Abstract
Changes in the oxidant/antioxidant environment of aging Leydig cells have been shown to be correlated with the reduced ability of these cells to produce testosterone. With this in mind, we hypothesized that the experimental depletion of glutathione (GSH), an abundant Leydig cell intracellular antioxidant, might result in reduced testosterone production. Incubation of Leydig cells isolated from the testes of adult Brown Norway rats with buthionine sulfoximine (BSO) reduced GSH content by more than 70% and testosterone production by about 40%. The antioxidants vitamin E, N-tert-butyl-α-phenylnitrone and Trolox countered BSO’s effect on steroidogenesis but not on GSH depletion. Together, BSO and glutathione ethyl ester maintained intracellular GSH and also testosterone production, whereas 1,2-dithiole-3-thione, which increases intracellular GSH, increased testosterone production. In vivo studies also were conducted. Young (4 month old) and old (24 month old) rats were injected with BSO twice a day for 7 d, after which Leydig cells were isolated and analyzed in vitro. BSO treatment reduced Leydig cell GSH content by 70% and the ability of the Leydig cells to produce testosterone by more than 50%. As with aging, decreases were seen in LH-stimulated cAMP production, steroidogenic acute regulatory protein, cholesterol side-chain cleavage, 3β-hydroxysteroid dehydrogenase, and 17α-hydroxylase/17,20-lyase. The results of these studies, taken together, are consistent with the hypothesis that alteration in the oxidant/antioxidant environment may play a significant, causative role in the age-related reduced ability of Leydig cells to produce testosterone.
AGING IN RODENTS and men is associated with reduced serum testosterone concentration (1,2). Testosterone is produced by the Leydig cells in the interstitial compartment of the mammalian testis. Studies of the Brown Norway rat have demonstrated that reduced steroidogenesis by individual Leydig cells, rather than loss of the cells, accounts for reduced serum levels of testosterone (3,4). Multiple defects have been identified in the steroidogenic pathway of old Leydig cells, including reductions in each of LH-stimulated cAMP production, the cholesterol transport proteins steroidogenic acute regulatory protein (StAR) and peripheral benzodiazepine receptor (also referred to as translocator protein), and downstream steroidogenic enzymes of the mitochondria and smooth endoplasmic reticulum (5,6,7,8).
Although the mechanism(s) by which age-related defects in the Leydig cell steroidogenic pathway occur remains uncertain, there is correlative evidence that changes in the oxidant/antioxidant status of the cells is involved. Thus, the antioxidant defense molecules superoxide dismutase-1 and -2, glutathione peroxidase, and glutathione (GSH) are significantly reduced as Leydig cells age (9,10). Additionally, the superoxide content of the mitochondria of aging Leydig cells is significantly increased, compared with young Leydig cells (11), as is lipid peroxidation (10). These correlative results, taken together, suggest that an alteration in the redox environment of aged Leydig cells may be involved in the reduced steroidogenesis that characterizes these cells.
GSH is a water-soluble tripeptide composed of glutamine, cysteine, and glycine. Reduced glutathione, the most abundant intracellular small molecule thiol present in mammalian cells, serves as a potent intracellular antioxidant (12,13). Intracellular biosynthesis of GSH is mediated by two ATP-dependent enzymes, γ-glutamylcysteine synthetase (the rate-limiting enzyme) and glutathione synthetase (14,15). Buthionine sulfoximine (BSO), a specific γ-glutamylcysteine synthetase inhibitor, can block the rate-limiting step of GSH biosynthesis and in doing so is able to deplete the intracellular GSH pool in both cultured cells and in whole animals (15,16). In the present study, we hypothesized that if alteration of the redox environment of aging Leydig cells indeed plays a role in age-related reductions in Leydig cell steroidogenesis, the experimental reduction of the Leydig cell intracellular GSH pool should increase oxidative stress in the cells and thus result in decreased testosterone production. To test this hypothesis, we examined the in vitro and in vivo effects of GSH depletion on Leydig cell steroidogenesis.
Materials and Methods
Chemicals
Dibutyryl cAMP (dbcAMP), l-buthionine-sulfoximine (BSO), glutathione ethyl ester (GSHEE), 25-hydroxycholesterol (25-HC), all-rac-α-tocopherol (vitamin E), N-tert-butyl-α-phenylnitrone (BPN), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), and monoclonal β-actin antibody were from Sigma (St. Louis, MO). 1,2-Dithiole-3-thione (D3T) was kindly provided by Dr. Rex Munday (Ruakura Agricultural Research Centre, Hamilton, New Zealand). 6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) was from OXIS International Inc (Portland, OR). Bovine lipoprotein was from MP Biomedicals Inc. (Solon, OH). M-199 medium was from Invitrogen (Carlsbad, CA). Type III collagenase was from Worthington (Freehold, NJ). [1,2,6,7,16,17-3H(N)]testosterone (115.3 Ci/mmol) was from PerkinElmer Life Sciences, Inc. (Boston, MA). Testosterone antibody was from ICN (Costa Mesa, CA). StAR antibody was from ABR, Inc. (Golden, CO). P450 side-chain cleavage (P450scc) antibody was from Chemicon International (Temecula, CA). The horseradish peroxidase-conjugated antirabbit donkey IgG, enhanced chemiluminescence Western blot detection, and [3H]cAMP assay kits were from Amersham Pharmacia Biotech (Piscataway, NJ). Bovine LH (USDA-bLH-B-6) was provided by the U.S. Department of Agriculture Animal Hormone Program (Beltsville, MD). All steroids, including pregnenolone, progesterone, 17-hydroxyprogesterone, androstenedione, and testosterone were from Steraloids (Newport, RI).
Animals
Young (4 months old) and aged (24 months old) male Brown Norway rats were obtained through the National Institute on Aging, supplied by Harlan Sprague Dawley Inc. (Indianapolis, IN). Rats were housed in the animal facilities of the Johns Hopkins Medical Institutions, under conditions of controlled light (14 h light, 10 h dark) and temperature (22 C) and with free access to rat chow and water. All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, according to protocols approved by the Johns Hopkins Animal Care and Use Committee.
Effects of BSO, GSHEE, and D3T on Leydig cell steroidogenesis in vitro
Leydig cells were isolated from 4-month-old rats (see below) and cultured in M-199 medium supplemented with 2.2 g/liter NaHCO3, 2.4 g/liter HEPES, 0.1% BSA, 0.25 g/liter bovine lipoprotein, and 25 mg/liter gentamicin (pH 7.4) for 48 h. The cells were maintained at 34 C in 5% CO2. BSO (0–100 μm) or D3T (100 μm) was added to the medium. Some of the cells incubated with 100 μm BSO also were incubated with GSHEE (4 mm) or the antioxidants vitamin E (all-rac-α-tocopherol; 2.5–40 μg/ml), BPN (1 mm), or Trolox (100 μm). At the end of the 48-h culture period, the cells were incubated in fresh media with maximally stimulating bovine LH (100 ng/ml) for 30 min (for cAMP production measurements) or 2 h (for testosterone production measurements). For testosterone measurements, media were collected at the end of treatment and stored at −80 C. Thawed samples were assayed for testosterone by RIA. The sensitivity of the RIA was 13 pg/tube, and the intraassay and interassay coefficients of variation were 8.9 and 13.6%, respectively. The cells were used to measured GSH content (see below).
Effects of BSO on Leydig cell steroidogenesis in vivo
Twenty-four animals were randomly divided into four groups of six animals each: young control, young treated, old control, and old treated. Treatment consisted of ip injections of the glutathione synthesis inhibitor BSO at a dosage of 2 mmol/kg body weight at 12-h intervals for 7 d. Control groups received injections of the vehicle (PBS). Testes were collected from each rat 2 h after the last injection. Leydig cells were isolated from the testes of each animal (see below) and the abilities of the cells to produce cAMP and testosterone were assessed in vitro. The purified cells (2 × 105 per 200 μl) were incubated in 96-well Falcon culture plates with LH (100 ng/ml), dbcAMP (2 mm), or 25-HC (12.5 μm) for 2 h. The media were collected for testosterone analysis. To assay the activities of the steroidogenic enzymes 3β-hydroxysteroid dehydrogenase (3β-HSD), 17α-hydroxylase/17,20-lyase (P450c17), and 17β-hydroxysteroid dehydrogenase (17β-HSD), the cells were incubated with pregnenolone (12.5 μm), progesterone (12.5 μm), or androstenedione (6.25 μm), their respective substrates, for 2 h, and all Δ4–3-ketosteroids were quantified by HPLC as reported previously (6,17). For example, to assay 3β-HSD activity, pregnenolone was supplied to the cells, and progesterone, 17α-hydroxyprogesterone, androstenedione, and testosterone were quantified by HPLC and added together as a measure of the ability of 3β-HSD to convert pregnenolone to progesterone. To assay the activity of P450c17, the cells were incubated with progesterone, and androstenedione and testosterone were quantified and added together. To assay 17β-HSD activity, the cells were incubated with androstenedione, and testosterone was quantified. Some of the cells were frozen in liquid nitrogen immediately after purification to assay their GSH content (see below).
Leydig cell isolation
Leydig cells were isolated from rat testes as previously described (18). In brief, the testicular artery was cannulated and testes were perfused with type III collagenase (1 mg/ml) in dissociation buffer [M-199 medium with 2.2 g/liter HEPES, 1.0 g/liter BSA, 25 mg/liter trypsin inhibitor, 0.7 g/liter sodium bicarbonate (pH 7.4)] to clear testicular blood. Testes then were decapsulated and digested in collagenase (0.25 mg/ml, 34 C) with slow shaking (90 cycles/min, 30 min). For in vitro experiments, the dissociated cells were purified by centrifugal elutriation and Percoll gradient centrifugation (18). The final purity of the Leydig cells, determined by staining the cells for 3β-HSD activity (19), was consistently about 95%. For in vivo studies in which animals received either BSO or PBS treatments, the dissociated cells were purified by Percoll gradient centrifugation without the centrifugal elutriation step. The final purity of the Leydig cells isolated this way consistently was about 80%.
cAMP production
For in vitro studies, purified Leydig cells were first treated with BSO for 48 h. The cells then were incubated with LH (100 ng/ml) in phenol red-free M-199 medium (2 × 105 cells per 50 μl) for 30 min to assay cAMP production (5). For in vivo studies, Leydig cells isolated from BSO-treated and control animals were preincubated in M-199 medium for 2 h and then were stimulated with LH (100 ng/ml) in phenol red-free M-199 medium (2 × 105 cells per 50 μl) for 30 min before assay for cAMP production. The medium in which the cells were incubated contained isobutyl-methylxanthine (100 μm) to inhibit phosphodiesterase activity. At the end of the incubation, 50 μl Tris buffer [0.05 m (pH 7.5)] containing 4 mm EDTA and 2 mg/ml theophylline were added to the culture medium to block cAMP degradation. The plates were frozen on dry ice and stored at −80 C before cAMP assays. cAMP levels were measured using a [3H]cAMP assay kit (Amersham, Piscataway, NJ) according to the manufacturer’s directions. The sensitivity of the assay was 0.05 pmol per assay tube.
GSH assay
GSH was measured as previously described (20). In brief, Leydig cells were suspended in sodium phosphate buffer [0.1 m (pH 8.0), with 5 mm EDTA] and sonicated. Protein was precipitated in 5% metaphosphoric acid and then centrifuged at 13,000 × g for 30 min to obtain the supernatant. The supernatant was diluted 10 times with sodium phosphate buffer. Diluted sample (10 μl) was incubated with 10 μl of o-phthalaldehyde (in methanol) and 180 μl of phosphate buffer for 15 min at room temperature. Fluorescence was read with a VersaFluor fluorometer (Bio-Rad laboratories, Hercules, CA) at an excitation wavelength of 350 nm and an emission wavelength of 420 nm. The cellular GSH content was calculated using a concurrently run GSH standard curve.
Western blot analysis
Freshly isolated Leydig cells from PBS- or BSO-treated rats were lysed with Tris-sodium dodecyl sulfate (SDS) buffer [62.5 mm Tris, 2% SDS, 50 mm dithiothreitol (pH 6.8)] and sonicated on ice. Lysates were mixed with 3× SDS loading buffer (New England BioLab, Ipswich, MA). Total proteins from equal numbers of cells (2 × 105) were separated by 10% SDS-PAGE and then transferred onto a nitrocellulose membrane. After incubation with primary antibody (1:400) and horseradish peroxidase-conjugated secondary antibody (1:5000), the signals were detected by the enhanced chemiluminescence Western blot kit from Amersham Pharmacia Biotech. The bound antibodies on the membranes were stripped by Restore Western blot stripping buffer (Pierce, Rockford, IL), and the membranes were reprobed with new antibodies in the sequence of StAR, P450scc, and β-actin. The proteins were quantified by densitometry scanning of the film and processed by National Institutes of Health Image software (Bethesda, MD). The total density of the proteins in each membrane was first corrected by β-actin and then normalized to the control sample, usually young untreated cells, in the same membrane. At least three pools of cells that came from different animals were analyzed for each treatment.
MTT assay
Cell viability was estimated using the MTT method (21). This assay measures the reduction of MTT (a yellow tetrazolium salt) to blue formazan in viable cells. Leydig cells (2 × 105) treated in vitro with BSO or freshly isolated from BSO-treated animals were incubated with 100 μl fresh medium containing 0.5 mg/ml MTT. After 1 h, the medium was removed and the reduced formazan was dissolved in 100 μl acidified (0.04 n HCl) isopropanol at room temperature for 20 min. The dissolved formazan was then removed to wells in a new 96-well plate and read in DTX800 Multimode Detector (Beckman, Coulter, Inc., Fullterton, CA) at 562-nm wavelength. Control (blank) wells contained isopropanol only. Three to six pools of cells from different animals were analyzed for each treatment.
Statistical analyses
Data are expressed as the mean ± sem. Group means were evaluated by one-way ANOVA. If group differences were revealed by ANOVA (P < 0.05), differences between individual groups were determined with the Student-Neuman-Kuels test, using SigmaStat software (Systat Software Inc., Richmond, CA). Values were considered significant at P < 0.05.
Results
Effects of reduced or increased GSH on Leydig cell steroidogenesis in vitro
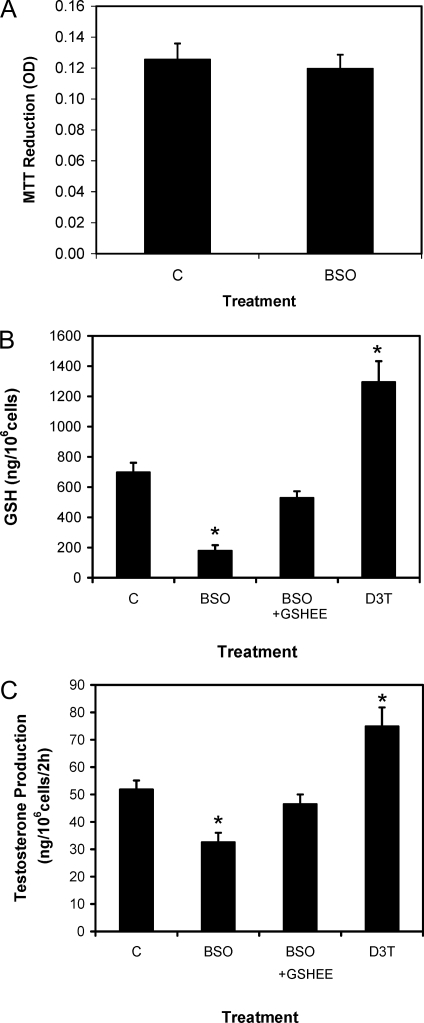
We first examined whether BSO treatment affects cell viability. To this end, Leydig cells were incubated with 0.5 mg/ml MTT for 1 h after prior incubation with BSO for 48 h. As seen in Fig. 1A, the ability of the cells to reduce MTT was not significantly affected by BSO treatment, indicating that in vitro BSO treatment did not affect cell viability. We next determined the extent to which culturing cells with BSO affects intracellular GSH levels. As shown in Fig. 1B, culturing young adult Leydig cells with BSO reduced intracellular GSH significantly, by more than 70%, compared with controls. Coincubation of the cells with GSHEE along with BSO maintained GSH levels in the cells. Incubation of the cells with the γ-glutamylcysteine synthetase-inducing agent D3T significantly increased intracellular GSH content. Figure 1C shows the effects of BSO, BSO plus GSHEE, and D3T on Leydig cell testosterone production. For this study, Leydig cells isolated from young adult rat testes were incubated in vitro with BSO, BSO plus GSHEE, or D3T for 48 h and then with maximally stimulating LH for 2 h. Testosterone was assayed in the media. The ability of cells incubated with BSO to produce testosterone was significantly reduced, by about 40%, compared with control cells. Incubation of the cells with GSHEE along with BSO prevented the BSO-induced reduction in testosterone production. Incubation of the cells with D3T significantly increased testosterone production, compared with control cells.
Figure 1.
Cell viability (A), GSH content (B), and testosterone production (C) assays using Leydig cells isolated from 4-month-old rats and cultured without (C) or with GSH modulating reagents (BSO, 100 μm; BSO plus GSHEE, 4 mm; or D3T, 100 μm) for 48 h. Control cells (C) were cultured with vehicle (0.1% alcohol) only. Cell viability was determined by measuring the ability of the cells to reduce MTT (0.5 mg/ml) for 1 h after an initial 48-h culture period. The ability of the cells to produce testosterone was determined after their incubation with maximally stimulating LH (100 ng/ml) for 2 h after the initial 48-h culture period. The data are expressed as mean ± sem of three experiments. *, Significantly different from control (C) cells at P < 0.05.
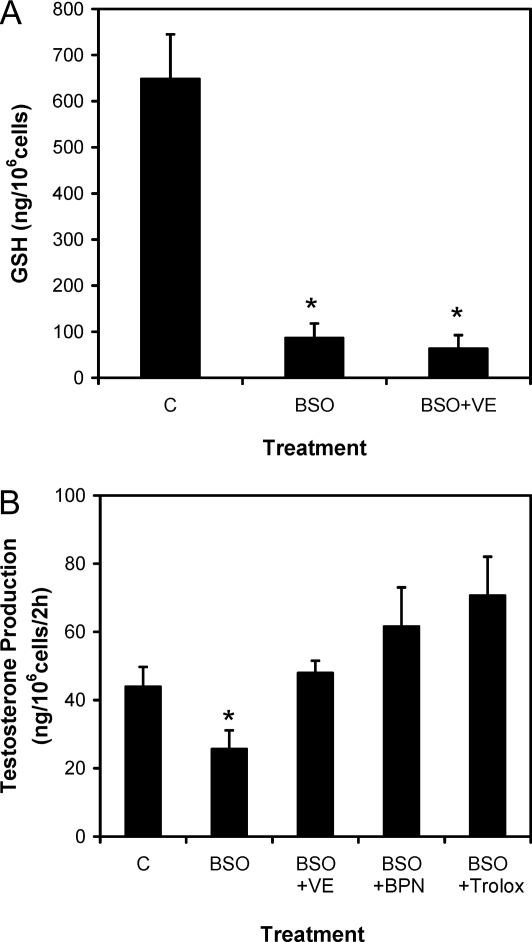
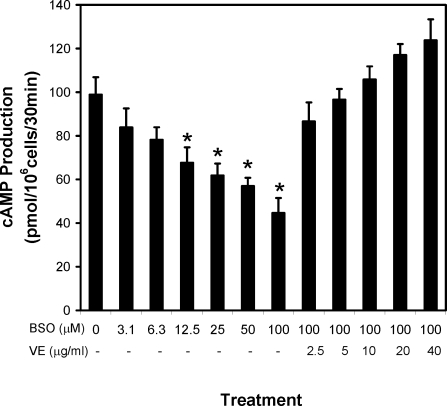
A series of studies was conducted to examine whether the inclusion of antioxidants in the culture medium would prevent the effect of BSO on Leydig cell steroidogenesis. Although vitamin E did not prevent the decrease in intracellular GSH concentration resulting from BSO treatment (Fig. 2A), its inclusion in the culture medium did prevent the BSO-induced reduction in testosterone production (Fig. 2B). The inclusion of the antioxidants BPN or Trolox in the culture medium also prevented the BSO-induced reduction in testosterone production (Fig. 2B). The effect of BSO treatment on the LH receptor-adenylyl cyclase cascade was examined by assessing the ability of the cells to produce cAMP after their incubation with increasing concentrations of BSO for 48 h and then with LH for 30 min. As shown in Fig. 3, BSO treatment decreased cAMP production in a dose-dependent manner. Coincubation of the cells with BSO and vitamin E (2.5–40 μg/ml) prevented the suppressive effect of BSO on cAMP production.
Figure 2.
GSH content (A) and testosterone production (B) by Leydig cells isolated from 4-month-old rats and cultured without (C) or with BSO (100 μm) or BSO plus the antioxidants vitamin E (VE; 40 μg/ml), BPN (1 mm), or Trolox (100 μm) (B). Control cells (C) were cultured with vehicle (0.1% alcohol) only. The ability of the cells to produce testosterone was determined after their incubation with maximally stimulating LH (100 ng/ml) for 2 h after the initial 48-h culture period. The data are expressed as mean ± sem of four experiments. *, Significantly different from control (C) cells at P < 0.05.
Figure 3.
Leydig cell cAMP production in vitro. Leydig cells isolated from the testes of 4-month-old rats were cultured without (0) or with BSO (3.1–100 μm) in vitro for 48 h. Some of the cells were cultured with 100 μm BSO and, additionally, the antioxidant vitamin E (VE; 2.5–40 μg/ml). The ability of the cells to produce cAMP was determined after their incubation with maximally stimulating LH (100 ng/ml) for 30 min after the initial 48-h culture period. The data are expressed as mean ± sem of four experiments. *, Significantly different from cells cultured without BSO at P < 0.05.
Effects of reduced GSH on Leydig cell steroidogenesis in vivo
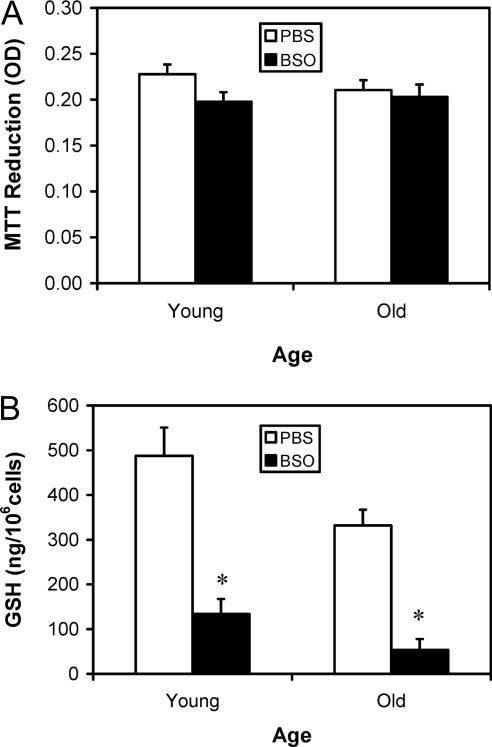
Having demonstrated that incubation of Leydig cells with BSO resulted in reduced intracellular GSH and testosterone production without affecting cell viability, we conducted a study to determine whether in vivo BSO treatment of rats also would result in reduced Leydig cell GSH and testosterone production without affecting cell viability. To this end, young and old rats were treated with BSO at a dose of 2 mmol/kg body weight at 12-h intervals for 7 d. Control rats received vehicle (PBS) treatment. Leydig cells were isolated from the testes 2 h after the last BSO administration. To determine whether BSO treatment of the rats affected Leydig cell viability, the freshly isolated cells were incubated with 0.5 mg/ml MTT for 1 h. As seen in Fig. 4A, the ability of the cells to reduce MTT did not change significantly with age or BSO treatment, indicating that BSO treatment of the whole animals had no effect on Leydig cell viability. Next, intracellular GSH levels in the isolated cells were measured. As seen in Fig. 4B, the GSH concentration in Leydig cells isolated from old control animals was significantly lower, by about 30%, than the concentration in young control cells. BSO treatment of young and old rats resulted in significantly reduced Leydig cell GSH content in Leydig cells from both ages, with reductions of 70%.
Figure 4.
Cell viability (A) and GSH content (B) of Leydig cells isolated from 4-month-old (young) and 24-month-old (old) rats that were treated with BSO or vehicle (PBS) for 7 d. Cell viability was determined by measuring the ability of the isolated cells to reduce MTT (0.5 mg/ml) for 1 h. The data are expressed as mean ± sem of six animals per group. *, Significantly different from cells isolated from age-matched control animals at P < 0.05.
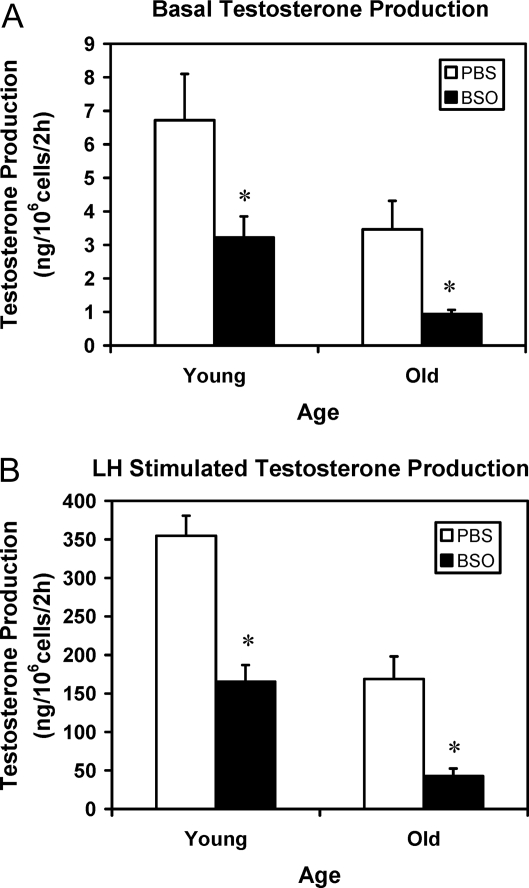
To determine the effect of BSO treatment and thus of reduced GSH in vivo on Leydig cell steroidogenesis, Leydig cells were isolated from young and old rats that had been treated with BSO for 7 d, and the ability of the isolated cells to produce testosterone was assayed in vitro by their incubation without (Fig. 5A) or with (Fig. 5B) maximally stimulating LH for 2 h. Leydig cells from old control rats produced significantly less testosterone than cells from young control animals under both basal (Fig. 5A) and LH-stimulated (Fig. 5B) conditions. With 1 wk of BSO treatment, cells isolated from rats of both ages produced significantly less testosterone than their age-matched controls whether or not the cells were stimulated with LH.
Figure 5.
Testosterone production by Leydig cells isolated from 4-month-old (young) and 24-month-old (old) rats that were treated with BSO or vehicle (PBS) for 7 d. The ability of the cells to produce testosterone was assayed in vitro by incubating the cells without (A) or with maximally stimulating LH (100 ng/ml) (B). The data are expressed as mean ± sem of six animals per group. *, Significantly different from cells isolated from age-matched control animals at P < 0.05.
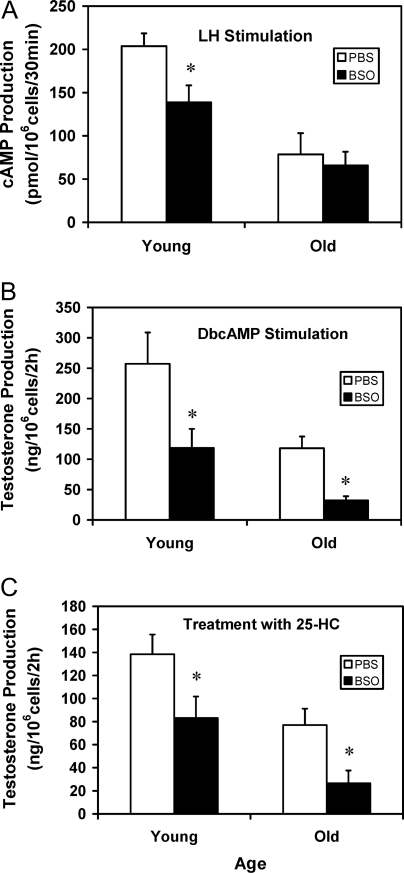
To determine the specific steps of the steroidogenic pathway that were affected by in vivo BSO administration, Leydig cells isolated from PBS- or BSO-treated young and old rats were incubated with LH (Fig. 6A), dbcAMP (Fig. 6B), or 25-HC (Fig. 6C). In response to LH, Leydig cells from old control rats produced significantly less cAMP than the young control cells. BSO treatment of young, but not old, rats resulted in significantly reduced cAMP production, compared with age-matched controls (Fig. 6A). Cells from young and old control and BSO-treated rats were incubated with dbcAMP to bypass LH signaling (Fig. 6B) or with 25-HC to bypass hormone-dependent cholesterol transport into the mitochondria (Fig. 6C). As with cells incubated with LH, old control cells incubated with either dbcAMP or 25-HC produced significantly less testosterone than young control cells. Cells from BSO-treated young and old rats produced significantly less testosterone than the cells from age-matched control animals when the cells were incubated with dbcAMP (Fig. 6B) or 25-HC (Fig. 6C).
Figure 6.
cAMP (A) and testosterone production (B and C) by Leydig cells isolated from 4-month-old (young) and 24-month-old (old) rats that were treated with BSO or vehicle (PBS) for 7 d. For cAMP assays (A), Leydig cells were incubated with LH (100 ng/ml) and the phosphodiesterase inhibitor isobutyl-methylxanthine (100 μm) for 30 min. For testosterone assays, cells were incubated with dbcAMP (2 mm, B) or 25-HC (12.5 μm, C) for 2 h. The data are expressed as mean ± sem of six animals per group. *, Significantly different from cells isolated from age-matched control animals at P < 0.05.
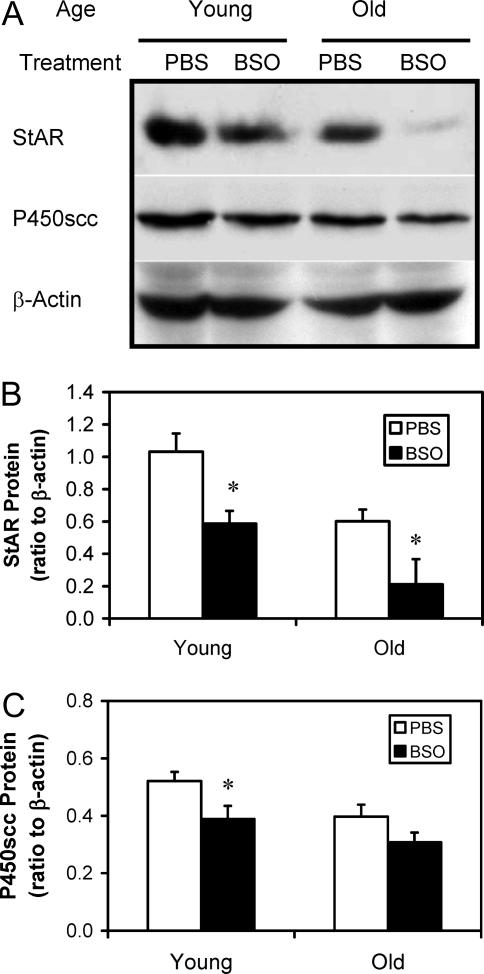
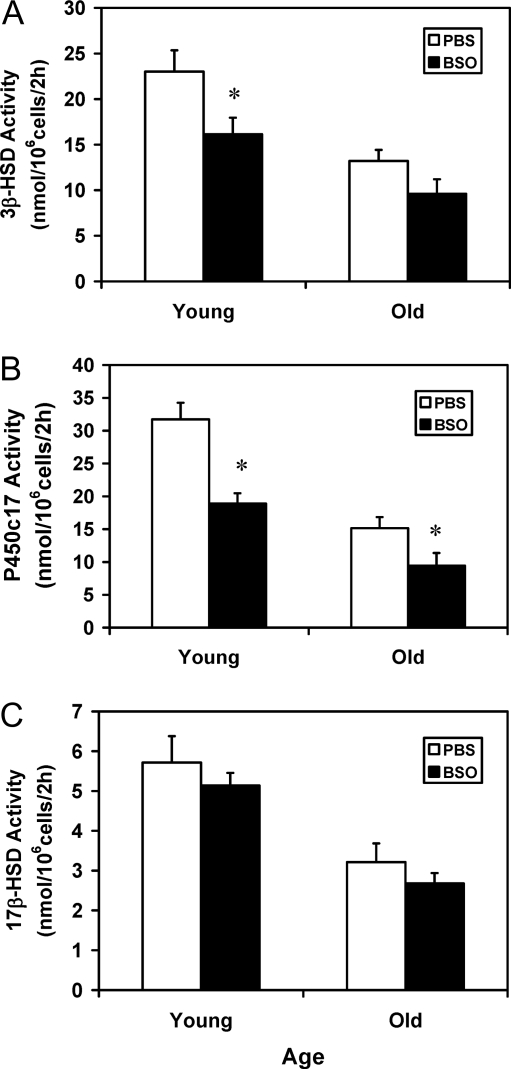
Figure 7 shows the effects of in vivo BSO treatment on StAR and P450scc enzyme in Leydig cells isolated from young and old control (PBS treated) and BSO-treated rats. StAR and P450scc were analyzed by Western blots (Fig. 7A) and quantified (Fig. 7, B and C, respectively). Cells isolated from old control animals (PBS treated) contained significantly less StAR and P450scc than cells isolated from young controls. Treatment of either young and old rats with BSO resulted in significantly reduced Leydig cell StAR, compared with age-matched controls. In the case of P450scc, BSO treatment resulted in significant reduction in Leydig cells from young rats but not in Leydig cells from old rats. BSO treatment also had downstream effects on the steroidogenic enzymes of the smooth endoplasmic reticulum. For these studies, the activities of 3β-HSD (Fig. 8A), P450c17 (Fig. 8B) and 17β-HSD (Fig. 8C) were assayed by incubating cells with the substrates for each enzyme and measuring and adding all enzymatic products. The activities of 3β-HSD, P450c17, and 17β-HSD were all significantly reduced between old and young control animals. BSO treatment, however, resulted in different effects on the three enzymes between young and old animals. In young animals, BSO resulted in significant reductions in 3β-HSD and P450c17 but not in 17β-HSD, compared with age-matched controls. For old animals, however, BSO resulted in significantly reduced P450c17 from the age-matched controls but not 3β-HSD or 17β-HSD.
Figure 7.
Representative Western blots of StAR protein and P450scc enzyme in Leydig cells isolated from 4- and 24-month-old rats that were treated with BSO or vehicle (PBS) for 7 d (A) and quantification of the blots (B and C). Total proteins from equal numbers of cells (2 × 105) were evaluated. β-actin served as the loading control. The data are expressed as mean ± sem of six animals per group. *, Significantly different from cells isolated from age-matched control animals at P < 0.05.
Figure 8.
The activities of the steroidogenic enzymes 3β-HSD (A), P450c17 (B), and 17β-HSD (C). Leydig cells were isolated from 4- and 24-month-old rats that were treated with either BSO or vehicle (PBS) for 7 d. The cells were incubated in vitro with the substrate for 3β-HSD (pregnenolone, 12.5 μm), P450c17 (progesterone, 12.5 μm), or 17β-HSD (androstenedione, 6.25 μm) for 2 h. The Δ4–3-ketosteroid products of each enzyme were quantified by HPLC. The data are expressed as mean ± sem of six animals per group. *, Significantly different from cells isolated from age-matched control animals at P < 0.05.
Discussion
Numerous studies have suggested that imbalance of prooxidants and antioxidants, and thus a shift in the redox state within a cell, can lead to DNA, protein and/or lipid damage, and thus to functional changes (22,23). During normal metabolism, cells produce reactive oxygen species (ROS). In steroidogenic cells, ROS production would be expected to be particularly high because, in addition to the mitochondrial electron transport chain, steroid hydroxylations by the cytochrome P450 enzymes produce ROS (24,25). This may have significance for Leydig cell function because ROS apparently can have detrimental effects on critical components of the steroidogenic pathway (26,27,28,29).
In normally aging Leydig cells, the superoxide content has been shown to increase (11) and the antioxidant defense molecules superoxide dismutase-1 and -2, glutathione peroxidase, and GSH to decrease (9,10). It is notable that lipid peroxidation also increases with Leydig cell aging (10), perhaps as a consequence of changes in the redox environment of aging Leydig cells. Increased lipid peroxidation also occurs in aged adrenal cells (30). The focus of our studies was on GSH, an abundant Leydig cell intracellular antioxidant that, as indicated above, decreases significantly as Leydig cells age. The Leydig cell is not novel in this regard; age-related reductions in GSH have been seen in a number of systems, including human serum (31), rat liver, and rat brain (32,33,34). Given the abundance of GSH in Leydig cells, we hypothesized that the experimental depletion of GSH would result in reduced steroidogenesis. Our objective in taking this experimental approach was to move beyond correlations of age-related changes in testosterone production with the Leydig cell redox environment to cause-effect relationships.
BSO supplementation in the culture medium significantly depleted the GSH pool in cultured Leydig cells by more than 70% in 48 h, and this was accompanied by significantly reduced steroidogenic function. Importantly, when the cells were incubated with GSHEE along with BSO, intracellular GSH levels were maintained and the ability of the cells to produce testosterone was not reduced. This suggests that the effect of BSO on Leydig cell testosterone production was through its reduction of intracellular GSH content rather than through a mechanism unrelated to the antioxidant environment. Two additional observations supported this conclusion. First, increasing the cellular content of GSH beyond control levels with D3T increased testosterone production beyond control levels. Second, incubating Leydig cells with the antioxidants vitamin E, BPN, or Trolox along with BSO also countered the BSO-dependent decrease in steroidogenesis, although GSH was reduced. Taken together, these studies indicate that BSO-induced depletion of intracellular GSH results in reduced steroidogenesis as a consequence of the antioxidant properties of GSH.
These in vitro results led us to determine whether there also is an effect of BSO treatment in vivo on Leydig cell steroidogenic function. To address this, young and old rats were treated with BSO for 7 d. Except for the observation that the BSO-treated young and old rats had slightly reduced body weights (by about 5% at both ages), compared with control rats treated with vehicle (PBS), the treated rats appeared and behaved normally. BSO treatment of young and old animals did not affect Leydig cell viability but was found to deplete the GSH concentration in Leydig cells by 70–80%, with similar reductions in other organs (liver, epididymis, seminal vesicles, prostate) as well (35 and our unpublished observations). The Leydig cells isolated from BSO-treated rats had significantly decreased steroidogenic function, compared with Leydig cells from the PBS-treated control rats. These observations were consistent with the results of the in vitro studies in which Leydig cells were cultured in the presence or absence of BSO. Glutathione depletion resulted in perturbation of steroidogenesis at multiple steps of the steroidogenic pathway. Thus, as in aged cells, the cells from BSO-treated young rats were characterized by reduced cAMP production, down-regulation of StAR, and reduced steroidogenic enzymes in the mitochondria and smooth endoplasmic reticulum.
A number of previous studies have shown that in vivo BSO treatment can deplete GSH in most tissues. It is possible, therefore, that reduced GSH in organs other than the testes might have an indirect effect on Leydig cell function. Thus, although the in vitro studies presented herein argue that BSO directly affects Leydig cell function, we cannot rule out the possibility of additional indirect effects in vivo.
In summary, the present study indicates that depletion of the intracellular GSH pool in both young and old cells can significantly decrease testosterone production. The effects of GSH depletion and aging on the steroidogenic pathway were similar, suggesting that the age-related reductions in GSH concentration, leading to alterations in the redox environment of the Leydig cell may be responsible, at least in part, for age-related decreases in testosterone production by Leydig cells.
Acknowledgments
We thank Dr. Rex Munday (Ruakura Agricultural Research Centre, New Zealand) for kindly providing us D3T.
Footnotes
This work was supported by Grants AG026721 (to H.C.), AG020999 (to T.R.B.), and AG21092 (to B.R.Z.) from the National Institute on Aging, National Institutes of Health.
Disclosure Statement: The authors have nothing to disclose.
First Published Online January 17, 2008
Abbreviations: BPN, N-tert-butyl-α-phenylnitrone; BSO, l-buthionine-sulfoximine; dbcAMP, dibutyryl cAMP; D3T, 1,2-dithiole-3-thione; GSH, glutathione; GSHEE, glutathione ethyl ester; 25-HC, 25-hydroxycholesterol; 3β-HSD, 3β-hydroxysteroid dehydrogenase; 17β-HSD, 17β-hydroxysteroid dehydrogenase; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; P450c17, 17α-hydroxylase/17,20-lyase; P450scc, P450 side-chain cleavage; ROS, reactive oxygen species; SDS, sodium dodecyl sulfate; StAR, steroidogenic acute regulatory protein; Trolox, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid.
References
- Vermeulen A, Kaufman JM 1995 Ageing of the hypothalamo-pituitary-testicular axis in men. Horm Res 43:25–28 [DOI] [PubMed] [Google Scholar]
- Zirkin BR, Chen H 2000 Regulation of Leydig cell steroidogenic function during aging. Biol Reprod 63:977–981 [DOI] [PubMed] [Google Scholar]
- Chen H, Hardy MP, Huhtaniemi I, Zirkin BR 1994 Age-related decreased Leydig cell testosterone production in the brown Norway rat. J Androl 15:551–557 [PubMed] [Google Scholar]
- Chen H, Huhtaniemi I, Zirkin BR 1996 Depletion and repopulation of Leydig cells in the testes of aging brown Norway rats. Endocrinology 137:3447–3452 [DOI] [PubMed] [Google Scholar]
- Chen H, Liu J, Luo L, Zirkin BR 2004 Dibutyryl cyclic adenosine monophosphate restores the ability of aged Leydig cells to produce testosterone at the high levels characteristic of young cells. Endocrinology 145:4441–4446 [DOI] [PubMed] [Google Scholar]
- Luo L, Chen H, Zirkin BR 1996 Are Leydig cell steroidogenic enzymes differentially regulated with aging? J Androl 17:509–515 [PubMed] [Google Scholar]
- Luo L, Chen H, Zirkin BR 2001 Leydig cell aging: steroidogenic acute regulatory protein (StAR) and cholesterol side-chain cleavage enzyme. J Androl 22:149–156 [PubMed] [Google Scholar]
- Culty M, Luo L, Yao ZX, Chen H, Papadopoulos V, Zirkin BR 2002 Cholesterol transport, peripheral benzodiazepine receptor, and steroidogenesis in aging Leydig cells. J Androl 23:439–447 [PubMed] [Google Scholar]
- Luo L, Chen H, Trush MA, Show MD, Anway MD, Zirkin BR 2006 Aging and the brown Norway rat Leydig cell antioxidant defense system. J Androl 27:240–247 [DOI] [PubMed] [Google Scholar]
- Cao L, Leers-Sucheta S, Azhar S 2004 Aging alters the functional expression of enzymatic and non-enzymatic anti-oxidant defense systems in testicular rat Leydig cells. J Steroid Biochem Mol Biol 88:61–67 [DOI] [PubMed] [Google Scholar]
- Chen H, Cangello D, Benson S, Folmer J, Zhu H, Trush MA, Zirkin BR 2001 Age-related increase in mitochondrial superoxide generation in the testosterone-producing cells of Brown Norway rat testes: relationship to reduced steroidogenic function? Exp Gerontol 36:1361–1373 [DOI] [PubMed] [Google Scholar]
- Roberts LR, Steinrauf LK, Blickenstaff RT 1980 New evaluation of potential methylmercury scavengers. J Pharm Sci 69:964–967 [DOI] [PubMed] [Google Scholar]
- Fang YZ, Yang S, Wu G 2002 Free radicals, antioxidants, and nutrition. Nutrition 18:872–879 [DOI] [PubMed] [Google Scholar]
- Griffith OW 1999 Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med 27:922–935 [DOI] [PubMed] [Google Scholar]
- Anderson ME 1998 Glutathione: an overview of biosynthesis and modulation. Chem Biol Interact 111–112:1–14 [DOI] [PubMed] [Google Scholar]
- Griffith OW, Meister A 1979 Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem 254:7558–7560 [PubMed] [Google Scholar]
- Wing TY, Ewing LL, Zirkin BR 1984 Effects of luteinizing hormone withdrawal on Leydig cell smooth endoplasmic reticulum and steroidogenic reactions which convert pregnenolone to testosterone. Endocrinology 115:2290–2296 [DOI] [PubMed] [Google Scholar]
- Klinefelter GR, Hall PF, Ewing LL 1987 Effect of luteinizing hormone deprivation in situ on steroidogenesis of rat Leydig cells purified by a multistep procedure. Biol Reprod 36:769–783 [DOI] [PubMed] [Google Scholar]
- Payne AH, Downing JR, Wong KL 1980 Luteinizing hormone receptor and testosterone synthesis in two distinct populations of Leydig cells. Endocrinology 106:1424–1429 [DOI] [PubMed] [Google Scholar]
- Hissin PJ, Hilf R 1976 A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 74:214–226 [DOI] [PubMed] [Google Scholar]
- Wilson AP 2000 Cytotoxicity and viability assays. In: Masters JRW, ed. Animal cell culture: a practical approach. 3rd ed. Oxford, UK: Oxford University Press; 175–219 [Google Scholar]
- Finkel T, Holbrook NJ 2000 Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247 [DOI] [PubMed] [Google Scholar]
- Drew B, Leeuwenburgh C 2002 Aging and the role of reactive nitrogen species. Ann NY Acad Sci 959:66–81 [DOI] [PubMed] [Google Scholar]
- Peltola V, Huhtaniemi I, Metsa-Ketela T, Ahotupa M 1996 Induction of lipid peroxidation during steroidogenesis in the rat testis. Endocrinology 137:105–112 [DOI] [PubMed] [Google Scholar]
- Hornsby PJ 1989 Steroid and xenobiotic effects on the adrenal cortex: mediation by oxidative and other mechanisms. Free Radic Biol Med 6:103–115 [DOI] [PubMed] [Google Scholar]
- Quinn PG, Payne AH 1984 Oxygen-mediated damage of microsomal cytochrome P-450 enzymes in cultured Leydig cells. Role in steroidogenic desensitization. J Biol Chem 259:4130–4135 [PubMed] [Google Scholar]
- Quinn PG, Payne AH 1985 Steroid product-induced, oxygen-mediated damage of microsomal cytochrome P-450 enzymes in Leydig cell cultures. Relationship to desensitization. J Biol Chem 260:2092–2099 [PubMed] [Google Scholar]
- Georgiou M, Perkins LM, Payne AH 1987 Steroid synthesis-dependent, oxygen-mediated damage of mitochondrial and microsomal cytochrome P-450 enzymes in rat Leydig cell cultures. Endocrinology 121:1390–1399 [DOI] [PubMed] [Google Scholar]
- Diemer T, Allen JA, Hales KH, Hales DB 2003 Reactive oxygen disrupts mitochondria in MA-10 tumor Leydig cells and inhibits steroidogenic acute regulatory (StAR) protein and steroidogenesis. Endocrinology 144:2882–2891 [DOI] [PubMed] [Google Scholar]
- Azhar S, Cao L, Reaven E 1995 Alteration of the adrenal antioxidant defense system during aging in rats. J Clin Invest 96:1414–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP, Mody Jr VC, Carlson JL, Lynn MJ, Sternberg Jr P 2002 Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med 33:1290–1300 [DOI] [PubMed] [Google Scholar]
- Sandhu SK, Kaur G 2002 Alterations in oxidative stress scavenger system in aging rat brain and lymphocytes. Biogerontology 3:161–173 [DOI] [PubMed] [Google Scholar]
- Liu H, Wang H, Shenvi S, Hagen TM, Liu RM 2004 Glutathione metabolism during aging and in Alzheimer disease. Ann NY Acad Sci 1019:346–349 [DOI] [PubMed] [Google Scholar]
- Liu RM 2002 Down-regulation of γ-glutamylcysteine synthetase regulatory subunit gene expression in rat brain tissue during aging. J Neurosci Res 68:344–351 [DOI] [PubMed] [Google Scholar]
- Zubkova EV, Robaire B 2004 Effect of glutathione depletion on antioxidant enzymes in the epididymis, seminal vesicles, and liver and on spermatozoa motility in the aging brown Norway rat. Biol Reprod 71:1002–1008 [DOI] [PubMed] [Google Scholar]