Abstract
MicroRNAs (miRNAs) are endogenous small noncoding RNAs that decrease the expression levels of specific genes by translational repression, sequestration, and degradation of their mRNAs. Angiotensin II is an important modulator of adrenal zona glomerulosa cell physiology, including steroidogenesis and proliferation among many other physiological processes. Because each miRNA may regulate the expression levels of multiple genes, thereby resembling the transcription regulatory networks triggered by transcription factors, we hypothesize that specific miRNAs may be involved in angiotensin II-mediated adrenocortical cell physiology. The human adrenocortical cell line H295R is the only adrenal cell line available with a steroid secretion pattern and regulation similar to freshly isolated adrenocortical cells. We screened for miRNAs regulated by angiotensin II in H295R cells and found that miRNA-21 expression levels were specifically modulated by angiotensin II. Angiotensin II time dependently increased miRNA-21 expression reaching a 4.4-fold induction after 24 h. Angiotensin II-mediated miRNA-21 expression resulted in biologically active miRNA-21, determined using a fusion mRNA reporter system carrying miRNA-21 target sequences in its 3′ untranslated region. Up-regulation of miRNA-21 intracellular levels increased aldosterone secretion but not cortisol. Elevation of miRNA-21 levels also increased cell proliferation in H295R cells. In summary, miRNA-21 is an endogenously expressed miRNA in human adrenal cells. miRNA-21 expression is up-regulated by angiotensin II, and its overexpression caused an increase in aldosterone secretion and cell proliferation. Alterations in miRNA-21 expression levels or function may be involved in dysregulation of angiotensin II signaling and abnormal aldosterone secretion by adrenal glands in humans.
THE MAMMALIAN ADRENAL cortex is composed of three distinct zones: the zona glomerulosa, the zona fasciculata, and the zona reticularis (1,2). The cells in the zona glomerulosa, underlying the adrenal capsule, secrete mineralocorticoids, primarily aldosterone. Aldosterone is mainly under the control of angiotensin II (Ang II), the end product of the renin-angiotensin system (RAS), although many other molecules also regulate its secretion (3,4). Aldosterone exerts its effect in not only epithelial target tissues, in which its main function is regulation of sodium, potassium, and water balance but also in nonepithelial tissues such as the brain and the heart. High circulating aldosterone levels, due to a dysregulation of normal physiological secretion, has serious deleterious effects in multiple systems. In experimental animal models, excess aldosterone causes hypertension and heart, brain, and kidney target organ damage (5,6,7,8,9). In humans, high circulating levels of aldosterone due to primary aldosteronism have been associated with hypertension and target organ injury in the heart and kidney (10,11). In addition, aldosterone levels in the highest quartile of the normal range for this steroid in nonhypertensive patients has been reported to correlate with an increased risk to suffer an increase in blood pressure or develop hypertension (12,13).
MicroRNAs (miRNAs) are endogenous, small (19–25 nucleotide long), noncoding RNAs that have important roles in development, proliferation, hematopoiesis, and apoptosis (14,15,16,17,18,19). miRNAs can be independent transcription units, embedded in the introns of coding RNAs, or in either the introns or exons of noncoding RNAs. miRNAs are transcribed by RNA polymerase II into a primary miRNA (pri-miRNA) transcript that is capped and contains a polyA-tail sequence. A pri-miRNA contains at least one stem structure constituted by a terminal loop and flanking single strand RNA sequences. The stem-loop sequence is cropped from the pri-miRNA to generate a pre-miRNA by a complex composed of the RNase III Drosha and its cofactor DGCR8. The pre-miRNA is then transported from the nuclei to the cytoplasm by the exportin-5 and Ran-GTP transport receptor complex for further processing. Pre-miRNAs are further processed by the RNase III Dicer to generate mature miRNAs approximately 22 nucleotides long. Mature miRNAs complex with RNA-induced silencing complex (RISC) to generate the RNA-protein complex miRISC. The miRISC complex recognizes its imperfectly matched target and binds in the 3′ untranslated region (UTR) of the target mRNA. The miRISC complex will specifically down-regulate the expression of the target mRNA by multiple mechanisms including mainly translational repression and sequestration in P bodies but also deadenylation, decapping, and degradation (20,21,22,23).
Although at the beginning they were considered a curiosity in nematodes, miRNAs have been described in viruses, plants, and animals, with more than 474 miRNAs reported in humans. Although the regulation of miRNA expression, their targets, and their physiological actions are not well understood, it is clear that they play a crucial role in multiple physiological processes. More than a decade ago, it was reported that lin-4 plays a crucial role in the progression from the first to the second larval stage in Caenorhabditis elegans (24,25). Evidence of miRNA importance in development was reinforced when it was found that the miRNA let-7 is essential for late-larval to adult cell progression in the same nematode experimental model (26,27). Recently multiple groups have shown that miRNAs are also required for normal mammal physiology. Knockdown of specific miRNAs causes severe pathologies in the immune and cardiac systems (28,29,30,31). miRNA mode of action targeting multiple genes and the important effects observed in specific miRNA knockout experimental models suggest that miRNAs may trigger gene expression regulatory networks similarly to the well-known transcription factors (32).
Due to the importance of miRNAs in the control of cell physiology in multiple experimental models and the lack of knowledge of the role of miRNAs in adrenal cell physiology, we studied whether Ang II, an important modulator of adrenal cell physiology, regulates the expression of specific miRNAs in human adrenocortical cells. We screened more than 200 miRNAs by microarray analysis and found that miRNA-21 was the only miRNA whose expression levels were up-regulated by Ang II treatment in H295R human adrenocortical cells. miRNA-21 is a highly conserved miRNA expressed from its own transcription unit (15,33,34). miRNA-21 expression is increased in tumor tissues and has been described as an oncogenic miRNA exhibiting antiapo-ptotic activity in various carcinomas (35,36). miRNA-21 is expressed, regulated, and functional in cardiac tissue (37,38,39). Cardiac expression of miRNA-21 has been reported to be increased in vivo in experimental models of cardiac hypertrophy and in vitro by Ang II and phenylephrine in neonatal rat cardiomyocytes (37,38). The physiological role of cardiac miRNA-21 is unclear because it has been reported to mediate and inhibit Ang II- and phenylephrine-mediated cardiomyocyte proliferation by different research groups (37,38).
The aim of the present study was to study the regulation of miRNA-21 expression and its physiological role in H295R human adrenocortical cells. We used the human adrenocortical cell line H295R, which is the only adrenal cell line that expresses all of the steroidogenic enzymes required for the synthesis of aldosterone from cholesterol, has a steroid secretion pattern and regulation similar to that of primary adrenal cells cultures, and has been proven to be an excellent model to study adrenal cell physiology (40,41). We report Ang II-mediated regulation of miRNA-21 in H295R cells, its biological activity, and role in the two main physiological effects of Ang II on adrenocortical cells, steroid secretion, and proliferation.
Materials and Methods
Materials
Ang II was obtained from American Peptide Co. (Sunnyvale, CA). miRNA primer pairs (miRNA-21: catalog no. 30102; U6: catalog no. 30303; S5: catalog no. 30302), miRNA precursors (pre-miRs, pre-miRNA-21: catalog no. 17100 ID: PM10206; pre-miR-negative control no. 1: catalog no. 17110; pre-miR-negative control no. 2: catalog no. 17111), and miRNA inhibitors (anti-miR-21: catalog no. 17000 ID: AM10206; anti-miR-negative control no. 1: catalog no. 17010) were obtained from Ambion (Austin, TX). Pre-miR-negative controls no. 1 and 2 and anti-miR-negative control are designed not to target any known gene sequence.
Plasmids
pMIR-REPORT-miRNA-21 and its control plasmid have been previously reported (42). pSEAP2-control plasmid was obtained from CLONTECH (Mountain View, CA). pCMV-miRNA-21 and its control has been previously reported (43).
Cell culture
H295R human adrenocortical cells (44) were cultured in H295R complete media containing DMEM:F12 (1:1) supplemented with 2% Ultroser G (Biosepra, Villeneuve-la-Garenne, France), Insulin/Transferrin/Selenium-Plus (Discovery Labware, Bedford, MA) and antibiotic/antimycotic mixture (Invitrogen, Carlsbad, CA) as we previously described (45), until subconfluent in 6-well plates. Media were replaced with 3 ml fresh media containing various agents and cultured for periods indicated in each experimental protocol.
miRNA extraction and real-time RT-PCR
miRNA was extracted with the mirVana miRNA isolation kit (Ambion) and miRNA-21 quantification was performed using the mirVana quantitative RT-PCR miRNA detection kit (Ambion) following the manufacturer’s suggested protocols. Total RNA (25 ng) was reversed transcribed using gene-specific primers. For real-time PCR, gene-specific reverse transcribed products were amplified with SuperTaq DNA polymerase (Ambion) and gene-specific primers. Reaction conditions were 3 min at 95 C followed by 50 cycles of 15 sec at 95 C and 30 sec at 60 C. Real-time data were obtained during the extension phase and threshold cycle values were obtained at the log phase of each gene amplification. PCR product quantification was performed by the relative quantification method (46) and standardized against U6 or S5 RNA. Efficiency for each primer pair was assessed by using serial dilutions of reverse transcriptase product. Results are expressed as arbitrary units and normalized against U6 or S5 RNA expression. The specificity of the PCR products was confirmed by melting temperature determination of the PCR product and high-resolution electrophoretic analysis in 4% NuSieve 3:1 agarose gels (Cambrex, Rockland, ME) of PCR products. Gene-specific primers were obtained from Ambion.
Reporter assays
H295R cells were plated in 24-well plates with media without antibiotic/antimycotics and grown until 90–95% confluent. H295R cells were transfected with 3 μg plasmid DNA/well (2.5 μg reporter plasmid plus 0.5 μg pSEAP2-control plasmid) and 2 μl/well Lipofectamine 2000 (Invitrogen) following the manufacturer’s suggested protocols. Cells were cultured overnight, media replaced with 1 ml/well fresh media with or without Ang II (100 nm), and cultured for an additional 24 h. Media were collected to measure secreted alkaline phosphatase using the Great EscAPe SEAP chemiluminescence kit (CLONTECH). Cells were lysed with Glo Lysis buffer (Promega, Madison, WI) and luciferase activity quantified with Bright-Glo luciferase assay kit (Promega).
Steroid secretion
H295R cells were transfected using Nucleofector technology (Amaxa Biosystems, Gaithersburg, MD). Three million log phase cells were resuspended in 100 μl Nucleofector solution R, mixed with 100 picomoles miRNA precursors, miRNAs inhibitors or 3 μg plasmid DNA, and electroporated using the proprietary program T-20. Cells were allowed to recover for 15 min in RPMI 1640 media at 37 C. Cells were plated in 24-well plates with 1 ml H295R complete media per well and allowed to recover for 16 h. Cell culture media was removed and replaced with media with or without Ang II (10 nm) and cells cultured for an additional 24 h. At the end of the incubation period, cell culture supernatants were saved for aldosterone and cortisol determination by ELISA as previously reported (47,48). Cells were lysed with M-PER lysis buffer (Pierce, Rockford, IL) and protein concentration measured by the bicinchoninic acid method (Pierce) using BSA as standard.
Cell proliferation
H295R cells were transfected using Nucleofector technology (Amaxa Biosystems). Three million log phase cells were resuspended in 100 μl Nucleofector Solution R, mixed with 100 pmol miRNA precursors, and electroporated using the proprietary program T-20. Cells were allowed to recover for 15 min in RPMI 1640 media at 37 C. Cells (104 cells/well) were plated in 96-well plates with 0.2 ml H295R complete media per well and allowed to recover for 16 h. Cell culture media were removed and replaced with media with or without Ang II (10 nm) and cells cultured for an additional 24 h. Cell proliferation was quantified using the CellTiter-Glo luminescent cell viability assay kit (Promega) following manufacturer suggested protocol.
Statistics
All results were expressed as mean ± sem. Two groups were compared by t test and multiple groups were analyzed by one-way ANOVA followed by Tukey’s post hoc comparisons or two-way ANOVA followed by Bonferroni comparisons. Time-response curves were tested by two-way ANOVA followed by Bonferroni comparisons. All experiments were performed at least twice in triplicates. Differences were considered statistically significant at P < 0.05. Statistical calculations were performed with GraphPad Prism package version 4.03 (GraphPad Software, Inc., San Diego, CA).
Results
miRNA-21 is up-regulated by Ang II in H295R cells
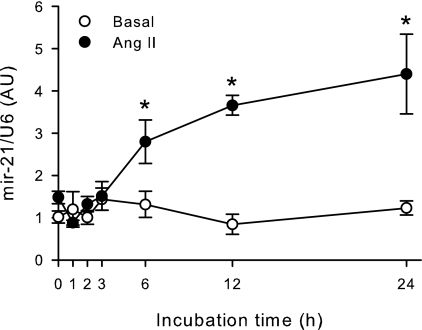
H295R human adrenocortical cells were incubated with 100 nm Ang II for periods of increasing duration up to 24 h, and miRNA-21 expression was quantified by real-time PCR (Fig. 1). Ang II increased miRNA-21 expression in H295R cells continuously between 6 and 24 h after hormone treatment reaching a maximal 4.4-fold stimulation after 24 h of treatment. The small RNA U6 was used as the housekeeping control to normalize miRNA-21 expression. To confirm Ang II-mediated miRNA-21 expression, we used another small RNA S5 as housekeeping control obtaining similar results (data not shown). To test the effect of another aldosterone secretagogue on miRNA-21 expression, H295R cells were treated with a maximal stimulatory concentration of potassium (16 mm) for up to 24 h. Potassium did not significantly modify miRNA-21 expression in H295R cells (data not shown).
Figure 1.
Ang II up-regulates miRNA-21 expression in H295R human adrenocortical cells. H295R cells were incubated with or without 100 nm Ang II for increasing periods up to 24 h, RNA extracted, and then quantified by real-time RT-PCR. miRNA-21 RNA was normalized by U6 RNA expression and expressed as fold increase vs. time = 0. *, P < 0.05 vs. basal, n = 3.
miRNA-21 is biologically active in H295R cells
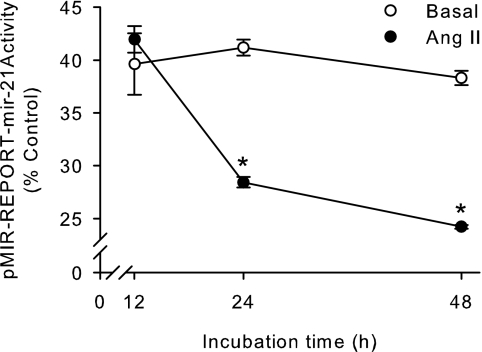
To test whether Ang II-mediated increase in miRNA-21 expression is indeed translated into an increase in biologically active miRNA-21 in adrenal cells, we performed reporter studies. We used a reporter plasmid that expresses, under the control of a constitutive promoter, a fusion mRNA that encodes for the luciferase reporter enzyme fused to miRNA-21 target sequences in its 3′ UTR. miRNA-21 became bound to its target sequences in the fusion mRNA, decreasing the concentration of luciferase and consequently its activity. Control plasmids did not carry the miRNA-21 target sequences. Ang II time-dependently decreased luciferase activity indicating that Ang II-mediated miRNA-21 expression is translated into an increase of biologically active miRNA-21 (Fig. 2). Nonstimulated cells transfected with the miRNA-21 reporter plasmid showed a reduction in luciferase activity due to the basal expression of miRNA-21 that did not change during the course of the experiment.
Figure 2.
Ang II up-regulates biologically active miRNA-21 expression in H295R human adrenocortical cells. H295R cells were transfected with pMIR-REPORT-miRNA-21 or pMIR-REPORT-control, allowed to recover overnight, and incubated in the presence or absence of 100 nm Ang II for increasing periods up to 48 h. Data are expressed as percentage of pMIR-REPORT-miRNA-21/pMIR-REPORT-control. *, P < 0.05 vs. basal, n = 6.
miRNA-21 increases aldosterone secretion by H295R cells
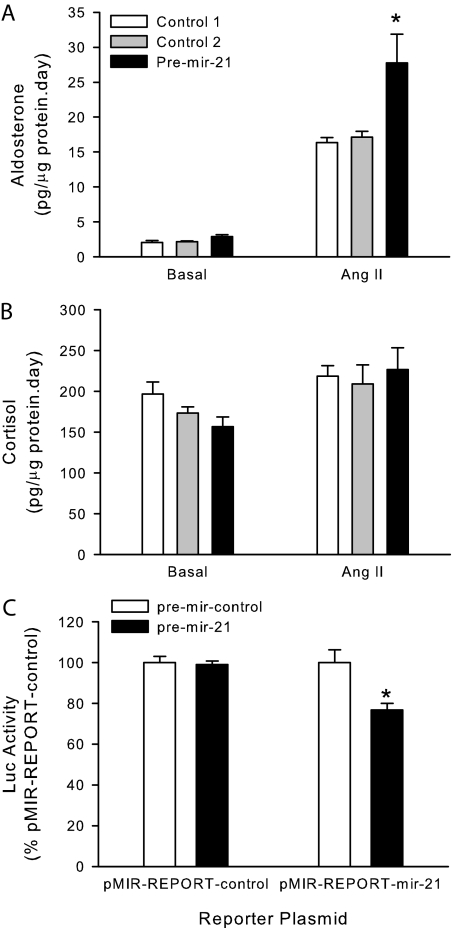
To study the role of Ang II-induced miRNA-21 expression in H295R cells, we performed transfection studies to analyze the effect of miRNA-21 up-regulation on aldosterone secretion. H295R cells were transfected with synthetic miRNA-21 precursors, allowed to recover, and then incubated in the presence or absence of a submaximal Ang II concentration (10 nm). Increased levels of miRNA-21 caused an almost 70% increase in aldosterone secretion under Ang II submaximal stimulatory conditions and did not cause any significant effect under basal conditions (Fig. 3A). To test the specificity of the miRNA-21 effect on steroid secretion, we quantified cortisol secretion in the same cell culture media. Increased levels of miRNA-21 did not modify cortisol secretion by H295R cells under basal or Ang II-stimulatory conditions (Fig. 3B).
Figure 3.
miRNA-21 up-regulates aldosterone secretion by H295R human adrenocortical cells. A and B, H295R cells were transfected by nucleofection with synthetic pre-miRNA-21 or pre-mir-control no. 1 or no. 2, allowed to recover overnight, and incubated in the presence or absence of 10 nm Ang II for 24 h. Aldosterone (A) and cortisol (B) were measured in cell culture supernatants by ELISA and cells lysed to quantify total protein. C, H295R cells were cotransfected by nucleofection with synthetic pre-miRNA-21 or pre-mir-control no. 1, pMIR-REPORT-miRNA-21 or pMIR-REPORT-control, and pSEAP2-control; allowed to recover overnight; media replaced; and incubated for an additional 24-h period. Data are expressed as percentage of pre-mir-control. *, P < 0.05 vs. pre-mir-control, n = 3.
To confirm that transfected synthetic miRNA-21 precursors translate into increased levels of biologically active miRNA-21, we performed cotransfection studies with miRNA-21 precursors and miRNA-21 reporter plasmids. Transfection of synthetic miRNA-21 precursors caused an almost 25% decrease in the activity of the miRNA-21 reporter plasmid indicating the success of the transfection conditions (Fig. 3C).
To further confirm the effect of increased levels of miRNA-21 on aldosterone secretion we up-regulated miRNA-21 intracellular concentration using a plasmid-driven overexpression system under the control of a constitutive promoter. Plasmid-driven miRNA-21 overexpression caused a 62% increase in aldosterone secretion under Ang II submaximal stimulatory conditions by H295R cells (8.23 ± 1.09 vs. 13.37 ± 1.93 pg aldosterone per microgram protein per day, P < 0.05, n = 3).
Because elevated intracellular levels of miRNA-21 consistently increased aldosterone secretion using several experimental approaches, we tested whether miRNA-21 inhibition would modulate basal or Ang II-mediated aldosterone secretion. H295R cells were transfected with miRNA-21 inhibitors (or their controls), allowed to recover, and then incubated in the presence or absence of a submaximal Ang II concentration (10 nm). Surprisingly, miRNA-21 inhibition did not modify either basal or Ang II-stimulated aldosterone or cortisol secretion.
miRNA-21 increases adrenal cell proliferation by H295R cells
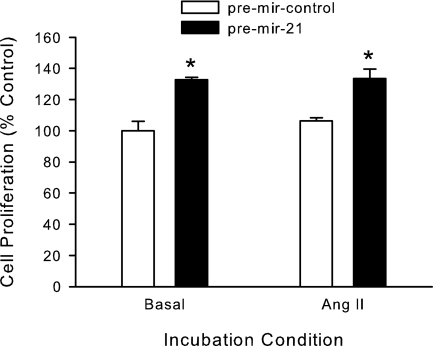
To study the role of miRNA-21 up-regulation in adrenal cell proliferation, we transfected H295R cells with miRNA-21 precursors and studied cell proliferation under basal and Ang II-stimulated conditions. Increased levels of miRNA-21 precursors increased H295R cells proliferation 33 and 26% under basal and Ang II stimulatory conditions, respectively (Fig. 4).
Figure 4.
miRNA-21 up-regulates cell proliferation by H295R human adrenocortical cells. H295R cells were transfected by nucleofection with synthetic pre-miRNA-21 or pre-mir-control no. 1, allowed to recover overnight, and incubated in the presence or absence of 10 nm Ang II for 24 h. Cell proliferation was quantified using the Cell-Titer Glo luminescent assay (Promega). *, P < 0.05 vs. pre-mir-control, n = 6.
Discussion
The main finding of this report is that the endogenous miRNA miRNA-21 is up-regulated by the aldosterone secretagogue Ang II in H295R human adrenocortical cells and its up-regulation caused a specific increase in aldosterone secretion and cell proliferation. To the best of our knowledge, this is the first report describing a physiological role of an endogenous miRNA in adrenocortical cells.
miRNAs are not new to mammalian genetics, although it has only been in recent years that they are beginning to be considered not as a curiosity but as a completely new layer in gene expression regulation. miRNAs act by several pathways, including translational repression and mRNA sequestration and degradation, but all ultimately result in the decrease of the protein levels of specific genes. Nevertheless, miRNAs have also been recently reported to be able to bind to the 3′ UTR of specific mRNAs up-regulating its translation (49). The limited current knowledge regarding the specific targets for each miRNA or the availability of reliable bioinformatic tools to predict them in silico does not diminish their fundamental role, as has been reported in multiple living organisms (50,51,52) including mammals (28,29,30,31).
In this report, we show that Ang II, the end product of the RAS, up-regulates miRNA-21 expression in human adrenocortical cells. The RAS is an important modulator of adrenal zona glomerulosa cell physiology (3,4). We also showed that Ang II-mediated miRNA-21 expression is translated into an increase in biologically active miRNA-21 expression capable of decreasing the expression/activity of a fusion mRNA reporter gene. miRNA-21 has been shown to be regulated not only in tumors but also more recently under physiological conditions in the cardiac tissue. Cardiac expression of miRNA-21 has been reported to be increased in in vivo experimental models of cardiac hypertrophy (37,38,39). In vitro, both Ang II and phenylephrine up-regulate miRNA-21 expression in neonatal rat cardiomyocytes (37,38). This finding may suggest that Ang II is a key modulator of miRNA-21 expression as has been reported in neonatal rat cardiomyocytes and as we have observed in human adrenocortical cells.
Surprisingly, potassium, another aldosterone secretagogue, did not modify miRNA-21 expression levels in H295R cells, suggesting that miRNA-21 is a specific target of Ang II action in human adrenocortical cells. We did not quantify the expression levels of other miRNAs under potassium treatment in H295R cells, raising the possibility that potassium may regulate the expression levels of other miRNAs, which may have similar or different mRNA targets.
TarBase, a database of experimentally validated miRNA targets, lists 102 translational repressed validated miRNA targets as well as 359 down-regulated or cleaved miRNA targets for the human (53). Three mRNA targets have been validated for miRNA-21 using mRNA fusion reporter genes with the 3′ UTR regions of tropomyosin 1, phosphatase and tensin homolog deleted on chromosome 10, and programmed cell death 4 mRNAs (54,55,56). Unfortunately, tropomyosin 1, phosphatase and tensin homolog deleted on chromosome 10, or programmed cell death 4 has not been studied in adrenal cortex cells in relation to steroid secretion or proliferation. Further studies are needed to address whether either of these validated miRNA-21 targets are also targeted by miRNA-21 in adrenocortical cells or whether any of them is responsible for the physiological effects observed with miRNA-21 up-regulation. We observed that miRNA-21 overexpression increased aldosterone secretion and proliferation in H295R cells. These results suggest that miRNA-21 may be down-regulating the expression of genes that are inhibitors of aldosterone secretion and proliferation. These inhibitory genes could be the same or different for the two physiological processes under study, aldosterone secretion and proliferation.
In contrast to our expectations, miRNA-21 inhibition did not modify aldosterone or cortisol secretion by H295R cells under either basal or Ang II-stimulatory conditions. Potential explanations for these apparently contradictory results are that miRNA-21 inhibition was not complete, and low levels of miRNA-21 are enough to sustain Ang II-mediated aldosterone secretion or the existence of compensatory intracellular mechanisms, which deal with decreased levels of bioactive miRNA-21, among many other explanations. Similar discordant results from experiments with miRNA-21 overexpression and down-regulation have been reported in other cell systems (38,39).
Excess circulating aldosterone levels are associated with hypertension in experimental animal models and humans. Hypertension is a well-established determinant of cardiovascular disease and a major cause of disability and death (57). The prevalence of hypertension in the adult population in the United States is approximately 29% (58). Hypertension is a worldwide health problem with an estimated 1 billion hypertensive adults worldwide, two thirds of them in economically developing countries (59). If the current trend persists, it is estimated that there would be a 60% increase in the number of hypertensive adults by 2025. Multiple epidemiological studies indicate that primary aldosteronism is the most common cause of secondary hypertension, accounting for approximately 10% of hypertensive patients (60,61,62). Current estimates indicate that there are 8.5 million people in the United States with primary aldosteronism.
Transcription factors have been implicated in multiple diseases in the last decades. miRNAs with similar potential of regulating multiple gene networks may be involved as the transcription factors in the modulation of the intracellular expression network whose dysregulation may lead to disease. We have shown that miRNA-21 is regulated by Ang II in human adrenal cells and that it is involved in the regulation of aldosterone secretion. Multiple questions about miRNA-21 remain open, including whether miRNA-21 endogenous levels are regulated by other aldosterone secretagogues such as endothelin-1 or ACTH, miRNA-21 is similarly regulated by Ang II in adrenocortical cells from other species, miRNA-21 levels are regulated in vivo by Ang II and adrenocortical cell physiological stimulators such as low-salt diet or elevated potassium, and the physiological targets of miRNA-21 and how these interact to specifically up-regulate aldosterone secretion, among many other questions. We hope this report will stimulate further research in these areas. The present report allows the speculation that miRNAs and specifically miRNA-21 may be involved in excess aldosterone secretion in patients with primary aldosteronism.
Acknowledgments
We thank Dr. W. E. Rainey (Medical College of Georgia, Augusta, GA) for generously providing H295R cells. We also thank Dr. Bryan R. Cullen (Duke University Medical Center, Durham, NC) and Lance P. Ford (Ambion, Austin, TX) for generously providing plasmids.
Footnotes
This work was supported by medical research funds from the Department of Veterans Affairs and National Institutes of Health Grants HL27255 and HL75321. C.A.C. was supported by Chilean Grant FONDECYT 1070876.
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 24, 2008
Abbreviations: Ang II, Angiotensin II; miRNA, microRNA; miRISC, miRNA complex with RISC; pri-miRNA, primary miRNA; RAS, renin-angiotensin system; RISC, RNA-induced silencing complex; UTR, untranslated region.
References
- Stewart PM 2003 The adrenal cortex. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, eds. Williams textbook of endocrinology. 10th ed. Philadelphia: Elsevier; 491–551 [Google Scholar]
- Nussey S, Whitehead S 2001 The adrenal gland. In: Nussey S, Whitehead S, eds. Endocrinology: an integrated approach. 1st ed. Oxford, UK: BIOS Scientific Publishers Ltd.; 115–170 [PubMed] [Google Scholar]
- Ehrhart-Bornstein M, Hinson JP, Bornstein SR, Scherbaum WA 1998 Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev 19:101–143 [DOI] [PubMed] [Google Scholar]
- Williams GH 2005 Aldosterone biosynthesis, regulation, and classical mechanism of action. Heart Fail Rev 10:7–13 [DOI] [PubMed] [Google Scholar]
- Garwitz ET, Jones AW 1982 Aldosterone infusion into the rat and dose-dependent changes in blood pressure and arterial ionic transport. Hypertension 4:374–381 [DOI] [PubMed] [Google Scholar]
- Brilla CG, Weber KT 1992 Mineralocorticoid excess, dietary sodium and myocardial fibrosis. J Lab Clin Med 120:893–901 [PubMed] [Google Scholar]
- Gomez-Sanchez EP 1986 Intracerebroventricular infusion of aldosterone induces hypertension in rats. Endocrinology 118:819–823 [DOI] [PubMed] [Google Scholar]
- Rocha R, Chander PN, Khanna K, Zuckerman A, Stier Jr CT 1998 Mineralocorticoid receptor antagonist protects stroke-prone hypertensive rats against vascular injury. Hypertension 31:451–458 [DOI] [PubMed] [Google Scholar]
- Rocha R, Chander PN, Zuckerman A, Stier CT 1999 Role of aldosterone in renal vascular injury in stroke-prone hypertensive rats. Hypertension 33:232–237 [DOI] [PubMed] [Google Scholar]
- Rossi GP, Sacchetto A, Pavan E, Palatini P, Graniero GR, Canali C, Pessina AC 1997 Remodeling of the left ventricle in primary aldosteronism due to Conn’s adenoma. Circulation 95:1471–1478 [DOI] [PubMed] [Google Scholar]
- Rossi GP, Bernini G, Desideri G, Fabris B, Ferri C, Giacchetti G, Letizia C, Maccario M, Mannelli M, Matterello MJ, Montemurro D, Palumbo G, Rizzoni D, Rossi E, Pessina AC, Mantero F 2006 Renal damage in primary aldosteronism: results of the PAPY Study. Hypertension 48:232–238 [DOI] [PubMed] [Google Scholar]
- Vasan RS, Evans JC, Larson MG, Wilson PW, Meigs JB, Rifai N, Benjamin EJ, Levy D 2004 Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med 351:33–41 [DOI] [PubMed] [Google Scholar]
- Newton-Cheh C, Guo CY, Gona P, Larson MG, Benjamin EJ, Wang TJ, Kathiresan S, O’Donnell CJ, Musone SL, Camargo AL, Drake JA, Levy D, Hirschhorn JN, Vasan RS 2007 Clinical and genetic correlates of aldosterone-to-renin ratio and relations to blood pressure in a community sample. Hypertension 49:846–856 [DOI] [PubMed] [Google Scholar]
- Rana TM 2007 Illuminating the silence: understanding the structure and function of small RNAs. Nat Rev Mol Cell Biol 8:23–36 [DOI] [PubMed] [Google Scholar]
- Du T, Zamore PD 2005 microPrimer: the biogenesis and function of microRNA. Development 132:4645–4652 [DOI] [PubMed] [Google Scholar]
- Kloosterman WP, Plasterk RH 2006 The diverse functions of microRNAs in animal development and disease. Dev Cell 11:441–450 [DOI] [PubMed] [Google Scholar]
- Ambros V 2004 The functions of animal microRNAs. Nature 431:350–355 [DOI] [PubMed] [Google Scholar]
- Bartel DP 2004 MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297 [DOI] [PubMed] [Google Scholar]
- He L, Hannon GJ 2004 MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5:522–531 [DOI] [PubMed] [Google Scholar]
- Pillai RS, Bhattacharyya SN, Filipowicz W 2007 Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol 17:118–126 [DOI] [PubMed] [Google Scholar]
- Jackson RJ, Standart N 2007 How do microRNAs regulate gene expression? Sci STKE 2007:re1 [DOI] [PubMed] [Google Scholar]
- Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R 2006 Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev 20:515–524 [DOI] [PubMed] [Google Scholar]
- Nilsen TW 2007 Mechanisms of microRNA-mediated gene regulation in animal cells. Trends Genet 23:243–249 [DOI] [PubMed] [Google Scholar]
- Lee RC, Feinbaum RL, Ambros V 1993 The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75:843–854 [DOI] [PubMed] [Google Scholar]
- Wightman B, Ha I, Ruvkun G 1993 Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75:855–862 [DOI] [PubMed] [Google Scholar]
- Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G 2000 The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403:901–906 [DOI] [PubMed] [Google Scholar]
- Slack FJ, Basson M, Liu Z, Ambros V, Horvitz HR, Ruvkun G 2000 The lin-41 RBCC gene acts in the C. elegans heterochronic pathway between the let-7 regulatory RNA and the LIN-29 transcription factor. Mol Cell 5:659–669 [DOI] [PubMed] [Google Scholar]
- van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN 2007 Control of stress-dependent cardiac growth and gene expression by a MicroRNA. Science 316:575–579 [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, Vetrie D, Okkenhaug K, Enright AJ, Dougan G, Turner M, Bradley A 2007 Requirement of bic/microRNA-155 for normal immune function. Science 316:608–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL, Schmidt-Supprian M, Rajewsky N, Yancopoulos G, Rao A, Rajewsky K 2007 Regulation of the germinal center response by microRNA-155. Science 316:604–608 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, Srivastava D 2007 Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1–2. Cell 129:303–317 [DOI] [PubMed] [Google Scholar]
- Chen K, Rajewsky N 2007 The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet 8:93–103 [DOI] [PubMed] [Google Scholar]
- Cai X, Hagedorn CH, Cullen BR 2004 Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA 10:1957–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler D, Brocke-Heidrich K, Pfeifer G, Stocsits C, Hackermuller J, Kretzschmar AK, Burger R, Gramatzki M, Blumert C, Bauer K, Cvijic H, Ullmann AK, Stadler PF, Horn F 2007 Interleukin-6-dependent survival of multiple myeloma cells involves the Stat3-mediated induction of microRNA-21 through a highly conserved enhancer. Blood 110:1330–1333 [DOI] [PubMed] [Google Scholar]
- Chan JA, Krichevsky AM, Kosik KS 2005 MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res 65:6029–6033 [DOI] [PubMed] [Google Scholar]
- Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY 2007 miR-21-mediated tumor growth. Oncogene 26:2799–2803 [DOI] [PubMed] [Google Scholar]
- Tatsuguchi M, Seok HY, Callis TE, Thomson JM, Chen JF, Newman M, Rojas M, Hammond SM, Wang DZ 2007 Expression of microRNAs is dynamically regulated during cardiomyocyte hypertrophy. J Mol Cell Cardiol 42:1137–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Ji R, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C 2007 MicroRNAs are aberrantly expressed in hypertrophic heart. Do they play a role in cardiac hypertrophy? Am J Pathol 170:1831–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN 2006 A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA 103:18255–18260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey WE, Bird IM, Mason JI 1994 The NCI-H295 cell line: a pluripotent model for human adrenocortical studies. Mol Cell Endocrinol 100:45–50 [DOI] [PubMed] [Google Scholar]
- Rainey WE, Saner K, Schimmer BP 2004 Adrenocortical cell lines. Mol Cell Endocrinol 228:23–38 [DOI] [PubMed] [Google Scholar]
- Cheng AM, Byrom MW, Shelton J, Ford LP 2005 Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res 33:1290–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Cullen BR 2003 Sequence requirements for micro RNA processing and function in human cells. RNA 9:112–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird IM, Hanley NA, Word RA, Mathis JM, McCarthy JL, Mason JI, Rainey WE 1993 Human NCI-H295 adrenocortical carcinoma cells: a model for angiotensin-II-responsive aldosterone secretion. Endocrinology 133:1555–1561 [DOI] [PubMed] [Google Scholar]
- Romero DG, Plonczynski M, Vergara GR, Gomez-Sanchez EP, Gomez-Sanchez CE 2004 Angiotensin II early regulated genes in H295R human adrenocortical cells. Physiol Genomics 19:106–116 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW 2001 A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Sanchez CE, Foecking MF, Ferris MW, Chavarri MR, Uribe L, Gomez-Sanchez EP 1987 The production of monoclonal antibodies against aldosterone. Steroids 49:581–587 [DOI] [PubMed] [Google Scholar]
- Romero DG, Vergara GR, Zhu Z, Covington GS, Plonczynski MW, Yanes LL, Gomez-Sanchez EP, Gomez-Sanchez CE 2006 Interleukin-8 synthesis, regulation, and steroidogenic role in H295R human adrenocortical cells. Endocrinology 147:891–898 [DOI] [PubMed] [Google Scholar]
- Vasudevan S, Tong Y, Steitz JA 2007 Switching from repression to activation: microRNAs can up-regulate translation. Science 318:1931–1934 [DOI] [PubMed] [Google Scholar]
- Jones-Rhoades MW, Bartel DP, Bartel B 2006 MicroRNAS and their regulatory roles in plants. Annu Rev Plant Biol 57:19–53 [DOI] [PubMed] [Google Scholar]
- Cullen BR 2006 Viruses and microRNAs. Nat Genet 38(Suppl):S25–S30 [DOI] [PubMed] [Google Scholar]
- Mallory AC, Vaucheret H 2006 Functions of microRNAs and related small RNAs in plants. Nat Genet 38(Suppl):S31–S36 [DOI] [PubMed] [Google Scholar]
- Sethupathy P, Corda B, Hatzigeorgiou AG 2006 TarBase: a comprehensive database of experimentally supported animal microRNA targets. RNA 12:192–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Si ML, Wu H, Mo YY 2007 MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1). J Biol Chem 282:14328–14336 [DOI] [PubMed] [Google Scholar]
- Meng F, Henson R, Lang M, Wehbe H, Maheshwari S, Mendell JT, Jiang J, Schmittgen TD, Patel T 2006 Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology 130:2113–2129 [DOI] [PubMed] [Google Scholar]
- Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H, MicroRNA-21 (miR-21) post-transcriptionally down-regulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 10.1038/sj.onc.1210856 [DOI] [PubMed] [Google Scholar]
- Oparil S, Zaman MA, Calhoun DA 2003 Pathogenesis of hypertension. Ann Intern Med 139:761–776 [DOI] [PubMed] [Google Scholar]
- Ong KL, Cheung BM, Man YB, Lau CP, Lam KS 2007 Prevalence, awareness, treatment, and control of hypertension among United States adults, 1999–2004. Hypertension 49:69–75 [DOI] [PubMed] [Google Scholar]
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J 2005 Global burden of hypertension: analysis of worldwide data. Lancet 365:217–223 [DOI] [PubMed] [Google Scholar]
- Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, Gomez-Sanchez CE, Veglio F, Young Jr WF 2004 Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab 89:1045–1050 [DOI] [PubMed] [Google Scholar]
- Mattsson C, Young Jr WF 2006 Primary aldosteronism: diagnostic and treatment strategies. Nat Clin Pract Nephrol 2:198–208 [DOI] [PubMed] [Google Scholar]
- Calhoun DA 2007 Is there an unrecognized epidemic of primary aldosteronism? Pro. Hypertension 50:447–453 [DOI] [PubMed] [Google Scholar]