Abstract
Activin is a pleiotropic growth factor with a broad pattern of tissue distribution that includes reproductive tissues. Although direct actions of activin have been described in gonadal and uterine tissues, actions in the myometrium have not been defined. In this study we have characterized the responsiveness of uterine tissue and myometrial cell lines to activin-A. Uterine tissue and two myometrial cell lines, PHM1 (pregnant human myometrial 1) and hTERT HM (telomerase reverse transcriptase-infected human myometrial) respond to activin-A as measured by phosphorylation of Smad-2. Those cell lines express a full complement of activin receptors, as well as activin βA subunit and follistatin. Activin inhibited proliferation of PHM1 and human telomerase reverse transcriptase-infected human myometrial cell line cells, with more extensive growth inhibition observed in PHM1s. In PHM1s, activin-A decreased oxytocin receptor and HoxA-10 mRNA expression but did not alter total progesterone receptor, cyclooxygenase-2 (Cox-2), and connexin 43 mRNA expression levels. Furthermore, treatment of PHM1 myometrial cells with activin-A attenuated oxytocin and thromboxaneA2 induced intracellular Ca2+ accumulation. In conclusion, myometrial cells are activin sensitive, and activin-A can regulate myometrial cell functions.
ACTIVIN AND INHIBIN are growth factors with important hormonal roles in both male and female reproductive tissues. Activin belongs to the TGF-β superfamily of growth factors, which also includes bone morphogenetic proteins, growth-differentiation factors, and myostatin. Activin-A is a dimer of two activin βA subunits. Activin binds to cell-surface receptor complexes containing two distinct classes of receptor serine kinases called type I and type II. Activin first binds to type II receptors, either ActRIIA or ActRIIB, and only binds type I receptors (ALK4) once bound to type II receptors. Within the receptor complex, type II receptors phosphorylate, and thereby activate, ALK4, which in turn phosphorylates intracellular substrates such as the Smad proteins. Activin induces ALK4 dependent phosphorylation of Smad 2 and Smad 3, which mediate transcriptional activation of activin target genes. Activin signaling is also regulated by several membrane (cripto) and extracellular factors, including the receptor antagonist inhibin and the activin binding protein follistatin (1).
Activin has actions in uterine tissues, both in cycling females and during pregnancy. Activin-A is expressed in glandular and surface endometrial epithelium throughout the menstrual cycle with maximal immunostaining detected in late secretory endometria. Activin-A is expressed in stromal cells only in the late secretory phase (2). Suggested actions of exogenous activin in the endometrial layer of the uterus include regulation of cell proliferation (3) and induction of stromal decidualization during the secretory phase in preparation for invasion by trophoblast if pregnancy occurs (4). During pregnancy, activin-A is strongly expressed in decidualized endometrium (5), placenta (6), and fetal maternal intrauterine membranes (7,8). During pregnancy, activin-A has been suggested to regulate trophoblast growth and differentiation (9), as well as embryo implantation (10).
In contrast to the well-established actions of activin in the endometrium, activin expression and activin actions in the myometrium are less clear. An initial study by Schneider-Kolsky et al. (11) demonstrated that high levels of activin βA subunit and all three of the activin receptor subtypes were detected in vascular endothelial cells within the human myometrium but found expression was low or absent in myometrial smooth muscle cells. However, a subsequent report found activin receptor expression in myometrial cells of neonatal ovine uterus (12), as well as a moderate immunostaining for activin βA subunit and low immunostaining for activin βB subunit. Another study reported that, during pregnancy, rat myometrial cells were capable of binding activin-A, suggesting that activin may have actions in myometrium during gestation (13).
The growth of uterine cells during pregnancy represents one of the most remarkable events in reproduction (14), with massive increases in both the size and number of myometrial smooth muscle cells in particular. Myometrial cell growth goes through a series of distinct phases. During early pregnancy myometrial cells divide in a proliferative phenotype, whereas in late pregnancy myometrial cells cease dividing, and increase in size and weight through hypertrophy and remodeling of the extracellular matrix. During both of these phases, myometrial quiescence and contractile unresponsiveness to stimuli such as oxytocin are essential. However, at term a switch from myometrial quiescence to myometrial activation is necessary to enable the synchronous, high-amplitude, high-frequency contractions of labor.
Circulating activin levels are highest during late-term pregnancy, which has led to the suggestion that activin may have a role in the initiation of myometrial contractility at term. In fact, several articles have studied maternal serum activin-A levels at parturition, but controversy exists. Although some studies found higher activin-A levels in preterm and term labor compared with gestational age-matched controls not in labor (8,15,16,17,18,19), other papers reported no difference in circulating activin-A levels between patients in labor and not in labor, matched for gestational age (20,21). However, many of activin’s roles are autocrine or paracrine, and it is possible that activin from local sources such as myometrium or endometrium rather than from the general circulation might gain access to the myometrium. None of these studies has directly examined possible effects of activin on myometrial contraction, nor have studies examined activin regulation of myometrial cell growth. Because it has been reported that TGF-βs affect myometrial cell proliferation (22), a contribution of activin is also suggested. Therefore, we have examined the effect of activin-A on myometrial cell proliferation and functionality, such as contractility.
Materials and Methods
Materials
NuPAGE gels, molecular weight standards, and CyQuant cell proliferation assay kits were purchased from Invitrogen (Carlsbad, CA). Anti-phospho-Smad 2 antibody was purchased from Cell Signaling Technology, Inc. (Danvers, MA). Oxytocin and laboratory chemicals were obtained from Sigma-Aldrich (St. Louis, MO). Rabbit polyclonal Smad 2/3 antibodies were raised against a peptide conserved between Smad 2 and Smad 3 spanning amino acids 159–175 of human Smad 3 and were produced by Joan Vaughan (Peptide Biology Laboratories, The Salk Institute). Activin βA subunit antiserum was generated toward the N terminus of the porcine activin βA subunit using the synthetic fragment 81–113 and was produced by Joan Vaughan. Recombinant human activin-A was generated using a stable activin-expressing cell line generously provided by Dr. J. Mather (Genentech, Inc., South San Francisco, CA) and was purified by Wolfgang Fischer (Peptide Biology Laboratory, The Salk Institute). Follistatin-288 was generously provided by Dr. Teresa Woodruff (Northwestern University, Chicago, IL). The pregnant human myometrial 1 cell line (PHM1) (23) was generously provided by Dr. Barbara Sanborn (Colorado State University, Fort Collins, CO), and human telomerase reverse transcriptase (hTERT)-infected human myometrial cell line (hTERT HM) (24) by Dr. William Rainey (University of Texas Southwestern Medical Center, Dallas, TX).
Tissue culture
The PHM1 and hTERT HM were grown on tissue culture-treated plastic (Corning, Corning, NY) in 5% CO2 in a 37 C humidified incubator. PHM1s were cultured in high-glucose DMEM (The Salk Institute) supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin glutamine, and 0.1 mg/ml geneticin. hTERT HMs were cultured in DMEM/F12 medium with HEPES supplemented with 10% FBS and 1% penicillin/streptomycin glutamine.
Protein extraction and immunoblotting
PHM1s and hTERT HMs were treated with activin-A and/or follistatin as indicated for 30 min or left untreated in subconfluent 10-cm plates. Cells were rinsed in ice-cold HEPES dissociation buffer (HDB) [25 mm HEPES (pH 7.4), 137 mm NaCl and 5 mm KCl, 0.7 mm Na2HPO4] and solubilized in 150 μl ice-cold radioimmunoprecipitation assay (RIPA) buffer [50 mm Tris-HCl (pH 7.4), 150 mm NaCl, 1 mm EDTA, 1% Igepal CA-630, 0.25% Na-deoxycholate] supplemented with protease inhibitors (set III; Calbiochem, San Diego, CA) and phosphatase inhibitors (set II; Calbiochem). Samples were rocked at 4 C for 30 min, and insoluble cellular materials were removed by centrifugation. Rat uteri were rapidly excised, cut into fragments, washed in hexadimethrine bromide, and treated with or without 10 nm activin-A for 45 min in 200 μl DMEM supplemented with 10% FBS, 1% penicillin/streptomycin glutamine. Uteri fragments were flash frozen on dry ice, stored at −20 C, until crushed while frozen, and suspended in 200 μl ice-cold radioimmunoprecipitation assay buffer supplemented with protease and phosphatase inhibitors. Samples were rocked at 4 C for 30 min, and insoluble cellular materials were removed by centrifugation.
Soluble protein was quantified using a Bradford protein assay (Bio-Rad, Richmond, CA), and equal amounts of proteins were loaded onto 10% NuPAGE gels and resolved by SDS-PAGE under reducing conditions. Proteins were transferred to 0.2-μm nitrocellulose membranes in an X-cell II apparatus (Invitrogen) according to the manufacturer’s instructions. After blocking of the membrane with 5% (wt/vol) nonfat milk powder in Tris-buffered saline with Tween 20 (TBST) [50 mm Tris-HCl (pH 7.4), 150 mm NaCl, 0.05% Tween 20], membranes were incubated overnight with 1:1000 dilutions of primary antibodies against activin-βA, phosphorylated-Smad 2, or Smad 2/3 as noted. Membranes were washed four times in 5% (wt/vol) nonfat milk powder in TBST and incubated with 1:10,000 dilutions of horseradish peroxidase-conjugated antirabbit IgG (Pierce, Rockford, IL) for 2 h. Blots were washed three times in 5% (wt/vol) nonfat milk powder in TBST, and immunoreactive proteins were visualized using Super Signal West Pico chemiluminescent substrate (Pierce).
Animals
Female Sprague Dawley rats (200–220 g), were used in the experiments described in this study. Animals were maintained on a 12-h light, 12-h dark cycle (lights on at 0600 h), and provided rat chow and water ad libitum. The Salk Institute animal use and care committee approved all procedures described in this study. Animals were killed by decapitation.
RNA extraction, PCR, and quantitative real-time PCR
Total RNA was extracted from PHM1s using an RNeasy Mini Kit (QIAGEN, Inc., Valencia, CA), with DNase treatment on-column using a RNase-free DNase Kit (QIAGEN) according to the manufacturer’s instructions. RT reactions were performed on 1 μg total RNA using SuperScript II (Invitrogen). For PCR to detect expression, control reactions were performed omitting either the RT enzyme or template RNA to test for contamination with genomic DNA or nonspecific amplification. A total of 35 cycles was performed of 1 min at 94 C, 1 min at 55 C, and 1 min at 72 C, and amplification products were visualized on 2% agarose gel and stained with ethidium bromide. For real-time PCR, primer sets were either previously described (25,26,27) or designed using Primer Express software from PE Applied Biosystems (Foster City, CA). For newly designed primer pairs, BLAST searches were performed to exclude homology to other human genes. Real-time PCR assays were validated for each primer set by confirming that single amplicons of predicted size and sequence were generated. Real-time PCR reaction mixtures used Universal Master Mix with SYBR Green (Sigma-Aldrich), 80 nm final concentration of each primer, and 2 ng cDNA in a final reaction volume of 25 μl. Reactions were performed in 96-well plates on an ABI PRISM 7700 with sequence detection system software (Applied Biosystems) with initial conditions of 50 C for 2 min, 95 C for 10 min, followed by 40 cycles of 95 C for 15 sec, 60 C for 1 min. For each biological sample, amplification reactions were performed in triplicate, and each sample was normalized to triplicate amplification of an 18S rRNA control. The primers used are listed in Table 1.
Table 1.
PCR primers
| Gene | Primer forward | Primer reverse | PCR fragment length (bp) | Reference |
|---|---|---|---|---|
| Activin-βA | catcacgtttgccgagtcag | gactgctccttttcctcatc | 495 | 56 |
| Follistatin | tgccacctgagaaaggctac | acagacaggctcatccgact | 201 | 57 |
| ALK4 | cgctccaggatctcgtctac | aaccaagaccgttcttcacg | 202 | |
| ActRII | gatcttcccactccaggaca | tgtgatgatgttccccttga | 199 | |
| Oxytocin receptor | agaagcactcgcgcctctt | aggtgatgtcccacagcaact | 102 | 25 |
| HoxA-10 | caagaggcaaagaaaagcgttaa | gtagatgcttgcagaaggaaagg | 52 | 25 |
| Progesterone receptor A-B | agcccacaatacagcttcgag | tttcgacctccaaggaccat | 254 | 25 |
| Cox-2 | gaatcattcaccaggcaaattg | tctgtactgcgggtggaaca | 66 | 25 |
| Connexin 43 | tttcaatggctgctcctc | tgctcacttgcttgcttgtt | 123 | 26 |
| 18S | cggctaccacatccaaggaa | gctggaattaccgcggct | 187 |
Cell proliferation assays and apoptotic frequency determination
Cellular growth curves were measured using the CyQuant cell proliferation assay kit according to the manufacturer’s instructions. PHM1s were seeded in 96-well plates either at various initial densities (see Fig. 3A), or at densities of 103 cells per well in a total volume of 100 μl DMEM supplemented with 10% FBS and doses of activin-A as noted. Cells were grown for the indicated number of days, with media replaced every 3 d. These conditions result in the linear range of the assay based on control experiments. At the indicated times, media were discarded, and plates were frozen. To assay, cells were lysed, and total cellular nucleic acid was measured using florescence at 520-nm emission after excitation at 480 nm. For direct counts of PHM1 numbers, cells were seeded in six-well plates in 2 ml DMEM supplemented with 10% FBS and 10 nm activin-A. After 20-d growth, wells were trypsinized, and cells were counted both manually by hemocytometer with Trypan blue staining to identify live cells, and cells were also mechanically counted in a Coulter counter (Multisizer 3; Beckman Coulter, Inc., Fullerton, CA).
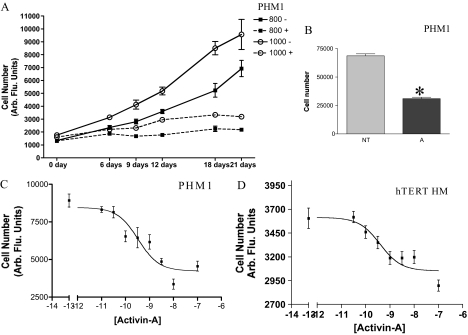
Figure 3.
Effect of activin-A on PHM1 myometrial cell proliferation. A, Changes in PHM1 number in cells seeded in a 96-well plate at a density of 800 and 1000 cells at different days with (+) or without (−) activin-A treatment. B, Direct count of PHM1 number after 21 d with (A) or without (NT) 10 nm activin-A treatment. C, Dose response of activin-A effect on PHM1 proliferation on cells seeded in a 96-well plate at a density of 1000. D, Dose response of activin-A on hTERT cell proliferation on cells seeded in a 96-well plate at a density of 1000 cells for each well. In A–D the cell proliferation is measured using the CyQuant cell proliferation assay kit. Arb Flu., Arbitrary fluorescence. *, P < 0.05.
To measure apoptotic frequency, cells were trypsinized, pelleted, and resuspended in DMEM, and then an equal volume of ice-cold fixative (3:1 ratio of 95% ethyl alcohol to glacial acetic acid) was added; cells were mounted on slides and stained with 4′,6-diamidino-2-phenylindole (DAPI). Apoptotic cells with condensed chromatin were easily distinguished; their number relative to undamaged cells was counted in 20 separate fields containing a minimum of 10 cells per field.
Caspase 3 activity was measured using the Caspase 3 colorimetric assay kit from Calbiochem. Briefly, equal amounts of proteins from lysates of adherent and nonadherent cells were assayed colorimetrically for caspase-3-like protease activities by measuring the absorbance of free p-Nitroanile (pNA) generated by cleavage of Ac-DEVD-pNA as a colorimetric substrate following the manufacturer’s instructions. Cellular extracts were incubated for 10 min at 37 C with or without 0.1 μm Caspase-3 Inhibitor I and analyzed using a spectrophotometer (ELISA microplate reader; Bio-Rad). Data were recorded at 45-min intervals for 225 min. Recombinant human caspase-3 protein was used as a positive control.
To quantitate cell size and volume changes in activin-treated cells, morphometric analyses were performed. Fields were randomly selected, and the area (μm2) occupied by the cells was measured using a morphological imaging system (LUCIA, version 4.5; Nikon Instruments, Tokyo, Japan). To measure cell volume, cells were trypsinized and fixed with 0.2% glutaraldehyde/0.005% Tween 20, and cell volume was individually determined using a Coulter Counter (Multisizer 3).
Measurement of free intracellular calcium
To induce cellular effects, PHM1s were treated with or without 10 nm activin-A in 10 ml DMEM supplemented with 10% FBS in 10-cm plates for the indicated times. Twenty-four hours before calcium measurement, control and activin-treated cells were trypsinized and seeded in poly-d-lysine coated 96-well black clear bottom plates (Thomson Instrument Co., San Diego, CA) in 100 μl volume at a density of 2 × 104 cells per well. The concentration of FBS and activin-A was maintained at this time. To quantify intracellular concentration variation in response to oxytocin and thromboxaneA2 (TXA2), the calcium 3 assay kit was used, and the fluorescence was measured using a FlexStation (Molecular Devices, Sunnyvale, CA). Briefly, media were aspirated from the cells, and 200 μl DMEM supplemented with 0.1% FBS and calcium 3 reagent, with or without 10 nm activin-A, was added to each well and allowed to equilibrate with the cells for 60 min at 37 C in a 5% CO2 humidified incubator. The concentration of activin-A was kept constantly at 10 nm in all the steps, whereas the serum concentration was reduced to 0.1% FBS according to the manufacturer’s instruction. A 96-well reservoir plate with a 5× concentration of oxytocin (500 nm, 1.5 μm, and 4.5 μm) or TXA2 (1.5 μm) was prepared, and 50 μl stock oxytocin solution was automatically dispensed to the assay well by the instrument during florescence measurement. Samples were excited at 485 nm, and emission at 525 nm was measured every 2 sec throughout a 120-sec measurement window. Data were analyzed using SoftmaxPro (Molecular Devices). To ensure equal cell numbers between treatments, the plate was successively assayed for cell number using the CyQuant cell proliferation assay.
Data analysis
Data are presented as the mean ± sem, and a two-tailed t test was used for data analysis. Differences were considered significant when P < 0.05. All of the experiments were done in triplicate, except for the cell proliferation assay in which n = 6, and experiments were repeated either two or three times.
Results
Activin-A responsiveness of uterine tissues and myometrial cell lines, and expression of activin pathway components and regulators in myometrial cell lines
To determine whether the myometrium is activin responsive, we first tested if rat uterus explants exhibit detectable Smad signaling on activin-A treatment. Intact tissue explants from rat uterus, which contained both endometrium and myometrium, were treated with carrier or 10 nm activin-A. As shown in Fig. 1A, rat uterus explants expressed both Smad 2 and Smad 3, and activin-A treatment did not alter Smad levels (lower panel). Basal Smad 2 phosphorylation was low to undetectable, whereas activin-A treatment induced Smad 2 phosphorylation (Fig. 1A, upper panel), demonstrating that these explants did respond to activin-A.
Figure 1.
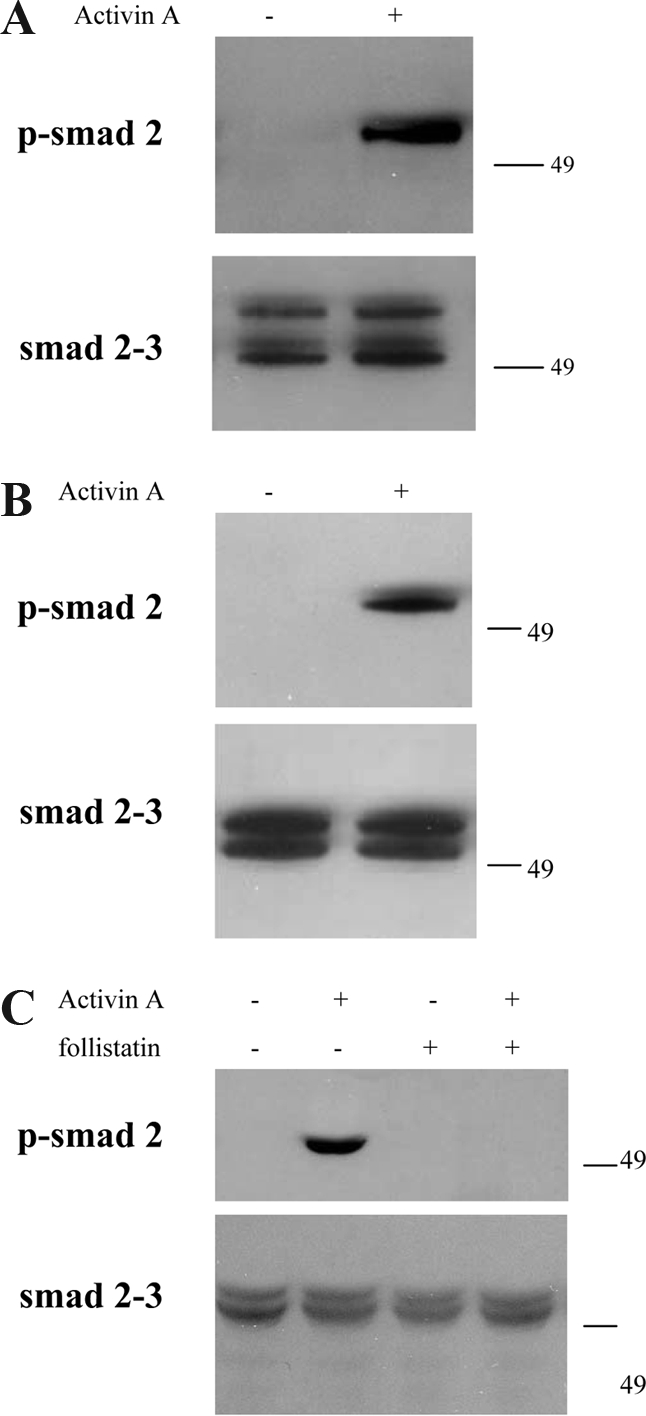
Myometrial responsiveness to activin-A. Activin-A induces phosphorylation of Smad 2 in rat uterus explants (A), and in two different myometrial cell lines, hTERT HM (B) and PHM1 (C). The levels of Smad 2 and 3, showed in the lower panels, instead resulted unchanged. In C the complete abrogation of the signal by follistatin cotreatment is shown.
Because these explants contained cells from both the endometrium and myometrium, this finding did not strictly confirm that myometrial cells respond to activin-A. Therefore, we also examined activin-A induced Smad phosphorylation in the myometrial-derived cell lines PHM1 and hTERT HM. PHM1s were derived from primary pregnant human myometrial cells infected with an adenovirus vector expressing the E6/E7 proteins of human papilloma virus 16 (23); hTERT HMs were derived from nonpregnant human myometrial cells engineered to express hTERT (24). hTERT HMs exhibited strongly induced phosphorylation of Smad 2 in response to activin-A (Fig. 1B, upper panel), with similar levels of total Smad 2 or Smad 3 (Fig. 1B, lower panel). Similarly, levels of total Smad 2 and Smad 3 were unchanged by activin treatment in PHM1s, whereas Smad 2 phosphorylation was strongly induced (Fig. 1C). Treatment of PHM1s with 30 nm follistatin did not induce Smad 2 phosphorylation, or alter Smad 2 or Smad 3 levels, but follistatin did block activin effects on Smad 2 phosphorylation (Fig. 1).
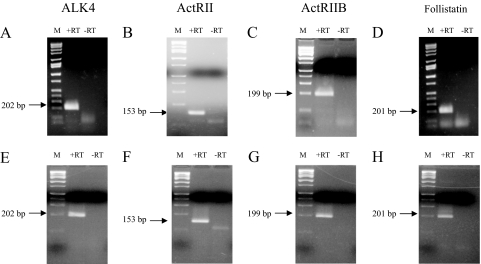
To define better the receptor and binding protein expression underlying this activin responsiveness, we examined expression of the known activin type I and type II receptors, and follistatin in PHM1s and hTERT HMs. Expression of the activin type I receptor ALK4 (Fig. 2, A and E), the activin type II receptors ActRII (Fig. 2, B and F) and ActRIIB (Fig. 2, C and G), and follistatin mRNAs (Fig. 2, D and H) were detected by RT-PCR. Both PHM1s (Fig. 2, A–C) and hTERT HMs (Fig. 2, E–G) expressed ALK4 and both type II receptor isoforms, implying that these cells contain the full complement of activin receptors. Follistatin mRNA expression was also detected in both PHM1 (Fig. 2D) and hTERT HMs (Fig. 2H).
Figure 2.
Expression of activin pathway components in PHM1 myometrial cells. Electrophoretic gel of the RT-PCR in PHM1s for the activin receptors ALK4 (A), ActRII (B), ActRIIB (C), and the activin binding protein follistatin (D), and in hTERT HM for the activin receptors ALK4 (E), ActRII (F), ActRIIB (G), and follistatin (H). −RT consists of amplifications performed in absences of reverse transcriptase enzyme. The molecular DNA mass marker (M) is Hi-Lo ladder (Minnesota Molecular, Minneapolis, MN).
Activin-A inhibits myometrial cell growth
Myometrial smooth muscle cells proliferate during each estrous cycle (28) and dramatically expand in number during the early stage of pregnancy (29). Therefore, we investigated whether activin-A could regulate myometrial cell growth using CyQuant assays, which effectively measured PHM1 and hTERT HM number (Fig. 3A; data not shown). PHM1s were plated in 96-well plates in standard growth media (containing 10% FBS) at various initial seeding densities from 100–1000 cells per well and treated with activin-A as shown. Activin-A treated cells (dotted lines) had decreased cell numbers compared with untreated wells, demonstrating that activin-A could inhibit PHM1 growth. Control experiments confirmed that our fluorescent cell density assay measured PHM1 numbers linearly within this cell density range (data not shown). Similar experiments in hTERT HMs also found activin-A inhibited proliferation, although the magnitude of growth inhibition was less than in PHM1s (data not shown). To ensure that we were actually measuring effects on cell number, we also directly counted cells. PHM1s were treated with or without 10 nm activin-A for 21 d, then wells were trypsinized, and living cells were counted manually by hemocytometer. Activin-A inhibited PHM1 growth in this assay (Fig. 3B). The magnitude of growth inhibition observed by cell counting was similar to that observed in CyQuant assays (compare Fig. 2B with density of 1000 cells per well after 21-d treatment in Fig. 3A).
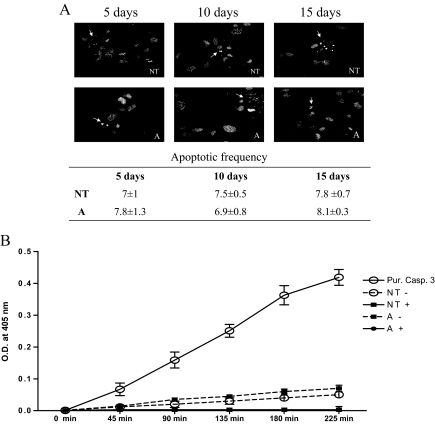
To exclude that the observed decrease in cell number was due to increased apoptosis, we determined the percentage of apoptotic PHM1s after 5, 10, and 15-d 10 nm activin treatment. As shown in Fig. 4A, activin-A treatment did not alter the rate of apoptosis as measured by DAPI staining of condensed chromatin in apoptotic cells. The low rate of apoptosis in activin-treated cells was also accessed by measurement of caspase-3 activity. PHM1s treated with activin-A for 15 d did not have significantly induced cellular caspase-3 activity compared with control cells (Fig. 4B). As controls, recombinant human caspase-3 exhibited high-caspase activity, and extracts treated with caspase-3 inhibitors had completely inhibited the low caspase-3 activity present in both untreated and activin-A treated cells. Quantification of DAPI stained nuclei in activin and control-treated cells corresponded well with the measurement of caspase-3 activity. Similar results were obtained in PHM1s treated with activin-A for 10 d (data not shown).
Figure 4.
Activin-A effect on PHM1 apoptosis. A, Photomicrographs of PHM1s stained with DAPI showing condensation of chromatin and damaged nuclei (arrows). The rate of apoptosis in untreated cells (NT) and in cells treated with 10 nm of activin-A (A) for 5, 10, or 15 d are shown. B, Cleavage of DEVD-pNA by cell extract and purified caspase-3. PHM1s were treated for 15 d with 10 nm activin-A (A) or left untreated (NT) and then lysed in assay buffer. Cell lysates were preincubated for 10 min at 37 C with (+) or without (−) 0.1 μm caspase-3 inhibitor. As a positive control, 15 U recombinant caspase-3 was added to assay buffer (Pur Casp 3). Samples were assayed for DEV-pNA cleavage with product absorbance measured at 405 nm every 45 min for 225 min.
To determine the potency of activin-A as a myometrial growth inhibitor, we examined a range of activin-A doses in PHM1s. After 21-d treatment, assays revealed that activin-A induced growth inhibition of PHM1s was dose dependent, with an EC50 of approximately 330 pm (Fig. 3C). This potency is similar to that observed in other activin responsive systems in the uterus (3), gonad (30), and pituitary (31). We also examined the potency of activin-A in the hTERT HM. As shown in Fig. 3D, activin-A also inhibited hTERT HM proliferation, with a measured EC50 of activin-A of approximately 390 pm. However, the magnitude of the activin induced growth arrest in hTERT HMs was significantly less than that in PHM1s.
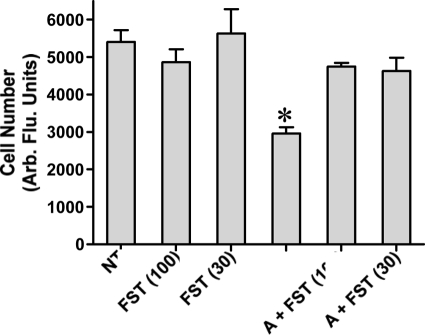
We performed RT-PCR to investigate activin βA subunit expression in PHM1s and detected bands consistent with expression of activin βA subunit mRNA (data not shown). Therefore, we tested whether PHM1s were under autocrine activin-A tone by using the activin antagonist follistatin. PHM1s were treated with activin-A, follistatin, or activin-A plus follistatin. After 18-d treatment, cell growth was measured using the CyQuant assay. As shown in Fig. 5, treatment of PHM1s with 10 nm activin-A suppressed growth, as expected, whereas treatment with either 30 or 100 nm follistatin did not alter the basal growth rate. Cotreatment with 10 nm activin-A and either 30 or 100 nm follistatin resulted in a cell number undistinguishable from untreated and follistatin-treated wells, demonstrating that these doses of follistatin were sufficient to suppress even super-maximal levels of activin-A. Together, these results implied that whereas PHM1s do express activin βA subunit, activin is not produced at sufficient levels to regulate proliferation of this cell line under these culture conditions.
Figure 5.
Follistatin blocking exogenous activin effect in PHM1 proliferation. Follistatin (FST), 30 and 100 nm, effect in PHM1 proliferation by itself and in combination with 10 nm activin-A (A). Arb. Flu., Arbitrary fluorescence. *, P < 0.05.
Activin regulation of myometrial functionality
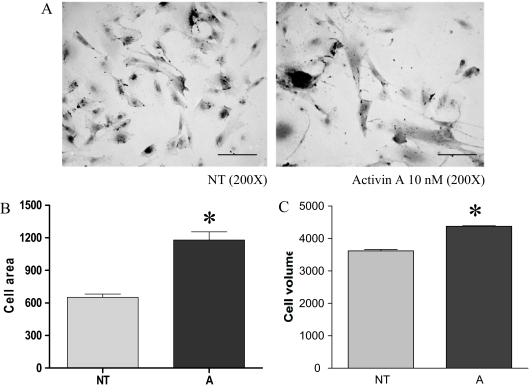
During pregnancy, myometrial cell proliferation is followed by arrest of myometrial cell growth, which is accompanied by cellular hypertrophy and differentiation (29). In this view an activin-A action in suppressing proliferation of myometrial cells could be consistent with activin acting as a myometrial differentiation factor. To examine this possibility, we investigated if activin-A regulated myometrial cell size or differentiated functions. PHM1s cultured with activin-A for 21 d appear larger than untreated control cells (Fig. 6A), and their area increased, as measured by morphometric analysis (Fig. 6B). This could be due to the increased volume of individual PHM1s or alternately due to changes in PHM1 morphology. To clarify these possibilities, we measured the volume of trypsinized control and activin-A treated PHM1s using a Coulter Counter (Multisizer 3). The measured volume of PHM1s treated with activin-A was statistically greater than untreated controls (Fig. 6C). The change in the microscopical appearance of activin-treated PHM1s (Fig. 6A) suggested that activin-A could have altered the levels of matrix protein. Surprisingly, however, real-time-PCR revealed no differences in expression of mRNAs levels of fibronectin, caldesmon, and collagenase in activin-treated or untreated cells (data not shown).
Figure 6.
Effect of activin-A on PHM1 morphology and volume. A, Representative photograph showing the effect of activin-A on PHM1 size as observed at light microscope. Magnification, ×200. B, Analysis of area of control and activin-A treated cells. C, Analysis of volume of trypsinized control and activin-A treated cells. A, 15 d with 10 nm activin-A; NT, left untreated. *, P < 0.05.
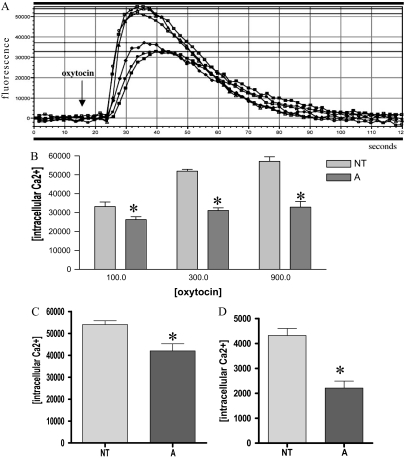
To investigate if activin-A affected the ability of PHM1s to sense and respond to contractile stimuli, we examined the ability of activin-A to induce intracellular Ca2+ accumulation by itself, or to modify oxytocin-induced intracellular Ca2+ accumulation in media containing 1% FBS. Treatment of PHM1s with 10 nm activin-A did not alter intracellular Ca2+ levels (data not shown), but pretreatment with 10 nm activin-A for 8 d resulted in attenuated intracellular Ca2+ accumulation in response to oxytocin. As shown in Fig. 7A, activin-A treated cells had a lower maximal Ca2+ accumulation in response to 300 nm oxytocin than cells with no pretreatment. As shown in Fig. 7B, intracellular Ca2+ accumulation was decreased in activin-A pretreated PHM1s over an oxytocin concentration ranging from 100–900 nm. Further experiments revealed that activin-A pretreatment of 24 h was also effective in suppressing oxytocin-mediated intracellular Ca2+ accumulation (Fig. 7C).
Figure 7.
Activin regulation of intracellular Ca2+ accumulation in PHM1s. A, An example of oxytocin-induced intracellular Ca2+ levels in PHM1 treated (A) or not (NT) for 8 d with 10 nm activin-A. The arrow shows the time of the oxytocin injection. B, Difference between baseline and maximal peak in untreated and 8-d activin-A pretreated cells in response to different oxytocin doses (100, 300, and 900 nm). C, Oxytocin (300 nm) induced intracellular Ca2+ levels in PHM1 treated or not for 24 h with 10 nm activin-A. D, TXA2 (300 nm) induced intracellular Ca2+ levels in PHM1 treated or not for 8 d with 10 nm activin-A. *, P < 0.05.
To confirm that activin regulates the intracellular calcium level, which can be involved in myometrial contractility and other myometrial functions, we also tested 11-deoxy prostaglandin F2α, a TXA2 analog. Pretreatment with 10 nm activin-A for 8 d resulted in attenuated intracellular Ca2+ accumulation in response to 300 nm TXA2 (Fig. 7D).
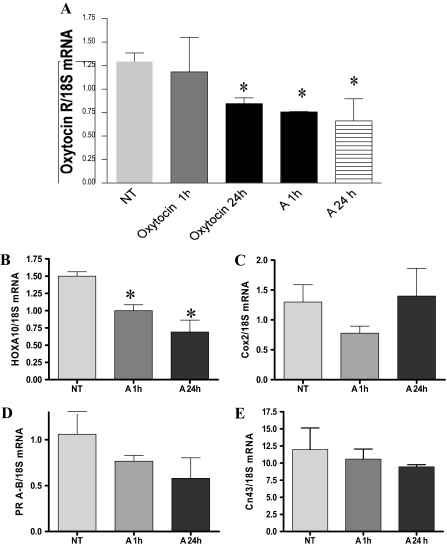
We also examined if activin-A treatment altered expression of PHM1 mRNAs involved in myometrial differentiation or contractility. Real-time RT-PCR experiments were performed to examine if activin-A treatment altered expression of oxytocin receptor, connexin 43 (a gap junction component), or some genes shown to be up-regulated during labor such as HoxA-10, cyclooxygenase (Cox)-2, and progesterone receptor (25). As shown in Fig. 8A, treatment of PHM1s with 10 nm activin-A resulted in a significant decrease in expression of oxytocin receptor mRNA within 1 h, with similar levels of suppression also seen after 24-h treatment (Fig. 8A), and at 8- and 15-d treatment (data not shown). As controls, we examined the effect of oxytocin treatment, which has been reported to result in transcriptional down-regulation of oxytocin receptor expression in myometrial cells (32). Three hundred nanomoles of oxytocin treatment resulted in significant suppression of oxytocin receptor expression after 24 h, which was similar in magnitude to that observed from activin-A treatment, although 1-h oxytocin treatment did not alter oxytocin receptor levels.
Figure 8.
Activin modulation of PHM1 mRNA expression of genes involved in myometrial differentiation or contractility. A, Representative results of real-time PCR for the oxytocin receptor mRNA levels in PHM1 in response to oxytocin and activin-A treatment for 1 and 24 h. Representative results of real-time PCR for HoxA-10 (B), Cox-2 (C), progesterone receptors A-B (D), and connexin 43 (E) in response to activin-A treatment for 1 and 24 h. All mRNAs were normalized with the housekeeping gene 18S mRNA. A, 15 d with 10 nm activin-A; NT, left untreated. *, P < 0.05.
Activin-A treatment also decreased the expression of HoxA-10 mRNA (Fig. 8B) but did not significantly alter Cox-2 mRNA (Fig. 8C), total progesterone receptor mRNA (Fig. 8D), or connexin 43 mRNA levels (Fig. 8E) after 24-h treatment.
Discussion
The myometrium is a dynamic tissue that is remodeled during each reproductive cycle (28) and also undergoes changes during pregnancy (14,29). Myometrial changes during pregnancy are particularly dramatic. An initial hyperplasic phase that results, depending on the species, on an increase in cell number of at least 10-fold (14) is followed by a synthetic phase that is characterized by cell hypertrophy, formation of gap junctions (33), and increased expression of proteins necessary for coordinated smooth muscle contractions that are the primary myometrial function at term (34).
Effects of activin on myometrium have not been reported; furthermore, activin binding to myometrium has been controversial, with binding of iodinated activin-A reported in rats (13), and expression of activin receptors detected in one study (12), but not detected in another study (11). We have initially characterized effects of activin-A on myometrial-derived cell lines. We detect expression of both isoforms of activin type II receptors, as well as activin type I receptor ALK4 in both PHM1 and hTERT HM myometrial cell lines. Furthermore, we demonstrate that these cell lines respond to activin-A with induction of Smad 2 phosphorylation, providing confirmation that these cells express sufficient levels of activin receptors to support activin signaling. Although we did not detect autocrine activin signaling in PHM1s, an autocrine role of activin-A in the myometrium is certainly plausible, given the level of activin βA subunit expression in myometrial cell lines. In the uterus, activins are also expressed in the endometrium adjacent to myometrial smooth muscle cells, suggesting that activin may have functional actions on the myometrium, even if autocrine signaling does not occur. The relative roles of autocrine, paracrine, and endocrine activin signaling are likely to differ under various physiological conditions and remain unclear.
To examine possible roles for activins in regulating myometrial cell function, we first examined the ability of activin-A to influence proliferation of myometrial cells. Myometrial cells undergo a wave of proliferation during each reproductive cycle (28) and also undergo an extended period of hyperplasia during the early phases of pregnancy (14). We found that activin-A reduced the total cell numbers of both PHM1 and hTERT HM myometrial cell lines in a time and dose-dependent manner. Because the rate of apoptosis (also measured by cytofluorimetric analysis of propidium iodide staining; data not shown) did not change, we assume that activin-A decreased the rate of PHM1 proliferation. Growth factors that promote myometrial cell proliferation have been identified, including epidermal growth factor, platelet-derived growth factor (35), and IGF (36). TGFβ-1, -2, and 3 were found to stimulate the rate of [3H]thymidine incorporation at low doses, whereas at higher doses they reduced the rate compared with the controls. Instead, TGF-βs had no significant effect on cell proliferation determined by cell counting (22). This suggests that activin-A is the main reported growth factor with cytostatic actions in myometrial cells. Anyway, even if we observe responses to activin that are considerably greater than those reported in response to TGF-β, we should compare these in the same system before drawing conclusions regarding which are the most efficacious.
PHM1s are an immortalized cell line generated through expression of the E6 and E7 proteins of the human papilloma 16 virus in myometrial cells derived from a pregnant woman (23). These cells have been previously used as model to study myometrial function because they did not appear to be transformed by viral infection, and they retain a considerable array of morphological and biochemical properties of the parent cells (37,38,39,40,41,42,43,44,45,46,47,48,49,50). On the other hand, hTERT HM is a cell line derived from nonpregnant human myometrial cells transfected with hTERT. hTERT HMs have also been used as a model system to study aspects of human myometrial function because they retain markers of differentiation that are observed in primary cultures of smooth muscle cells, and they express various smooth muscle/myometrium cell markers (24,51).
It is not clear if the differences in the extent of growth inhibition in response to activin-A between PHM1 and hTERT HMs involve the methods used to generate the cell lines (i.e. expression of viral oncogenes vs. telomerase expression), or alternately if differences involve one cell line originating from pregnant tissue and one being derived from nonpregnant tissue. In either case, activin-A responsiveness is clearly seen in both cell lines, suggesting that activin responsiveness is a common feature of myometrial cells. If the greater cytostatic response in PHM1s is due to PHM1 growth reflecting myometrial expansion seen early in pregnancy, then activin-A is a candidate signal to curtail myometrial hyperplasia. One possible model is that activin suppression of myometrial proliferation could be a component of activin acting as a myometrial differentiation factor during mid to late pregnancy.
PHM1s are reported to maintain smooth muscle morphology and functional characteristics seen during pregnancy, such as responsiveness to oxytocin (23,38,45,46). Therefore, we examined if activin-A had other actions in PHM1s consistent with a role for activin in myometrial differentiation during pregnancy. During early- and midpregnancy, uterine quiescence to contractile stimuli is crucial (52). Only at term is there a switch to the contractile phenotype essential to enable myometrial smooth muscle to generate the synchronous, high-amplitude, high-frequency contractions of labor. Although not itself stimulating intracellular Ca2+ accumulation, activin-A attenuated oxytocin and TXA2 induced intracellular Ca2+ accumulation in PHM1s.
A role for activin-A in triggering myometrial contractility at labor has been suggested, primarily based on the observation that maternal serum activin-A level increases during pregnancy (8,15,16,17,18,19,21), with the most marked increase being during the third trimester (53,54). Near term, the ratio of circulating activin-A and follistatin shifts to favor excess free, bioactive activin-A (54,55). However, attempts to correlate serum activin levels or activin/follistatin ratio with the onset of labor have not produced consistent results (8,15,16,17,18,19,20,21). Of course, possible local production could not be reflected in circulation. Our results demonstrate clearly that activin-A does not increase expression of genes that are up-regulated at labor such as HoxA-10, Cox-2, or progesterone receptor, and did not render PHM1s more sensitive to contractile stimuli. These findings do not support the model that activin promotes myometrial contraction at or near term. On the contrary, the decrease in oxytocin receptor and HoxA-10 expression observed on activin-A treatment suggests that activin-A, if anything, may be contributing to maintain the myometrium in a noncontractile phenotype.
In conclusion, activin plays a role if myometrial cells are exposed to this protein. In summary, we show that myometrial cells express activin receptors and signaling mediators, and that activin-A can induce myometrial cell responses, as measured by Smad 2 phosphorylation, inhibition of cellular proliferation, and modulation of sensitivity to contractile agents.
Acknowledgments
We thank Drs. Barbara Sanborn and Chun-Ying Ku of Colorado State University for generously providing the pregnant human myometrial 1 cell line, Dr. William Rainey of University of Texas Southwestern Medical Center for the human telomerase reverse transcriptase-infected human myometrial cell line, Dr. Teresa Woodruff of Northwestern University for the follistatin, and Dr. Louise Bilezikjian for her critical reading of the manuscript.
Footnotes
This work was supported in part by grants from the National Institutes of Health (Grant HD13527), The Adler Foundation, and The Clayton Medical Research Foundation, Inc.
Present address for P.C.: Institute of Normal Human Morphology, Faculty of Medicine, Polytechnic University of Marche, via Tronto 10/a, 60020 Ancona, Italy.
Disclosure Summary: P.C. and E.W. have nothing to declare. W.V. is a cofounder, consultant, equity holder, and member of the Board of Directors of Neurocrine Biosciences and Acceleron Pharma.
First Published Online January 31, 2008
Abbreviations: Cox, Cyclooxygenase; DAPI, 4′,6-diamidino-2-phenylindole; FBS, fetal bovine serum; hTERT, human telomerase reverse transcriptase; hTERT HM, human telomerase reverse transcriptase-infected human myometrial cell line; PHM1, pregnant human myometrial 1 cell line; pNA, p-Nitroanile; TBST, Tris-buffered saline with Tween 20; TXA2, thromboxaneA2.
References
- Vale W, Wiater E, Gray P, Harrison C, Bilezikjian L, Choe S 2004 Activins and inhibins and their signaling. Ann NY Acad Sci 1038:142–147 [DOI] [PubMed] [Google Scholar]
- Leung PH, Salamonsen LA, Findlay JK 1998 Immunolocalization of inhibin and activin subunits in human endometrium across the menstrual cycle. Hum Reprod 13:3469–3477 [DOI] [PubMed] [Google Scholar]
- Di Simone N, Schneyer AL, Caliandro D, Castellani R, Caruso A 2002 Regulation of endometrial adenocarcinoma cell proliferation by Activin-A and its modulation by 17β-estradiol. Mol Cell Endocrinol 192:187–195 [DOI] [PubMed] [Google Scholar]
- Jones RL, Salamonsen LA, Findlay JK 2002 Activin A promotes human endometrial stromal cell decidualization in vitro. J Clin Endocrinol Metab 87:4001–4004 [DOI] [PubMed] [Google Scholar]
- Jones RL, Salamonsen LA, Critchley HO, Rogers PA, Affandi B, Findlay JK 2000 Inhibin and activin subunits are differentially expressed in endometrial cells and leukocytes during the menstrual cycle, in early pregnancy and in women using progestin-only contraception. Mol Hum Reprod 6:1107–1117 [DOI] [PubMed] [Google Scholar]
- Meunier H, Rivier C, Evans RM, Vale W 1988 Gonadal and extragonadal expression of inhibin α, β A, and β B subunits in various tissues predicts diverse functions. Proc Natl Acad Sci USA 85:247–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraglia F, Anceschi MM, Calza L, Garuti GC, Fusaro P, Giardino L, Gennazzani AR, Vale W 1993 Inhibin and activin in human fetal membranes: evidence for a local effect on prostaglandin release. J Clin Endocrinol Metab 77:542–548 [DOI] [PubMed] [Google Scholar]
- Petraglia F, Gallinelli A, De Vita D, Lewis K, Mathews L, Vale W 1994 Activin at parturition: changes of maternal serum levels and evidence for binding sites in placenta and fetal membranes. Obstet Gynecol 84:278–282 [PubMed] [Google Scholar]
- Caniggia I, Lye SJ, Cross JC 1997 Activin is a local regulator of human cytotrophoblast cell differentiation. Endocrinology 138:3976–3986 [DOI] [PubMed] [Google Scholar]
- He ZY, Liu HC, Mele CA, Barmat L, Veeck LL, Davis O, Rosenwaks Z 1999 Expression of inhibin/activin subunits and their receptors and binding proteins in human preimplantation embryos. J Assist Reprod Genet 16:73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Kolsky M, Manuelpillai U, Gargett C, Wallace EM 2001 Activin βA-subunit and activin receptors in human myometrium at term and during labour. BJOG 108:869–874 [DOI] [PubMed] [Google Scholar]
- Hayashi K, Carpenter KD, Gray CA, Spencer TE 2003 The activin-follistatin system in the neonatal ovine uterus. Biol Reprod 69:843–850 [DOI] [PubMed] [Google Scholar]
- Draper LB, Chong H, Wang E, Woodruff TK 1997 The uterine myometrium is a target for increased levels of activin A during pregnancy. Endocrinology 138:3042–3046 [DOI] [PubMed] [Google Scholar]
- Johansson B 1984 Different types of smooth muscle hypertrophy. Hypertension 6(6 Pt 2):III64–III68 [DOI] [PubMed] [Google Scholar]
- Petraglia F, De Vita D, Gallinelli A, Aguzzoli L, Gennazzani AR, Romero R, Woodruff TK 1995 Abnormal concentration of maternal serum activin-A in gestational diseases. J Clin Endocrinol Metab 80:558–561 [DOI] [PubMed] [Google Scholar]
- Woodruff TK, Sluss P, Wang E, Janssen I, Mersol-Barg MS 1997 Activin A and follistatin are dynamically regulated during human pregnancy. J Endocrinol 152:167–174 [DOI] [PubMed] [Google Scholar]
- Gallinelli A, Gallo R, Genazzani AD, Matteo ML, Caruso A, Woodruff TK, Petraglia F 1996 Episodic secretion of activin A in pregnant women. Eur J Endocrinol 135:340–344 [DOI] [PubMed] [Google Scholar]
- Petraglia F, Di Blasio AM, Florio P, Gallo R, Gennazzani AR, Woodruff TK, Vale W 1997 High levels of fetal membrane activin β A and activin receptor IIB mRNAs and augmented concentration of amniotic fluid activin A in women in term or preterm labor. J Endocrinol 154:95–101 [DOI] [PubMed] [Google Scholar]
- Farina A, Lambert-Messerlian GM, Canick JA, Banzola I, Carletti A, Concu M, Tempesta A, Gabrielli S, Morano D, Rizzo N 2006 Total activin A in maternal blood as a marker of preterm delivery in low-risk asymptomatic patients. Prenat Diagn 26:277–281 [DOI] [PubMed] [Google Scholar]
- Schneider-Kolsky M, D’Antona D, Evans LW, Taylor N, O’Connor A, Groome NP, de Kretser D, Wallace EM 2000 Maternal serum total activin A and follistatin in pregnancy and parturition. BJOG 107:995–1000 [DOI] [PubMed] [Google Scholar]
- Plevyak MP, Lambert-Messerlian GM, Farina A, Groome NP, Canick JA, Silver HM 2003 Concentrations of serum total activin A and inhibin A in preterm and term labor patients: a cross-sectional study. J Soc Gynecol Investig 10:231–236 [DOI] [PubMed] [Google Scholar]
- Tang XM, Dou Q, Zhao Y, McLean F, Davis J, Chegini N 1997 The expression of transforming growth factor-β s and TGF-β receptor mRNA and protein and the effect of TGF-β s on human myometrial smooth muscle cells in vitro. Mol Hum Reprod 3:233–240 [DOI] [PubMed] [Google Scholar]
- Monga M, Ku CY, Dodge K, Sanborn BM 1996 Oxytocin-stimulated responses in a pregnant human immortalized myometrial cell line. Biol Reprod 55:427–432 [DOI] [PubMed] [Google Scholar]
- Condon J, Yin S, Mayhew B, Word RA, Wright WE, Shay JW, Rainey WE 2002 Telomerase immortalization of human myometrial cells. Biol Reprod 67:506–514 [DOI] [PubMed] [Google Scholar]
- Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R 2002 Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab 87:2924–2930 [DOI] [PubMed] [Google Scholar]
- Deglise S, Martin D, Probst H, Saucy F, Hayoz D, Waeber G, Nicod P, Ris HB, Corpataux JM, Haefliger JA 2005 Increased connexin43 expression in human saphenous veins in culture is associated with intimal hyperplasia. J Vasc Surg 41:1043–1052 [DOI] [PubMed] [Google Scholar]
- Lee MJ, Ma Y, LaChapelle L, Kadner SS, Guller S 2004 Glucocorticoid enhances transforming growth factor-β effects on extracellular matrix protein expression in human placental mesenchymal cells. Biol Reprod 70:1246–1252 [DOI] [PubMed] [Google Scholar]
- Burroughs KD, Fuchs-Young R, Davis B, Walker CL 2000 Altered hormonal responsiveness of proliferation and apoptosis during myometrial maturation and the development of uterine leiomyomas in the rat. Biol Reprod 63:1322–1330 [DOI] [PubMed] [Google Scholar]
- Shynlova O, Oldenhof A, Dorogin A, Xu Q, Mu J, Nashman M, Lye SJ 2006 Myometrial apoptosis: activation of the caspase cascade in the pregnant rat myometrium at midgestation. Biol Reprod 74:839–849 [DOI] [PubMed] [Google Scholar]
- Shikone T, Matzuk MM, Perlas E, Finegold MJ, Lewis KA, Vale W, Bradley A, Hsueh A 1994 Characterization of gonadal sex cord-stromal tumor cell lines from inhibin-α and p53-deficient mice: the role of activin as an autocrine growth factor. Mol Endocrinol 8:983–995 [DOI] [PubMed] [Google Scholar]
- Billestrup N, Gonzalez-Manchon C, Potter E, Vale W 1990 Inhibition of somatotroph growth and growth hormone biosynthesis by activin in vitro. Mol Endocrinol 4:356–362 [DOI] [PubMed] [Google Scholar]
- Phaneuf S, Asboth G, Carrasco MP, Europe-Finner GN, Saji F, Kimura T, Harris A, Lopez Bernal A 1997 The desensitization of oxytocin receptors in human myometrial cells is accompanied by down-regulation of oxytocin receptor messenger RNA. J Endocrinol 154:7–18 [DOI] [PubMed] [Google Scholar]
- Shynlova O, Mitchell JA, Tsampalieros A, Langille BL, Lye SJ 2004 Progesterone and gravidity differentially regulate expression of extracellular matrix components in the pregnant rat myometrium. Biol Reprod 70:986–992 [DOI] [PubMed] [Google Scholar]
- Hertelendy F, Zakar T 2004 Regulation of myometrial smooth muscle functions. Curr Pharm Des 10:2499–2517 [DOI] [PubMed] [Google Scholar]
- Rossi MJ, Chegini N, Masterson BJ 1992 Presence of epidermal growth factor, platelet-derived growth factor, and their receptors in human myometrial tissue and smooth muscle cells: their action in smooth muscle cells in vitro. Endocrinology 130:1716–1727 [DOI] [PubMed] [Google Scholar]
- Tang XM, Rossi MJ, Masterson BJ, Chegini N 1994 Insulin-like growth factor I (IGF-I), IGF-I receptors, and IGF binding proteins 1–4 in human uterine tissue: tissue localization and IGF-I action in endometrial stromal and myometrial smooth muscle cells in vitro. Biol Reprod 50:1113–1125 [DOI] [PubMed] [Google Scholar]
- Barhoumi R, Awooda I, Mouneimne Y, Safe S, Burghardt RC 2006 Effects of benzo-a-pyrene on oxytocin-induced Ca2+ oscillations in myometrial cells. Toxicol Lett 165:133–141 [DOI] [PubMed] [Google Scholar]
- Burghardt RC, Barhoumi R, Sanborn BM, Andersen J 1999 Oxytocin-induced Ca2+ responses in human myometrial cells. Biol Reprod 60:777–782 [DOI] [PubMed] [Google Scholar]
- Burghardt RC, Barhoumi R, Stickney M, Monga M, Ku CY, Sanborn BM 1996 Correlation between connexin43 expression, cell-cell communication, and oxytocin-induced Ca2+ responses in an immortalized human myometrial cell line. Biol Reprod 55:433–438 [DOI] [PubMed] [Google Scholar]
- Dodge KL, Carr DW, Sanborn BM 1999 Protein kinase A anchoring to the myometrial plasma membrane is required for cyclic adenosine 3′,5′-monophosphate regulation of phosphatidylinositide turnover. Endocrinology 140:5165–5170 [DOI] [PubMed] [Google Scholar]
- Dodge KL, Sanborn BM 1998 Evidence for inhibition by protein kinase A of receptor/G α(q) /phospholipase C (PLC) coupling by a mechanism not involving PLCβ2. Endocrinology 139:2265–2271 [DOI] [PubMed] [Google Scholar]
- Madsen G, Zakar T, Ku CY, Sanborn BM, Smith R, Mesiano S 2004 Prostaglandins differentially modulate progesterone receptor-A and -B expression in human myometrial cells: evidence for prostaglandin-induced functional progesterone withdrawal. J Clin Endocrinol Metab 89:1010–1013 [DOI] [PubMed] [Google Scholar]
- Massrieh W, Derjuga A, Doualla-Bell F, Ku CY, Sanborn BM, Blank V 2006 Regulation of the MAFF transcription factor by proinflammatory cytokines in myometrial cells. Biol Reprod 74:699–705 [DOI] [PubMed] [Google Scholar]
- Merlino AA, Welsh TN, Tan H, Yi LJ, Cannon V, Mercer BM, Mesiano S 2007 Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocrinol Metab 92:1927–1933 [DOI] [PubMed] [Google Scholar]
- Monga M, Campbell DF, Sanborn BM 1999 Oxytocin-stimulated capacitative calcium entry in human myometrial cells. Am J Obstet Gynecol 181:424–429 [DOI] [PubMed] [Google Scholar]
- Shlykov SG, Sanborn BM 2004 Stimulation of intracellular Ca2+ oscillations by diacylglycerol in human myometrial cells. Cell Calcium 36:157–164 [DOI] [PubMed] [Google Scholar]
- Shlykov SG, Yang M, Alcorn JL, Sanborn BM 2003 Capacitative cation entry in human myometrial cells and augmentation by hTrpC3 overexpression. Biol Reprod 69:647–655 [DOI] [PubMed] [Google Scholar]
- Yang M, Gupta A, Shlykov SG, Corrigan R, Tsujimoto S, Sanborn BM 2002 Multiple Trp isoforms implicated in capacitative calcium entry are expressed in human pregnant myometrium and myometrial cells. Biol Reprod 67:988–994 [DOI] [PubMed] [Google Scholar]
- Zhong M, Ku CY, Sanborn BM 2005 Pathways used by relaxin to regulate myometrial phospholipase C. Ann NY Acad Sci 1041:300–304 [DOI] [PubMed] [Google Scholar]
- Zhong M, Yang M, Sanborn BM 2003 Extracellular signal-regulated kinase 1/2 activation by myometrial oxytocin receptor involves Gα(q)Gβγ and epidermal growth factor receptor tyrosine kinase activation. Endocrinology 144:2947–2956 [DOI] [PubMed] [Google Scholar]
- Hardy DB, Janowski BA, Corey DR, Mendelson CR 2006 Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-κB activation of cyclooxygenase 2 expression. Mol Endocrinol 20:2724–2733 [DOI] [PubMed] [Google Scholar]
- Challis JRG, Matthews SG, Gibb W, Lye SJ 2000 Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev 21:514–550 [DOI] [PubMed] [Google Scholar]
- Muttukrishna S, Fowler PA, George L, Groome NP, Knight PG 1996 Changes in peripheral serum levels of total activin A during the human menstrual cycle and pregnancy. J Clin Endocrinol Metab 81:3328–3334 [DOI] [PubMed] [Google Scholar]
- O’Connor AE, McFarlane JR, Hayward S, Yohkaichiya T, Groome NP, de Kretser DM 1999 Serum activin A and follistatin concentrations during human pregnancy: a cross-sectional and longitudinal study. Hum Reprod 14:827–832 [DOI] [PubMed] [Google Scholar]
- Fowler PA, Evans LW, Groome NP, Templeton A, Knight PG 1998 A longitudinal study of maternal serum inhibin-A, inhibin-B, activin-A, activin-AB, pro-αC and follistatin during pregnancy. Hum Reprod 13:3530–3536 [DOI] [PubMed] [Google Scholar]
- Florio P, Severi FM, Luisi S, Ciarmela P, Calonaci G, Cobellis L, Petraglia F 2003 Endometrial expression and secretion of activin A, but not follistatin, increase in the secretory phase of the menstrual cycle. J Soc Gynecol Investig 10:237–243 [DOI] [PubMed] [Google Scholar]
- Ciarmela P, Florio P, Sigurdardottir M, Toti P, Maguer-Satta V, Rimokh R, Altomare A, Tosi P, Petraglia F 2004 Follistatin-related gene expression, but not follistatin expression, is decreased in human endometrial adenocarcinoma. Eur J Endocrinol 151:251–257 [DOI] [PubMed] [Google Scholar]