Abstract
The role of progesterone receptor (PR) signaling in skeletal metabolism is controversial. To address whether signaling through the PR is necessary for normal bone growth and turnover, we performed histomorphometric and microcomputed tomography analyses of bone from homozygous female PR knockout (PRKO) mice at 6, 12, and 26 wk of age. These mice possess a null mutation of the PR locus, which blocks the gene expression of A and B isoforms of PR. Body weight gain, uterine weight gain, and tibia longitudinal bone growth were normal in PRKO mice. In contrast, total, cancellous, and cortical bone mass were increased in the humerus of 12-wk-old PRKO mice, whereas cortical and cancellous bone mass in the tibia was normal. At 26 wk of age, cancellous bone area in the proximal tibia metaphysis of PRKO mice was 153% greater than age matched wild-type mice. The improved cancellous bone balance in 6-month-old PRKO mice was associated with elevated bone formation and a tendency toward reduced osteoclast perimeter. Taken together, these findings suggest that PR signaling in mice is not essential for bone growth and turnover. However, at some skeletal sites, PR signaling attenuates the accumulation of cortical and cancellous bone mass during adolescence.
THE SEX STEROIDS 17β-estradiol and progesterone are produced in the ovaries of mammals under tight regulation by the pituitary-hypothalamic-gonadal axis. The synthesis of these hormones increases at puberty, whereas their production and systemic levels rapidly decline in humans after menopause. Puberty and menopause represent stages of rapid gain and loss of bone mass, respectively. Although the effects of estrogen on the skeleton are well documented, those of progesterone have received comparatively little attention. Consequently, the physiological importance of progesterone signaling in bone growth, turnover, and loss remains unclear (1).
Clinical studies report variable effects of progesterone on bone mass. In postmenopausal women, progesterone was reported to reduce cortical but not cancellous bone loss, and combined estrogen and progestin therapy was reported to be as effective as high-dose estrogen alone in preventing overall bone loss (2,3,4). Part of the effect to reduce bone resorption may have been due to displacement of cortisol by progesterone from the osteoblast glucocorticoid receptor (5). Progesterone treatment was reported to increase spinal cancellous bone density in premenopausal women with menstrual disturbances (6). In these patients, the serum levels of estrogen are variable, but the levels of progesterone are universally diminished. On the other hand, the synthetic progesterone medroxyprogesterone acetate (MPA), which is used to suppress ovulation by decreasing pituitary gonadotropin output, is associated with a decrease in bone mineral density (7). The reduction in bone mass associated with MPA has been variously attributed to decreased estrogen production (7) and occupation by MPA of the glucocorticoid receptor (8).
Studies of laboratory animals generally support weak anabolic effects of progesterone on the skeleton. In contrast to ovariectomy (OVX), pseudopregnancy (a condition of estrogen deficiency with elevated progesterone) was not associated with cancellous bone loss in the rat (9). Moreover, in OVX rodents and dogs, physiological concentrations of progesterone have been reported to prevent bone loss from multiple sites and in some cases actually stimulate new bone formation, particularly of cortical bone (10,11). Pharmacological concentrations of progesterone administered to OVX rodents was reported to increase periosteal bone formation, inhibit bone resorption, and enhance estrogen-induced bone formation at endocortical surfaces (12,13).
However, not all studies report beneficial effects of progesterone on bone metabolism in postmenopausal women or OVX rats. Specifically, some investigators have failed to demonstrate an effect of progesterone, with or without estrogen, on OVX-induced bone loss in rats (14,15). Furthermore, the antiprogesterone RU486 was shown to reduce bone loss in OVX rats (16). Finally, more recent clinical trials dispute the earlier claims that progesterone replacement therapy improves bone density in postmenopausal, premenopausal, OVX, or amenorrheic women (1,17,18).
At the cellular level, progesterone exerts its physiological effects by binding to and activating the progesterone receptor (PR), a member of the steroid nuclear receptor transcription factor family. The PR subsequently binds directly or indirectly to the regulatory regions of target genes and modulates transcription (19). Of the various cell types present in the bone microenvironment, osteoblasts have been shown to express PR (20,21), and the level of PR expression in these cells can be stimulated by estrogen (20,21,22). As a result, it is possible that some of the effects on bone metabolism attributed to estrogen may be mediated by progesterone.
In an attempt to clarify the role of progesterone signaling in bone, we analyzed bone growth and turnover in mice carrying a null mutation in the PR locus [PR knockout (PRKO mice] (23). PRKO mice lack transcripts and protein for both PR-A and PR-B isoforms and exhibit abnormalities in the brain and multiple reproductive tissues, including the ovary, uterus, and mammary gland, leading to infertility in homozygous females. Here we report that PRKO mice exhibit site-specific increases in cancellous and cortical bone mass during adolescence.
Materials and Methods
Mice
Female isogenic wild-type (WT) and PRKO mice were developed in the laboratory of J. P. Lydon (Department of Cell Biology, Baylor College of Medicine, Houston, TX). Animals were maintained and experimental procedures on animals were conducted in accordance with guidelines outlined by the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee. Bones from WT and PRKO mice were analyzed by bone histomorphometry or microcomputed tomography (μCT) at three ages: 6 wk (five animals per group), 12 wk (10 animals per group), and 26 wk (five animals per group). Fluorochrome markers were used to label mineralizing bone surfaces. Each mouse was administered (perivascular tail vein injection) tetracycline (20 mg/kg body weight) followed by calcein (20 mg/kg body weight) 7 d apart; the final label being given 2–3 d before animals were killed. Mice were killed by cardiectomy under anesthesia (50 μl ketamine hydrochloride, 100 mg/ml) and blood and urine samples collected. Tibiae and humeri were dissected free of soft tissue and fixed in 70% ethanol, and uteri were weighed.
Histological processing
Whole tibiae were fixed in 70% ethanol for at least 72 h at 4 C. For analysis of cancellous bone, proximal tibiae were dehydrated in a graded ethanol series, infiltrated, and then embedded in plastic resin at room temperature. The resin comprised 88.5% (vol/vol) methyl methacrylate (Fisher Scientific, Pittsburgh, PA), 7% (vol/vol) hydroxyethyl methacrylate, 4.4% (vol/vol) dibutyl phthalate (Polysciences, Warrington, PA), 8.8% (wt/vol) polyethylene glycol distearate (Polysciences), and 0.8% (wt/vol) benzoyl peroxide (Aldrich Chemical Co., Milwaukee, WI). The undecalcified bone was then sectioned at 5 μm on a Reichert-Jung Supercut 2050 microtome. For analysis of cortical bone, diaphyseal cross-sections of tibiae (150 μm thick) were cut at the tibia-fibular synostosis, ground to 15–20 μm on a roughened glass plate, and mounted in Permount.
Histomorphometry
Bone histomorphometry was used to assess static and dynamic skeletal changes. Histomorphometric measurements were performed using the OsteoMeasure Analysis system (OsteoMetrics, Atlanta, GA). Longitudinal growth rate in 6-, 12-, and 26-wk-old mice was calculated in the proximal tibia from the mean distance between the most proximal label and the distal edge of the growth plate measured at five different sites across the metaphysis. Cancellous bone area/tissue area was measured in the proximal tibial epiphysis and metaphysis. Trabecular number (per millimeter), trabecular thickness (μm), and trabecular separation (μm) were calculated as described (24,25). Osteoclasts were identified by staining for tartrate-resistant acid phosphatase (TRAP) using an azo-dye coupling method with naphthol AS-TR phosphate and acetate-buffered saline (pH 5.5) as substrate. Sections were counterstained with methyl-green thionine in citrate buffer (pH 5.8). Fluorochrome-based indices of cancellous bone formation were measured and included 1) mineralizing perimeter/bone perimeter (cancellous bone perimeter covered with double plus half single fluorochrome label), 2) mineral apposition rate (the distance between two fluorochrome markers that comprise a double label divided by the number of days between label administration, micrometers per day), and 3) bone formation rate (calculated by multiplying mineralizing perimeter by mineral apposition rate) normalized to bone perimeter (square micrometers per micrometer per day) and tissue area (percent per day). For cortical bone, cross-sectional area and medullary area were measured and cortical area calculated as the difference between the two endpoints. All histomorphometric data were collected by the same individual and are reported in accordance with standard nomenclature (24).
μCT
μCT was used for nondestructive three-dimensional evaluation of bone architecture in 12-wk-old mice. Humeri were scanned using a Scanco μCT40 scanner (Scanco Medical AG, Basserdorf, Switzerland) at a voxel size of 12 × 12 × 12 μm and a threshold of 256 (gray scale, 0–1000). The threshold value was determined empirically. Entire humeri (cancellous plus cortical bone) were evaluated followed by evaluation of cancellous bone in the distal humeral epiphysis and cortical bone at the humeral midshaft. For the humeral epiphysis, about 20 slices (0.24 mm) of bone were measured and included cancellous bone only. Direct cancellous bone measurements included 1) cancellous bone volume/tissue volume (volume of tissue occupied by cancellous bone, percent), 2) trabecular number (number of trabeculae within the sampled tissue, per millimeter), 3) trabecular thickness (mean thickness of individual trabeculae, micrometers), and 4) trabecular separation (the distance between trabeculae, micrometers) (26). For the humeral midshaft, 20 slices (0.24 mm) of cortical bone were evaluated and total cross-sectional tissue volume (cortical and marrow volume, cubic millimeters), cortical volume (cubic millimeters), and marrow volume (cubic millimeters) determined.
Statistical analysis
Statistical significance was determined between WT and PRKO mice and across age groups using ANOVA followed by a Tukey’s post hoc test. A P value of <0.05 was considered statistically significant. All data are expressed as mean ± se.
Results
Bones from female PRKO mice were analyzed at three ages: 1) at the peripubertal age of 6 wk when the skeleton is in a phase of rapid growth, 2) at the postpubertal age of 12 wk when the reproductive system is fully developed and skeletal growth has slowed considerably, and 3) at 26 wk when longitudinal bone growth has ceased.
Body and uterine weight
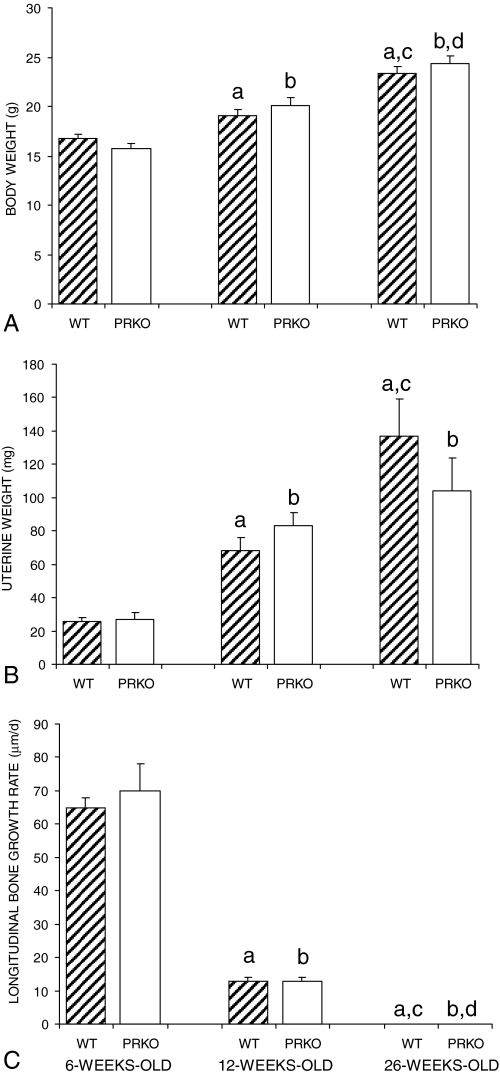
Body and uterine wet weights of both PRKO and WT mice were measured at the time of killing and are shown in Fig. 1, A and B, respectively. Both body weight and uterine weight increased progressively during the experiment. However, significant differences in either body weight or uterine weight between PRKO and WT control mice were not detected at any age examined. The lack of a difference in uterine weight is consistent with earlier findings that abnormalities in the size and morphology of the PRKO mouse uterus only become evident after OVX and hormonal stimulation (23).
Figure 1.
Total body weights (A), uterine wet weights (B), and proximal tibia longitudinal growth rates (C) of WT and PRKO mice measured at 6, 12, and 26 wk of age. Ablation of PR signaling had no effect on body weight gain, uterine weight gain, or the age-related slowing of bone growth. Longitudinal growth rate was below the limit of detection in 26-wk-old mice of either genotype (<5 μm/d). Values are mean ± se. a, Significantly different from WT at 6 wk; b, significantly different from PRKO at 6 wk; c, significantly different from WT at 12 wk; d, significantly different from PRKO at 12 wk; P < 0.01.
Longitudinal bone growth
Longitudinal bone growth at the proximal tibial growth plate is shown in Fig. 1C. Differences in longitudinal bone growth were not detected between WT and PRKO mice at any of the age groups examined. By 12 wk of age, the growth plates were narrow and growth rates had slowed markedly. At 26 wk of age, longitudinal bone growth in the tibiae had ceased in both genotypes.
Cancellous bone histomorphometry: proximal tibial epiphysis
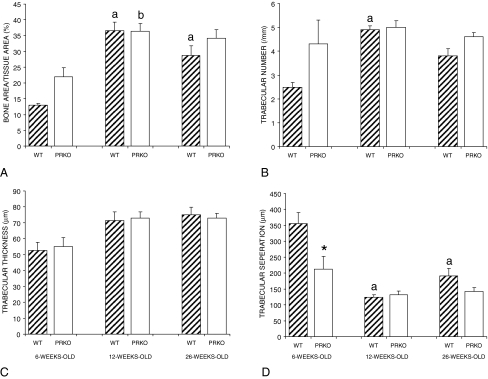
Cancellous bone architecture in the proximal tibial epiphysis is shown in Fig. 2. Cancellous bone area normalized to tissue area in WT mice was higher in 12- and 26-wk-old than in 6-wk-old animals (Fig. 2A). In the PRKO mice, cancellous bone area/tissue area was higher in 12-wk-old animals than in 6-wk-old animals. Significant differences in bone area/tissue area between PRKO and WT control mice were not detected at any age. Trabecular number increased in WT mice between 6 and 12 wk of age, whereas this endpoint did not change with age in PRKO mice (Fig. 2B). Trabecular thickness did not change with age irrespective of genotype (Fig. 2C). At 6 wk of age, trabecular separation was larger in WT compared with age-matched PRKO mice (Fig. 2D). Trabecular separation was also lower in 12- and 26-wk-old WT mice than in 6-wk-old WT mice. Differences in trabecular separation were not detected in PRKO mice with age. Fluorochrome labels were measured to evaluate the effects of age and genotype on bone formation at the proximal tibia epiphysis (data not shown). In both genotypes, the cancellous mineral apposition rate increased between 12 and 26 wk of age, whereas there were no changes in the extent of mineralizing perimeter. Similarly, bone formation increased in both genotypes between 12 and 26 wk of age. These findings suggest that the number of active osteoblasts on trabecular surfaces does not change during the first 26 wk of age in the epiphysis, but the activity of individual cells increases. The cancellous bone formation rate increased in parallel with the mineral apposition rate, and thus the age-related increase in cancellous bone formation was principally due to an increase in osteoblast activity.
Figure 2.
Cancellous bone architecture at the proximal tibia epiphysis as a function of age in WT and PRKO mice. Values are mean ± se. a, Significantly different from WT at 6 wk; b, significantly different from PRKO at 6 wk; *, significantly different from age-matched WT; P < 0.01.
Cancellous bone histomorphometry: proximal tibial metaphysis
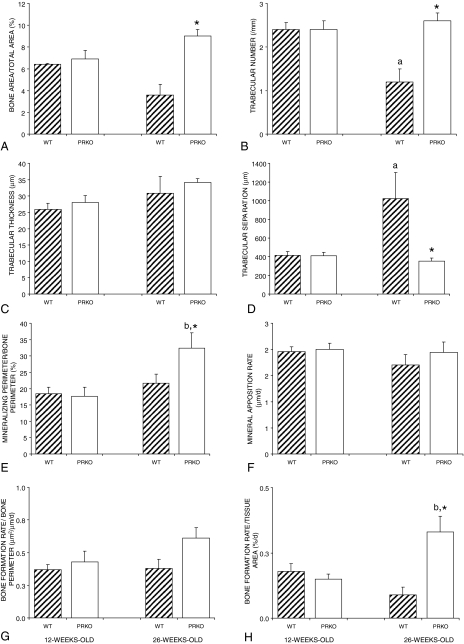
Cancellous bone architecture in the proximal tibial metaphysis of WT and PRKO mice is shown in Fig. 3, A–D. Between 12 and 26 wk of age, trabecular number decreased, whereas trabecular separation increased in WT mice. There were no significant changes with age in bone area/tissue area or trabecular thickness. Also, there were no changes with age for any architectural endpoints in PRKO mice. However, bone area/tissue area and trabecular number were greater and trabecular separation was lower in 26-wk-old PRKO compared with age-matched WT mice.
Figure 3.
Cancellous bone architecture and dynamic histomorphometry at the proximal tibia metaphysis as a function of age in WT and PRKO mice. Values are mean ± se. a, Significantly different from WT at 12 wk; b, significantly different from PRKO at 12 wk; *, significantly different from age-matched WT; P < 0.01.
Dynamic histomorphometry was also performed on tibia from 12- and 26-wk-old mice to determine whether changes in bone formation contributed to the age-related changes in bone architecture in WT mice. Indices of bone formation, which consisted of mineralizing perimeter/bone perimeter (Fig. 3E), mineral apposition rate (Fig. 3F), bone formation rate/bone perimeter (Fig. 3G), and bone formation rate/tissue area (Fig. 3H) did not differ in WT mice between 12 and 26 wk of age. During the same time interval, mineralizing perimeter/bone perimeter increased by 83% and bone formation rate/tissue area increased by 121% in PRKO mice. Mineral apposition rate and bone formation rate/bone perimeter did not change with age in PRKO mice. At 26 wk of age, mineralizing perimeter/bone perimeter and bone formation rate/tissue area was greater in PRKO compared with WT mice.
To assess possible differences in osteoclasts at the cancellous bone surface between WT and PRKO mice, sections from proximal tibial epiphysis and metaphysis of 12- and 26-wk-old mice were stained for TRAP activity. At 12 wk of age, no differences in the frequency of TRAP+ mono- or multinucleated cells were observed between the genotypes (data not shown) in either the epiphysis or metaphysis. However, at 26 wk, there was a tendency for PRKO mice to have a lower osteoclast perimeter/bone perimeter (−22%, P = 0.1) and a lower osteoclast perimeter/tissue area (−39%, P = 0.1) compared with WT mice.
Cortical bone histomorphometry: tibial diaphysis
There was a significant increase in tibia cross-sectional area between 6 and 26 wk of age in PRKO but not WT mice (Table 1). Progressive increases in cortical bone area were observed for both genotypes between 6 and 12 and 12 and 26 wk of age. In contrast, there were no age-related changes in medullary area for either genotype.
Table 1.
Histological analysis of cortical bone at the tibia-fibula synostosis in WT and PRKO mice at 6, 12, and 26 wk of age
| Endpoint | 6 wk old
|
12 wk old
|
26 wk old
|
ANOVA, P | |||
|---|---|---|---|---|---|---|---|
| WT | PRKO | WT | PRKO | WT | PRKO | ||
| Cross-sectional area (mm2) | 0.82 ± 0.03 | 0.83 ± 0.02 | 0.87 ± 0.03 | 0.88 ± 0.03 | 0.94 ± 0.04 | 1.00 ± 0.04b | <0.010 |
| Cortical area (mm2) | 0.49 ± 0.01 | 0.50 ± 0.03 | 0.58 ± 0.02a | 0.59 ± 0.02b | 0.68 ± 0.03a,c | 0.74 ± 0.02b,d | <0.0001 |
| Marrow area (mm2) | 0.32 ± 0.02 | 0.33 ± 0.01 | 0.30 ± 0.01 | 0.28 ± 0.02 | 0.27 ± 0.01 | 0.26 ± 0.02 | <0.164 |
Data are mean ± se.
Significantly different from WT at 6 wk; P < 0.01.
Significantly different from PRKO at 6 wk; P < 0.01.
Significantly different from WT at 12 wk; P < 0.01.
Significantly different from PRKO at 12 wk; P < 0.01.
μCT: humerus
A comparison of three-dimensional bone architecture of the humerus of 12-wk-old WT and PRKO mice is shown in Table 2. PRKO mice had 14% higher total humeral bone volume than WT mice. Cancellous bone volume at the epiphysis was 20% higher in PRKO compared with WT mice. The higher cancellous volume in the distal humerus was due to greater trabecular thickness. Significant differences in trabecular number and trabecular separation were not detected with genotype. Cortical bone volume at the midshaft was 21% higher in PRKO compared with WT mice, and there was a tendency (P = 0.09) for cross-sectional volume to be higher in PRKO compared with WT mice.
Table 2.
μCT analysis of humeri from WT and PRKO mice
| Endpoint | WT | PRKO | P |
|---|---|---|---|
| Total humerus (cortical + cancellous bone) | |||
| Bone volume (mm3) | 8.50 ± 0.24 | 9.65 ± 0.34 | <0.014 |
| Distal humerus epiphysis (cancellous bone) | |||
| Bone volume/tissue volume (%) | 31.0 ± 1.0 | 37.2 ± 1.7 | <0.005 |
| Trabecular number (mm−1) | 12.2 ± 0.2 | 11.9 ± 0.2 | <0.343 |
| Trabecular thickness (μm) | 55 ± 2 | 62 ± 2 | <0.013 |
| Trabecular separation (μm) | 97 ± 2 | 99 ± 2 | <0.636 |
| Midshaft humerus (cortical bone) | |||
| Cross-sectional volume (mm3) | 0.19 ± 0.005 | 0.21 ± 0.009 | <0.088 |
| Cortical volume (mm3) | 0.12 ± 0.003 | 0.14 ± 0.005 | <0.002 |
| Marrow volume (mm3) | 0.07 ± 0.003 | 0.07 ± 0.003 | <0.665 |
Compared with 12-wk-old WT mice, age-matched PRKO mice had higher total, cancellous, and cortical bone mass in the humerus. Data are mean ± se.
Discussion
In the PRKO animals, both A and B isoforms of PR are ablated, and consequently the homozygous mutant mice cannot signal via PR. Our histomorphometric analysis of the tibia from intact female PRKO mice shows that the loss of both PR-A and PR-B function has age- and compartment-specific effects on the skeleton. There was virtually no effect of PR knockout on longitudinal bone growth. However, at 12 wk of age, the PRKO mice did exhibit higher humeral cortical and cancellous bone volume compared with age-matched WT mice. At skeletal maturity, cancellous bone area/tissue area and tissue level bone formation were increased in the proximal tibia metaphysis in PRKO compared with WT mice.
Physiological concentrations of progesterone, when administered to osteopenic OVX rats, have been reported in some studies to produce a weak anabolic effect on cortical bone mass with no changes in resorption (10). At pharmacological concentrations, progestagens administered to OVX mice increase periosteal, but not endocortical, bone formation and may also reduce endocortical resorption (12). In the rat, progesterone has been reported to enhance the bone-preserving effects of estrogen by augmenting its suppression of bone resorption (27). However, none of these studies address whether signaling through PR is essential in normal bone growth and bone turnover.
The increased cortical bone mass observed in PRKO mice in the present study suggests that PR signaling may slightly antagonize radial bone growth during puberty, contributing to the sexual dimorphism of the skeleton. Our results in PRKO mice are qualitatively similar but smaller in magnitude than changes observed in OVX rats. In the rat tibia, reduced gonadal hormone levels are associated with substantial increases in periosteal bone formation and cortical bone area (28). The cortical bone changes in the 12-wk-old PRKO mice are also reminiscent of those in postpubertal estrogen receptor-β (ERβ) KO female mice (29), suggesting that estrogen signaling via ERβ and progesterone signaling via PR act cooperatively to reduce radial bone growth in females.
The cancellous bone volume, bone formation indices, and trabecular architecture were largely unaffected at the proximal tibial epiphysis by PR gene deletion. This indicates that, in ovary-intact animals, PR signaling is not required at this location for cancellous bone growth and turnover. The circulating concentrations of progesterone, estrogen, and FSH are within the normal range in PRKO mice (30). Also, uterine weight, a sensitive indicator of gonadal hormone status, showed parallel increases with age in the two genotypes. However, we cannot be certain there are no changes in the levels of other hormones that may exert compensatory effects on bone growth and turnover. For example, PRKO mice have elevated levels of prolactin and LH (30).
Our failure to detect age- and genotype-related changes in cancellous bone area in the proximal tibial epiphysis contrasts with the changes observed in the proximal tibial metaphysis. In the metaphysis, trabecular number decreased precipitously between 12 and 26 wk of age in WT but not in PRKO mice. This finding is in agreement with previous analysis of the effects of age on cancellous bone architecture in long bones of various strains of mice (31). Similarly, OVX in rats results in preferential loss of cancellous bone from the metaphysis but not the epiphysis (32). In rats, this differential response of the cancellous bone compartments to gonadal hormone insufficiency was attributed to the higher strain energy levels in the epiphysis, and the same mechanism may apply to mice.
No genotype-related differences in the microarchitecture of the epiphysis of skeletally mature mice were noted between WT and PRKO mice. However, the bone formation rate increased in both genotypes between 12 and 26 wk of age. As described below, a similar age-related increase in bone formation occurred at proximal tibial metaphysis in PRKO but not WT mice.
Compared with age-matched WT mice, bone formation in the proximal tibial metaphysis was increased in 26-wk-old PRKO mice. In addition, the PRKO mice showed a tendency for decreased osteoclast number. Taken together, these data indicate that PR signaling in WT mice prevents the compensatory increase in bone formation required to maintain cancellous bone architecture in adult mice.
Interestingly, disturbances in uterine growth/physiology only became evident in the PRKO female mice after OVX and hormonal stimulation (23), and therefore it would be of interest to determine whether the skeletal response of OVX PRKO mice to estrogen is impaired relative to OVX WT mice. This approach would address the possible involvement of PR-mediated secondary responses in the effects of estrogen on bone. In addition, progesterone may play a role in the regulation of calcium homeostasis and bone turnover during pregnancy and lactation, when progesterone levels are elevated. Pregnancy is associated with a positive calcium balance and increased cancellous bone volume, whereas it has been shown that lactation in rats and humans can lead to a net loss of calcium from bone, increased turnover, and cancellous bone loss (33,34). Unfortunately, we cannot use this model to assess the role of progesterone in calcium homeostasis and bone turnover during pregnancy, because PRKO animals are infertile.
Progesterone has been reported to stimulate markers of bone formation in fetal rat calvarial organ culture and in a model of bone matrix-induced endochondral bone formation (35,36). In contrast, the steroid had minimal effects, either alone or in combination with estradiol, on mature osteoblast-like cells cultured in vitro (37). However, a modest increase in cell proliferation and differentiation has been observed in vitro by some investigators (38,39,40). The differences observed between in vivo and in vitro model systems and those observed among different in vitro models suggest that PR signaling interacts with other regulatory pathways and that some of these pathways may be absent from some of the in vitro systems.
The extent of progesterone’s effect on bone may not be fully appreciated in the present analysis of PRKO mice. It is now known that human PR exists in two isoforms, A and B, which are generated from a single gene through alternate promoters, both of which are estrogen inducible (41). The PR-A isoform is transcriptionally less active than PR-B and under certain circumstances can inhibit the transcriptional activity of PR-B as well as the estrogen and mineralocorticoid receptors (42,43,44). Progesterone exhibited antiestrogenic growth-inhibitory activity on uterine epithelia in WT mice due to PR-A-mediated suppression of both ER and PR-B activity. Selective ablation of the PR-A isoform (PRAKO mice) was demonstrated to enhance the responsiveness of uterine epithelium to progesterone (45). In the PRAKO mouse, progesterone alone (through PR-B) increased uterine cell proliferation and enhanced the stimulatory effect of estrogen. Consequently, and in an analogous manner, the response of bone in vivo, and of osteoblasts in vitro, to progesterone may be altered by null mutation of PR-A. The progesterone response in bone may thus be impaired by PR-A, as it is in the uterus. In support of this possibility, osteoblasts in vitro express the A and B isoforms of PR at comparable levels, and both isoforms are induced by estrogen treatment (22). An analysis of the skeleton of PRAKO mice is therefore necessary to elucidate the relative roles of PR-A and -B in progesterone action on bone.
The present studies are directed toward the role of PR signaling in growing and young adult mice. It will be useful to perform studies in aged PRKO mice to investigate the possible role of the PR in the etiology of senile and/or postmenopausal osteoporosis.
In summary, gross abnormalities in bone growth and turnover were not observed between female WT and PRKO mice. However, ablation of the PR had effects on bone growth and turnover at selective skeletal sites. Specifically, PR signaling reduced accumulation of cortical and cancellous bone before peak bone mass.
Footnotes
This work was supported by National Institutes of Health (NIH) Grant AG04875 (to T.C.S. and S.K.), NIH Grant AA011140 (to R.T.T.), and NIH Training Grant DK07352 (to D.J.R.).
Current address for D.J.R.: Musculoskeletal Diseases Biology, GlaxoSmithKline, 1250 S. Collegeville Road, Collegeville, Pennsylvania 19426.
Current address for K.M.W.: Computational Biology and Bioinformatics, Pacific Northwest National Laboratory, PO Box 999 MS K7-90, Richland, Washington 99352.
Disclosure Statement: The authors have nothing to disclose.
First Published Online February 14, 2008
Abbreviations: ER-β, Estrogen receptor-β; μCT, microcomputed tomography; KO, knockout; MPA, medroxyprogesterone acetate; OVX, ovariectomized; PR, progesterone receptor; TRAP, tartrate-resistant acid phosphatase; WT, wild type.
References
- Prior JC 1990 Progesterone as a bone-trophic hormone. Endocr Rev 11:386–398 [DOI] [PubMed] [Google Scholar]
- Gallagher JC, Kable WT, Goldgar D 1991 Effect of progestin therapy on cortical and trabecular bone: comparison with estrogen. Am J Med 90:171–178 [PubMed] [Google Scholar]
- Lindsay R, Hart DM, Purdie D, Ferguson MM, Clark AS, Kraszewski A 1978 Comparative effects of oestrogen and a progestogen on bone loss in postmenopausal women. Clin Sci Mol Med 54:193–195 [DOI] [PubMed] [Google Scholar]
- Lobo RA, McCormick W, Singer F, Roy S 1984 Depo-medroxyprogesterone acetate compared with conjugated estrogens for the treatment of postmenopausal women. Obstet Gynecol 63:1–5 [PubMed] [Google Scholar]
- Gallagher J 1981 Biochemical effects of estrogen and progesterone on calcium metabolism. In: DeLuca H, Parfitt A, Jee W, Johnston C, eds. Recent advances in osteoporosis. Baltimore: University Park Press; 215–224 [Google Scholar]
- Prior JC, Vigna YM, Barr SI, Rexworthy C, Lentle BC 1994 Cyclic medroxyprogesterone treatment increases bone density: a controlled trial in active women with menstrual cycle disturbances. Am J Med 96:521–530 [DOI] [PubMed] [Google Scholar]
- Albertazzi P, Bottazzi M, Steel SA 2006 Bone mineral density and depot medroxyprogesterone acetate. Contraception 73:577–583 [DOI] [PubMed] [Google Scholar]
- Ishida Y, Ishida Y, Heersche JN 2002 Pharmacologic doses of medroxyprogesterone may cause bone loss through glucocorticoid activity: an hypothesis. Osteoporos Int 13:601–605 [DOI] [PubMed] [Google Scholar]
- Bowman BM, Miller SC 1996 Elevated progesterone during pseudopregnancy may prevent bone loss associated with low estrogen. J Bone Miner Res 11:15–21 [DOI] [PubMed] [Google Scholar]
- Barengolts EI, Gajardo HF, Rosol TJ, D’Anza JJ, Pena M, Botsis J, Kukreja SC 1990 Effects of progesterone on postovariectomy bone loss in aged rats. J Bone Miner Res 5:1143–1147 [DOI] [PubMed] [Google Scholar]
- Snow GR, Anderson C 1985 The effects of continuous progestogen treatment on cortical bone remodeling activity in beagles. Calcif Tissue Int 37:282–286 [DOI] [PubMed] [Google Scholar]
- Bain SD, Jensen E, Celino DL, Bailey MC, Lantry MM, Edwards MW 1993 High-dose gestagens modulate bone resorption and formation and enhance estrogen-induced endosteal bone formation in the ovariectomized mouse. J Bone Miner Res 8:219–230 [DOI] [PubMed] [Google Scholar]
- Barbagallo M, Carbognani A, Palummeri E, Chiavarini M, Pedrazzoni M, Bracchi PG, Passeri M 1989 The comparative effect of ovarian hormone administration on bone mineral status in oophorectomized rats. Bone 10:113–116 [DOI] [PubMed] [Google Scholar]
- Fujimaki T, Kurabayashi T, Yamamoto Y, Yasuda M, Tojo Y, Yahata T, Tanaka K 1995 Effects of progesterone on the metabolism of cancellous bone in young oophorectomized rats. J Obstet Gynaecol 21:31–36 [DOI] [PubMed] [Google Scholar]
- Kalu DN, Salerno E, Liu CC, Echon R, Ray M, Garza-Zapata M, Hollis BW 1991 A comparative study of the actions of tamoxifen, estrogen and progesterone in the ovariectomized rat. Bone Miner 15:109–123 [DOI] [PubMed] [Google Scholar]
- Barengolts EI, Lathon PV, Lindh FG 1995 Progesterone antagonist RU 486 has bone-sparing effects in ovariectomized rats. Bone 17:21–25 [DOI] [PubMed] [Google Scholar]
- De Souza MJ, Miller BE, Sequenzia LC, Luciano AA, Ulreich S, Stier S, Prestwood K, Lasley BL 1997 Bone health is not affected by luteal phase abnormalities and decreased ovarian progesterone production in female runners. J Clin Endocrinol Metab 82:2867–2876 [DOI] [PubMed] [Google Scholar]
- PEPI 1996 Effects of hormone therapy on bone mineral density: results from the postmenopausal estrogen/progestin interventions (PEPI) trial. The Writing Group for the PEPI. JAMA 276:1389–1396 [PubMed] [Google Scholar]
- Conneely OM 2001 Perspective: female steroid hormone action. Endocrinology 142:2194–2199 [DOI] [PubMed] [Google Scholar]
- Eriksen EF, Colvard DS, Berg NJ, Graham ML, Mann KG, Spelsberg TC, Riggs BL 1988 Evidence of estrogen receptors in normal human osteoblast-like cells. Science 241:84–86 [DOI] [PubMed] [Google Scholar]
- Wei LL, Leach MW, Miner RS, Demers LM 1993 Evidence for progesterone receptors in human osteoblast-like cells. Biochem Biophys Res Commun 195:525–532 [DOI] [PubMed] [Google Scholar]
- MacNamara P, O’Shaughnessy C, Manduca P, Loughrey HC 1995 Progesterone receptors are expressed in human osteoblast-like cell lines and in primary human osteoblast cultures. Calcif Tissue Int 57:436–441 [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery Jr CA, Shyamala G, Conneely OM, O’Malley BW 1995 Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266–2278 [DOI] [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR 1987 Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610 [DOI] [PubMed] [Google Scholar]
- Parfitt AM, Mathews CH, Villanueva AR, Kleerekoper M, Frame B, Rao DS 1983 Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis. Implications for the microanatomic and cellular mechanisms of bone loss. J Clin Invest 72:1396–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen JS, Laib A, Koller B, Prohaska S, Mosekilde L, Gowin W 2005 Stereological measures of trabecular bone structure: comparison of 3D micro computed tomography with 2D histological sections in human proximal tibial bone biopsies. J Microsc 218:171–179 [DOI] [PubMed] [Google Scholar]
- Schmidt IU, Wakley GK, Turner RT 2000 Effects of estrogen and progesterone on tibia histomorphometry in growing rats. Calcif Tissue Int 67:47–52 [DOI] [PubMed] [Google Scholar]
- Turner RT, Backup P, Sherman PJ, Hill E, Evans GL, Spelsberg TC 1992 Mechanism of action of estrogen on intramembranous bone formation: regulation of osteoblast differentiation and activity. Endocrinology 131:883–889 [DOI] [PubMed] [Google Scholar]
- Windahl SH, Vidal O, Andersson G, Gustafsson JA, Ohlsson C 1999 Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERβ−/− mice. J Clin Invest 104:895–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell PE, Lydon JP, Conneely OM, O’Malley BW, Levine JE 1997 Endocrine defects in mice carrying a null mutation for the progesterone receptor gene. Endocrinology 138:4147–4152 [DOI] [PubMed] [Google Scholar]
- Iwaniec UT, Yuan D, Power RA, Wronski TJ 2006 Strain-dependent variations in the response of cancellous bone to ovariectomy in mice. J Bone Miner Res 21:1068–1074 [DOI] [PubMed] [Google Scholar]
- Westerlind KC, Wronski TJ, Ritman EL, Luo ZP, An KN, Bell NH, Turner RT 1997 Estrogen regulates the rate of bone turnover but bone balance in ovariectomized rats is modulated by prevailing mechanical strain. Proc Natl Acad Sci USA 94:4199–4204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent GN, Price RI, Gutteridge DH, Smith M, Allen JR, Bhagat CI, Barnes MP, Hickling CJ, Retallack RW, Wilson SG, Devlin RD, Davies C, St. John A 1990 Human lactation: forearm trabecular bone loss, increased bone turnover, and renal conservation of calcium and inorganic phosphate with recovery of bone mass following weaning. J Bone Miner Res 5:361–369 [DOI] [PubMed] [Google Scholar]
- Miller SC, Shupe JG, Redd EH, Miller MA, Omura TH 1986 Changes in bone mineral and bone formation rates during pregnancy and lactation in rats. Bone 7:283–287 [DOI] [PubMed] [Google Scholar]
- Burnett CC, Reddi AH 1983 Influence of estrogen and progesterone on matrix-induced endochondral bone formation. Calcif Tissue Int 35:609–614 [DOI] [PubMed] [Google Scholar]
- Manzi DL, Pilbeam CC, Raisz LG 1994 The anabolic effects of progesterone on fetal rat calvaria in tissue culture. J Soc Gynecol Investig 1:302–309 [DOI] [PubMed] [Google Scholar]
- Slootweg MC, Ederveen AG, Schot LP, Schoonen WG, Kloosterboer HJ 1992 Oestrogen and progestogen synergistically stimulate human and rat osteoblast proliferation. J Endocrinol 133:R5–R8 [DOI] [PubMed] [Google Scholar]
- Ishida Y, Heersche JN 1999 Progesterone- and dexamethasone-dependent osteoprogenitors in bone cell populations derived from rat vertebrae are different and distinct. Endocrinology 140:3210–3218 [DOI] [PubMed] [Google Scholar]
- Tremollieres FA, Strong DD, Baylink DJ, Mohan S 1992 Progesterone and promegestone stimulate human bone cell proliferation and insulin-like growth factor-2 production. Acta Endocrinol (Copenh) 126:329–337 [DOI] [PubMed] [Google Scholar]
- Verhaar HJ, Damen CA, Duursma SA, Scheven BA 1994 A comparison of the action of progestins and estrogen on the growth and differentiation of normal adult human osteoblast-like cells in vitro. Bone 15:307–311 [DOI] [PubMed] [Google Scholar]
- Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P 1990 Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J 9:1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus WL, Weis KE, Katzenellenbogen BS 1995 Inhibitory cross-talk between steroid hormone receptors: differential targeting of estrogen receptor in the repression of its transcriptional activity by agonist-and antagonist-occupied progestin receptors. Mol Cell Biol 15:1847–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung L, Mohamed MK, Hoeffler JP, Takimoto GS, Horwitz KB 1993 Antagonist-occupied human progesterone B-receptors activate transcription without binding to progesterone response elements and are dominantly inhibited by A-receptors. Mol Endocrinol 7:1256–1265 [DOI] [PubMed] [Google Scholar]
- Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O’Malley BW, McDonnell DP 1993 Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol 7:1244–1255 [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM 2000 Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science 289:1751–1754 [DOI] [PubMed] [Google Scholar]