FOXO3a core binding [(A/G)(C/T)AAA(C/T)A] sites (labeled A–D), the three uPA primers used in the ChIP assay, and the region of the promoter used in a luciferase assay (Fig. 6). For the ChIP-chip data, the vertical lines represent a score calculated for a window around each probe by the MAT software. Ethanol and Dex-treated samples are shown after normalization with their respective input samples. *, Represents a region (solid horizontal black bar) that shows a significant ChIP-chip binding region as determined using MAT (P < 10−3; uPA, 2.74 × 10−4). B, Immunoprecipitation (IP)/Western blot showing anti-FOXO3a and control IgG immunoprecipitates from MCF10A-Myc cells starved for 72 h and treated with Dex (10−6 m) or vehicle [ethanol (Et)] for 24 h. C, PCR products from input DNA, chromatin immunoprecipitated anti-FOXO3a, and control IgG using TRAIL control primers and the three pairs of uPA primers. The experiment was performed three independent times with similar results.
After 72-h starvation, MCF10A-Myc cells were treated with Dex (10−6 m) or vehicle for 24 h. Whole cell lysates were then collected, and a conventional ChIP assay was performed. In a parallel experiment, the FOXO3a immunoprecipitated lysates were run on a Western blot, and the specificity of FOXO3a immunoprecipitation was confirmed (Fig. 3B). Slightly more FOXO3a was immunoprecipitated from the Dex-treated cells, consistent with previous observations showing a slight increase in steady-state FOXO3a protein levels after Dex treatment (9).
As a positive control, we used PCR primers for a region of the TRAIL promoter previously shown to be bound by FOXO3a in the absence of Dex (26). For this primer pair, we confirmed FOXO3a promoter occupancy in vehicle but not Dex-treated immunoprecipitates (Fig. 3C). In addition, uPA primer 1 did not amplify the far upstream uPA region, suggesting that this region is not a FOXO3a target. uPA primers 2 and 3 did exhibit vehicle-specific FOXO3a promoter occupancy. Together, the ChIP and ChIP-chip experiments suggest a region (−1262/−770 nt) of the uPA promoter that is occupied by FOXO3a in MCF10A-Myc cells.
Constitutively active FOXO3a increases uPA mRNA and protein steady-state levels and inhibits Dex-mediated uPA repression
Having identified putative FOXO3a binding sites in the uPA promoter, we then asked whether or not FOXO3a plays a functional role in uPA expression. MCF10A-Myc and MDA-MB-231 cells were stably transfected with either vector alone (pLPCX), pLPCX-WT-FOXO3a, or a pLPCX-constitutively active FOXO3a (TM-FOXO3a), in which three FOXO3a phosphorylation sites (Thr-32, Ser-253, and Ser-315) were mutated to alanines, thereby preventing phosphorylation and nuclear exclusion (27). C-28/I2 cells were transiently transfected with the same vectors.
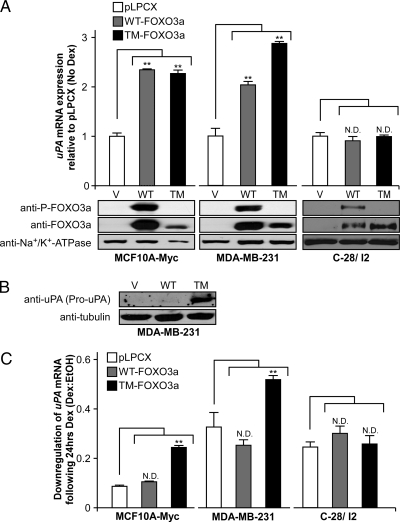
In all three transfected cell lines, significant levels of ectopic FOXO3a proteins were observed (Fig. 4A). As expected, there was significant FOXO3a phosphorylation in the cells expressing WT-FOXO3a, but not in the cells expressing TM-FOXO3a. We found that ectopic expression of either WT- or TM-FOXO3a significantly increased the steady-state levels of uPA mRNA in MCF10A-Myc and MDA-MB-231, but somewhat surprisingly, not in C-28/I2 cells (Fig. 4A). This suggests that FOXO3a increases uPA expression in a cell type-dependent fashion.
Figure 4.
uPA mRNA and protein levels after ectopic FOXO3a expression. MCF10A-Myc and MDA-MB-231 cells were stably transfected with either pLPCX, pLPCX-WT-FOXO3a, or constitutively active pLPCX-TM-FOXO3a and C28/I2 cells were transiently transfected with the same vectors. A, MCF10A-Myc, MDA-MB-231, and C-28/I2 uPA mRNA expression without Dex treatment evaluated by quantitative real-time RT-PCR. Data are shown relative to pLPCX alone after normalization to GAPDH and reported as a mean ± se of triplicate experiments. Lysates were also evaluated for FOXO3a and phospho-FOXO3a expression by Western blot. B, Lysates from MDA-MB-231 cells in the absence of Dex treatment were evaluated by Western blot to determine pro-uPA protein levels. C, MCF10A-Myc, MDA-MB-231, and C-28/I2 uPA mRNA expression with 24 h Dex (10−6 m) or vehicle (ethanol) treatment determined by quantitative real-time RT-PCR. The fold change of gene expression is shown relative to vehicle after normalization to GAPDH and reported as a mean ± se of triplicate experiments. **, P < 0.05 for FOXO3a constructs compared with control pLPCX using a two-tailed unpaired Student’s t test. ND, No significant difference compared with pLPCX; TM, TM-FOXO3a; V, pLPCX vector; WT, WT-FOXO3a.
uPA mRNA is transcribed into a minimally active pro-uPA form, which is secreted and then cleaved into active uPA outside of the cell (28). To determine whether or not FOXO3a regulation of uPA mRNA leads to increased uPA protein expression in the MDA-MB-231 cells, we examined uPA steady-state protein levels by Western analysis. Significantly more pro-uPA protein (55 kD) was identified in the cells expressing TM-FOXO3a (Fig. 4B). Interestingly, in comparison, WT-FOXO3a expression did not significantly increase uPA protein, perhaps because the level of steady-state mRNA did not increase enough in MDA-MB-231 cells to show a difference in protein expression.
We next examined whether or not WT-FOXO3a or TM-FOXO3a expression could reverse Dex-mediated down-regulation of uPA mRNA in these cell lines. We hypothesized that unlike the breast cell lines, constitutively active TM-FOXO3a would not affect GC-mediated uPA down-regulation in the C-28/I2 cells because TM-FOXO3a did not induce uPA expression in this cell type. Indeed, we found that the constitutively active TM-FOXO3a reversed the repression by GCs only in MCF10A-Myc and MDA-MB-231 cells (Fig. 4C). Furthermore, GCs likely inactivated ectopic WT-FOXO3a activity through induction of SGK-1, whereas TM-FOXO3a remained active. In summary, these results suggest that FOXO3a induces uPA expression in breast epithelial and cancer cells, and that FOXO3a inactivation contributes to GC-mediated down-regulation of uPA mRNA and protein steady-state levels.
After GR activation, SGK-1 expression contributes to uPA down-regulation
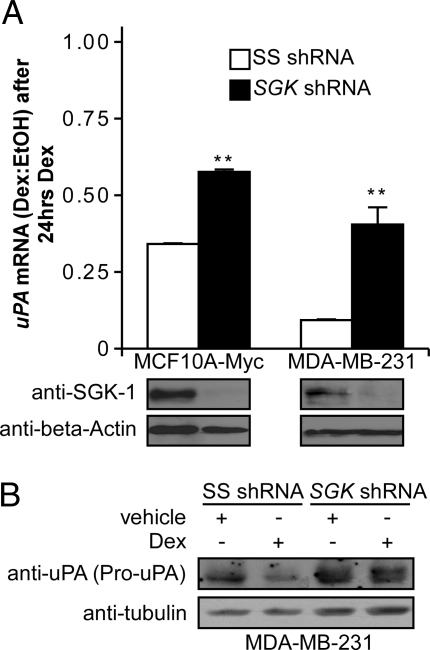
SGK-1 and Akt-1 can coordinately mediate FOXO3a phosphorylation and inhibit FOXO3a activity by causing its nuclear exclusion (11). After Dex treatment we have previously observed phosphorylation and nuclear exclusion of FOXO3a that appears to be dependent on SGK-1 induction alone (9). To test the hypothesis that GC-mediated down-regulation of uPA mRNA requires SGK-1, we examined whether or not inhibiting SGK-1 expression affects uPA down-regulation. MCF10A-Myc and MDA-MB-231 cells were stably transfected with either SS shRNA or SGK shRNA. Western analysis demonstrated that SGK-1 was efficiently decreased in both cell lines (Fig. 5A).
Figure 5.
Effect of SGK-1 knockdown on uPA mRNA and protein levels. A, Lysates from MCF10A-Myc and MDA-MB-231 cells stably transfected with a vector encoding SGK shRNA or SS shRNA were evaluated by Western blot. Cell lines were then treated with Dex (10−6 m) or vehicle (ethanol) for 24 h, and mRNA levels were determined by quantitative real-time RT-PCR. The fold change of gene expression is shown relative to vehicle after normalization to GAPDH, and data are reported as a mean ± se of triplicate experiments. **, P < 0.05 for SGK shRNA values compared with control SS shRNA using a two-tailed unpaired Student’s t test. B, Lysates from the MDA-MB-231 stably transfected cells evaluated for pro-uPA protein expression by Western blot after vehicle or Dex treatment.
We then used quantitative real-time RT-PCR to detect uPA mRNA levels after 24 h Dex or vehicle treatment. Consistent with the hypothesis that SGK-1 is required for Dex-mediated uPA down-regulation in breast epithelial cells, both cell lines expressing SGK shRNA exhibited significantly higher (∼2- to 3-fold) uPA mRNA (Fig. 5A). We did not see a full reversal of uPA mRNA down-regulation by Dex, most likely because additional transcription factors contribute to baseline uPA expression in these cells. However, the shRNA results suggest that SGK-1 induction is required for full mediation of uPA down-regulation after GR activation in breast epithelial and cancer cells.
We also examined uPA protein expression in the MDA-MB-231 cells by Western blot. In agreement with our mRNA results, intracellular pro-uPA protein was significantly decreased by Dex in control shRNA cells, but not in SGK shRNA cells (Fig. 5B). The near full reversal of the Dex-mediated down-regulation of uPA protein, in comparison to the partial reversal of uPA mRNA levels (Fig. 5A), suggests that SGK-1 may also have posttranscriptional effects on uPA expression.
FOXO3a can activate the uPA promoter-luciferase construct through a conserved FOXO3a binding sequence
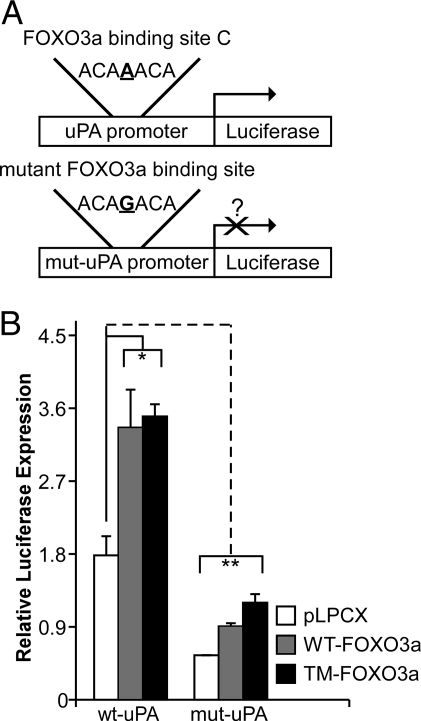
All of the four identified putative FOXO3a sites in the 2000-bp region upstream of the uPA TSS were in the region identified by ChIP-chip (Fig. 3A) and verified by conventional ChIP (Fig. 3C). We further studied the central site (Fig. 3A, site “C”) to determine whether or not FOXO3a binds to and activates the uPA promoter. Luciferase constructs containing a 1270-bp (−1252/+18, Fig. 3A) region of the uPA promoter were generated either containing the WT or a single nucleotide mutation in the core sequence (Fig. 6A). The WT or mutant construct was then cotransfected with a pCMV-β-galactosidase reporter into SK-BR-3 breast cancer cells along with either a pLPCX control, WT-FOXO3a, or TM-FOXO3a vector.
Figure 6.
FOXO3a regulation of uPA promoter activity. A, WT and mutant (mut) uPA promoter-luciferase constructs. B, Relative luciferase activity after expression of either control pLPCX (white bars), pLPCX-WT-FOXO3a (gray bars), or pLPCX-TM-FOXO3a constructs (black bars). Luciferase data are reported as a mean ± se of triplicate experiments. Asterisk (*) signifies that WT-FOXO3a and TM-FOXO3a increase WT-uPA promoter activity significantly (P < 0.05, using a two-tailed unpaired Student’s t test). Double asterisk (**) signifies that the mutant-uPA promoter has significantly less activity in the context of either the empty pLPCX, WT-FOXO3a, and TM-FOXO3a encoding vectors than the WT promoter (P < 0.05, using a two-tailed unpaired Student’s t test).
Figure 6B shows that WT-uPA promoter activity increased 2-fold after ectopic WT-FOXO3a or TM-FOXO3a expression. The mutated (mut-uPA) FOXO3a binding site promoter construct exhibited significantly less activity, suggesting that the WT sequence at binding site “C” is critical for regulating uPA promoter activity. Furthermore, ectopic expression of WT-FOXO3a or TM-FOXO3a was not able to restore the mutant promoter activity back to baseline. This is consistent with our ChIP data showing occupancy of the uPA promoter by FOXO3a. The ability of WT-FOXO3a and TM-FOXO3a to slightly increase activity despite the mutant binding site suggests that additional FOXO3a sites may participate in regulating uPA expression.
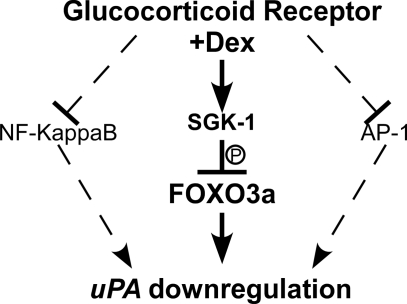
Overall, these data support the hypothesis that SGK-1-mediated phosphorylation and inactivation of FOXO3a are important mechanisms contributing to decreased uPA expression after GR activation in breast epithelial cells. Interestingly, GCs appear to use a common mechanism, i.e. transcription factor inhibition (AP-1, NF-κB, and FOXO3a), to down-regulate genes in a cell type-specific manner (Fig. 7).
Figure 7.
Regulation of uPA expression in breast epithelial and cancer cells. Cartoon depicting pathways implicated in the down-regulation of uPA by GCs, including the SGK-1/FOXO3a pathway reported in this paper.
Discussion
In this report we identify a novel mechanism contributing to GC-mediated down-regulation of uPA expression in breast epithelial and cancer cell lines. We demonstrate that GR activation leads to down-regulation of uPA mRNA levels that is mediated in part by SGK-1 and in turn by FOXO3a inactivation in breast epithelial cells. Furthermore, we show that active FOXO3a increases uPA transcription through binding to the proximal uPA promoter in these cells.
uPA expression also appears to correlate with the clinical severity of arthritis (29). GCs are used to alleviate arthritic symptoms and also down-regulate uPA in synovial cells (30), chondrocytes (25), and mononuclear cells (31), potentially accounting for their effectiveness in treatment. Because of this we examined the mechanism of uPA regulation by GCs in a human chondrocyte cell line. Interestingly, our data suggest that unlike breast cancer cells, uPA does not appear to be a FOXO3a target in chondrocytes. This is consistent with evidence suggesting that FOXO activity at target promoters is cell type dependent (32).
In cancer therapy, SGK-1 (33) and uPA (34) are considered to be potential therapeutic targets. Our data suggest that inhibiting SGK-1 might actually result in higher uPA levels, which could promote cancer cell invasion under certain conditions. Increased uPA production and concomitant cellular invasion might also be predicted upon inhibiting Akt-1, another phosphatidylinositol 3-kinase pathway member that phosphorylates and inactivates FOXO3a (35). In fact, consistent with the hypothesis that Akt-1 activation could result in down-regulated uPA expression via FOXO3a inactivation, Akt-1 activity inhibits tumor cell invasion in an in vitro assay (36). Akt-1’s inhibitory effects on tumor cell invasion have been suggested to result from its ability to cause the ubiquitin modification and degradation of nuclear factor of activated T cells (37). In turn, nuclear factor of activated T-cell inactivation results in the down-regulation of the pro-invasion gene cyclooxygenase-2 (38). Our data suggest that the observed inhibition of invasion after Akt-1 expression could also be a result of decreased uPA expression. Furthermore, in an in vivo mouse model, constitutively active Akt-1 expression appears to have opposing effects on mammary gland tumor growth and metastatic progression: Akt-1 accelerates tumorigenesis, while suppressing tumor invasiveness (39).
The difference in mechanism of uPA regulation by GCs in breast epithelial cells vs. chondrocytes suggests that confirming bona fide FOXO3a target genes will require cell type-specific verification. Furthermore, the discovery that FOXO3a contributes to uPA regulation implies that in addition to its well-described role in promoting apoptosis in cancer cells, FOXO3a may also play a role in tumor invasion.
Acknowledgments
We thank Dr. Michael Greenberg (Harvard University, Boston, MA) for the wild-type-forkhead box O3a-hemagglutinin and triple mutant-forkhead box O3a-hemagglutinin plasmids, and Dr. Geoffrey Greene’s laboratory (University of Chicago, Chicago, IL) for advice with luciferase and chromatin immunoprecipitation assays. We also thank Dr. Mary Goldring (Hospital for Special Surgery, Cornell University, New York, NY) and Dr. Hee-Jeong Im Sampen (Rush University, Chicago, IL) for the gift of C-28/I2 cells, and Dr. Dejuan Kong (Wayne State University School of Medicine, Detroit, MI) for help with antiurokinase plasminogen activator Western blots. Finally, we thank the University of Chicago Functional Genomics Core Facility for assistance in performing both quantitative real-time RT-PCR and chromatin immunoprecipitation microarray assays.
Footnotes
This work was supported by National Institutes of Health Grants CA90459 and CA89208 (to S.D.C.), and by the University of Chicago Cancer Research Foundation Auxiliary Board. T.P. was also supported in part by a summer undergraduate research fellowship from The Endocrine Society.
Disclosure Statement: The authors have nothing to disclose.
First Published Online January 31, 2008
Abbreviations: AP-1, Activator protein-1; ChIP, chromatin immunoprecipitation; ChIP-chip, chromatin immunoprecipitation microarray; Dex, dexamethasone; FOXO3a, forkhead box O3a; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GC, glucocorticoid; GR, glucocorticoid receptor; MAT, Model-based Analysis of Tiling-array; mut, mutation; NF-κB, nuclear factor κ-B; SGK, serum and glucocorticoid regulated kinase; shRNA, short hairpin RNA; SS, scrambled sequence; TM, triple mutant; TRAIL, TNF-related apoptosis inducing ligand; TSS, transcriptional start site; uPA, urokinase plasminogen activator; WT, wild type.
References
- Franchimont D 2004 Overview of the actions of glucocorticoids on the immune response: a good model to characterize new pathways of immunosuppression for new treatment strategies. Ann NY Acad Sci 1024:124–137 [DOI] [PubMed] [Google Scholar]
- Distelhorst CW 2002 Recent insights into the mechanism of glucocorticosteroid-induced apoptosis. Cell Death Differ 9:6–19 [DOI] [PubMed] [Google Scholar]
- Greenstein S, Ghias K, Krett NL, Rosen ST 2002 Mechanisms of glucocorticoid-mediated apoptosis in hematological malignancies. Clin Cancer Res 8:1681–1694 [PubMed] [Google Scholar]
- Moran TJ, Gray S, Mikosz CA, Conzen SD 2000 The glucocorticoid receptor mediates a survival signal in human mammary epithelial cells. Cancer Res 60:867–872 [PubMed] [Google Scholar]
- Mikosz CA, Brickley DR, Sharkey MS, Moran TW, Conzen SD 2001 Glucocorticoid receptor-mediated protection from apoptosis is associated with induction of the serine/threonine survival kinase gene, sgk-1. J Biol Chem 276:16649–16654 [DOI] [PubMed] [Google Scholar]
- Runnebaum IB, Bruning A 2005 Glucocorticoids inhibit cell death in ovarian cancer and up-regulate caspase inhibitor cIAP2. Clin Cancer Res 11:6325–6332 [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Tajima K, Sasson R 2002 Cell-specific regulation of apoptosis by glucocorticoids: implication to their anti-inflammatory action. Biochem Pharmacol 64:843–850 [DOI] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D 1998 Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95:14863–14868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Zou M, Brickley DR, Pew T, Conzen SD 2006 Glucocorticoid receptor activation signals through forkhead transcription factor 3a in breast cancer cells. Mol Endocrinol 20:2304–2314 [DOI] [PubMed] [Google Scholar]
- Webster MK, Goya L, Ge Y, Maiyar AC, Firestone GL 1993 Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol Cell Biol 13:2031–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME 2001 Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol Cell Biol 21:952–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dano K, Behrendt N, Hoyer-Hansen G, Johnsen M, Lund LR, Ploug M, Romer J 2005 Plasminogen activation and cancer. Thromb Haemost 93:676–681 [DOI] [PubMed] [Google Scholar]
- Andreasen PA, Kjoller L, Christensen L, Duffy MJ 1997 The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer 72:1–22 [DOI] [PubMed] [Google Scholar]
- Busso N, Belin D, Failly-Crepin C, Vassalli JD 1986 Plasminogen activators and their inhibitors in a human mammary cell line (HBL-100). Modulation by glucocorticoids. J Biol Chem 261:9309–9315 [PubMed] [Google Scholar]
- Busso N, Belin D, Failly-Crepin C, Vassalli JD 1987 Glucocorticoid modulation of plasminogen activators and of one of their inhibitors in the human mammary carcinoma cell line MDA-MB-231. Cancer Res 47:364–370 [PubMed] [Google Scholar]
- Nerlov C, Rorth P, Blasi F, Johnsen M 1991 Essential AP-1 and PEA3 binding elements in the human urokinase enhancer display cell type-specific activity. Oncogene 6:1583–1592 [PubMed] [Google Scholar]
- Beppu M, Ikebe T, Shirasuna K 2002 The inhibitory effects of immunosuppressive factors, dexamethasone and interleukin-4, on NF-κB-mediated protease production by oral cancer. Biochim Biophys Acta 1586:11–22 [DOI] [PubMed] [Google Scholar]
- Kennedy SG, Wagner AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN, Hay N 1997 The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev 11:701–713 [DOI] [PubMed] [Google Scholar]
- Wu W, Pew T, Zou M, Pang D, Conzen SD 2005 Glucocorticoid receptor-induced MAPK phosphatase-1 (MPK-1) expression inhibits paclitaxel-associated MAPK activation and contributes to breast cancer cell survival. J Biol Chem 280:4117–4124 [DOI] [PubMed] [Google Scholar]
- Johnson WE, Li W, Meyer CA, Gottardo R, Carroll JS, Brown M, Liu XS 2006 Model-based analysis of tiling-arrays for ChIP-chip. Proc Natl Acad Sci USA 103:12457–12462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, Li W, Song J, Wei L, Liu XS 2006 CEAS: cis-regulatory element annotation system. Nucleic Acids Res 34:W551–W554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Chaudhuri S, Brickley DR, Pang D, Karrison T, Conzen SD 2004 Microarray analysis reveals glucocorticoid-regulated survival genes that are associated with inhibition of apoptosis in breast epithelial cells. Cancer Res 64:1757–1764 [DOI] [PubMed] [Google Scholar]
- Blais A, Dynlacht BD 2005 Constructing transcriptional regulatory networks. Genes Dev 19:1499–1511 [DOI] [PubMed] [Google Scholar]
- Busso N, Collart M, Vassalli JD, Belin D 1987 Antagonist effect of RU 486 on transcription of glucocorticoid-regulated genes. Exp Cell Res 173:425–430 [DOI] [PubMed] [Google Scholar]
- Augustine AJ, Oleksyszyn J 1997 Glucocorticosteroids inhibit degradation in bovine cartilage explants stimulated with concomitant plasminogen and interleukin-1 α. Inflamm Res 46:60–64 [DOI] [PubMed] [Google Scholar]
- Ghaffari S, Jagani Z, Kitidis C, Lodish HF, Khosravi-Far R 2003 Cytokines and BCR-ABL mediate suppression of TRAIL-induced apoptosis through inhibition of forkhead FOXO3a transcription factor. Proc Natl Acad Sci USA 100:6523–6528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME 1999 Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857–868 [DOI] [PubMed] [Google Scholar]
- Crippa MP 2007 Urokinase-type plasminogen activator. Int J Biochem Cell Biol 39:690–694 [DOI] [PubMed] [Google Scholar]
- Brommer EJ, Dooijewaard G, Dijkmans BA, Breedveld FC 1992 Plasminogen activators in synovial fluid and plasma from patients with arthritis. Ann Rheum Dis 51:965–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitti G, Hamilton JA 1988 Modulation of urokinase-type plasminogen activator messenger RNA levels in human synovial fibroblasts by interleukin-1, retinoic acid, and a glucocorticoid. Arthritis Rheum 31:1046–1051 [DOI] [PubMed] [Google Scholar]
- Hamilton JA, Hart PH, Leizer T, Vitti GF, Campbell IK 1991 Regulation of plasminogen activator activity in arthritic joints. J Rheumatol Suppl 27:106–109 [PubMed] [Google Scholar]
- Chen J, Yusuf I, Andersen HM, Fruman DA 2006 FOXO transcription factors cooperate with δ EF1 to activate growth suppressive genes in B lymphocytes. J Immunol 176:2711–2721 [DOI] [PubMed] [Google Scholar]
- Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, Franklin RA, McCubrey JA 2003 Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia 17:590–603 [DOI] [PubMed] [Google Scholar]
- Pillay V, Dass CR, Choong PF 2007 The urokinase plasminogen activator receptor as a gene therapy target for cancer. Trends Biotechnol 25:33–39 [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME 1999 Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857–868 [DOI] [PubMed] [Google Scholar]
- Toker A, Yoeli-Lerner M 2006 Akt signaling and cancer: surviving but not moving on. Cancer Res 66:3963–3966 [DOI] [PubMed] [Google Scholar]
- Yoeli-Lerner M, Yiu GK, Rabinovitz I, Erhardt P, Jauliac S, Toker A 2005 Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol Cell [Erratum (2006) 22:145] 20:539–550 [DOI] [PubMed] [Google Scholar]
- Yiu GK, Toker A 2006 NFAT induces breast cancer cell invasion by promoting the induction of cyclooxygenase-2. J Biol Chem 281:12210–12217 [DOI] [PubMed] [Google Scholar]
- Hutchinson JN, Jin J, Cardiff RD, Woodgett JR, Muller WJ 2004 Activation of Akt-1 (PKB-α) can accelerate ErbB-2-mediated mammary tumorigenesis but suppresses tumor invasion. Cancer Res 64:3171–3178 [DOI] [PubMed] [Google Scholar]
- Irizarray RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP 2003 Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249–264 [DOI] [PubMed] [Google Scholar]