Abstract
We tested the hypothesis that epidermal growth factor (EGF) limits hypoxia-induced apoptosis in cultured human trophoblasts by phosphorylation of the proapoptotic protein Bcl-2-associated death promoter (BAD). Cytotrophoblasts were isolated from placentas of uncomplicated pregnancies at 38–40 wk gestation. Primary trophoblasts or transfected JEG3 trophoblast cells were cultured in less than 1 or 20% oxygen in the presence or absence of EGF and signaling pathway inhibitors. BAD, green fluorescent protein (GFP)-BAD, 14-3-3, Bcl-XL, and neoepitopes formed during apoptotic cleavage of cytokeratin 18 intermediate filaments were quantified using immunoblotting. Cultures immunostained by fluorescent antibodies were analyzed by confocal microscopy for BAD and GFP. Fluorescence resonance energy transfer was used to detect molecular interaction between endogenous BAD and GFP-BAD. We found EGF increased the phosphorylation of BADser112 under standard culture conditions. Whereas hypoxia enhanced apoptosis and increased phosphorylation of both BADser136 and BADser155, hypoxia diminished phosphorylation of BADser112, and this effect was reversible by EGF. Transfected GFP-BAD, which directly interacted with endogenous BAD by colocalization and fluorescence resonance energy transfer, enhanced hypoxia-induced apoptosis in JEG3 cells. EGF reduced apoptosis in hypoxic JEG3 cells that overexpressed GFP-BAD but not in cells overexpressing GFP-BAD that harbored a serine-to-alanine mutation at the 112 site. Coimmunoprecipitation studies showed that EGF reduced the proapoptotic interaction of BAD with Bcl-XL. The effect of EGF on phosphorylation of BADser112 was dependent on the action of p38 MAPK. We conclude that EGF signals via p38 MAPK to increase phosphorylation of BADser112 and thereby limit trophoblast apoptosis.
APOPTOSIS CONTRIBUTES to trophoblast turnover in normal placental villi (1). Enhanced apoptosis occurs in clinical conditions in which placental underperfusion and villous hypoxia associate with suboptimal pregnancy outcomes (2,3,4). Trophoblasts isolated from placentas of pregnancies complicated by preeclampsia or intrauterine growth restriction (IUGR) exhibit enhanced apoptosis in response to hypoxic and cytokine stimuli, compared with trophoblasts from uncomplicated pregnancies (5).
Epidermal growth factor (EGF) and the EGF receptor play key roles in placental development and function. Mice deficient in egf develop IUGR (6) that varies, depending on the genetic background of the mouse. Egfr homozygous null mice die at d 11.5 due to abnormal placental development of the spongiotrophoblast and labyrinthine placenta (7). EGF receptor is expressed in human placenta, and altered expression associates with preeclampsia, IUGR, and pathological trophoblast invasion into the basal plate as placenta accreta (8,9,10,11,12). Collectively these studies indicate that EGF effects are important in normal placental function.
EGF also functions in another role to attenuate villous trophoblast apoptosis induced by exogenous insults such as TNF-α (13), ceramide (14), and hypoxia (15). Although recent studies identified pathways by which EGF limits cytokine-induced apoptosis (16,17), the mechanisms by which EGF limits hypoxia-induced apoptosis in villous trophoblasts are unknown.
Transduction of apoptotic stimuli proceeds through receptor-mediated cascades or mitochondrial mechanisms, with abundant cross talk between the two pathways. The Bcl-2 family of proteins transduces the death response from many stimuli that induce apoptosis (18). These proteins form heterodimers that either protect or enhance the formation of the permeability transition pore in mitochondria and thereby affect apoptosis. The 24 members of the Bcl-2 family are subclassified by the presence of one or more homology domains, BH1–4, within Bcl-2. A pivotal member within the Bcl-2 family is Bcl-2-associated death promoter (BAD), which contains only the BH3 homology domain. The antiapoptotic and proapoptotic activity of this protein is modulated by selective phosphorylation of one or more serine residues in a cell-dependent and stimulus-specific manner (18). The protective effect of EGF on hypoxia-induced trophoblast apoptosis and the pivotal position of BAD in growth factor modulation of apoptosis led us to test the hypothesis that EGF reduces hypoxia-induced apoptosis in human trophoblasts by selective phosphorylation of BAD.
Materials and Methods
Cell isolation and culture
The Institutional Review Board of Washington University School of Medicine (St. Louis, MO) approved this study. Human placentas were obtained from singleton pregnancies at 38–40 wk gestation by reliable obstetrical dates after an uncomplicated pregnancy and vaginal or abdominal delivery. Villous tissue was harvested from the placenta after removal of the basal and chorionic plates, and villous cytotrophoblasts were isolated by the method of Kliman et al. (19) with modifications previously described (20). Elimination of coisolated fragments of syncytium was done as described by Guilbert et al. (21) after 4 h to allow cell attachment. Cytotrophoblasts were cultured at a density of 3.5 × 105 cells/cm2 in DMEM (Tissue Culture Facility, Washington University, St. Louis, MO)-based medium, as described (20). JEG3 human choriocarcinoma cells were maintained in MEM containing 10% fetal bovine serum and antibiotics, and the medium was replaced every 24 h. Standard conditions for cell culture were 5% CO2-95% air (FiO2 = 20%) at 37 C in an incubator (Nuaire, Plymouth, MN), and cells were cultured for the times listed in the legends in the absence or presence of vehicle alone; EGF (100 ng/ml; Upstate, Lake Placid, NY); AG1478 (10 μm; Calbiochem, San Diego, CA); or the kinase inhibitors (all purchased from Calbiochem) phosphatidylinositol 3 kinase (PI-3 kinase; AKT) inhibitor LY294002 (10 μm), the p42/44 (ERK 1/2) kinase inhibitor PD98059 (20 μm), the p38 MAPK kinase (MEK; p38) inhibitor SB203580 (10 μm), c-Jun N-terminal kinase (JNK) inhibitor SP600125 (10 μm), or the sphingosine kinase inhibitor dimethylsphingosine (20 μm).
Exposure to hypoxia
Cells were maintained in hypoxia in a glove box-type anaerobic chamber (Thermo Forma, Marietta, OH). The hypoxic atmosphere was less than 1% O2, 5% CO2, 10% H2, 85% N2 with continuous computerized monitoring indicating a partial pressure of O2 less than 15 mm Hg at 37 C. An attached interchange chamber ensured that the atmosphere of the main chamber remained hypoxic. The media inside the main chamber were purged of dissolved oxygen by bubbling with the above-listed hypoxic gas mixture for 5 min per 100 ml medium. Primary trophoblasts or JEG3 cells were exposed to standard conditions of 20% oxygen or to hypoxia in vehicle control or 100 ng/ml EGF.
DNA plasmids and transfection
Dr. Aviva Tolkovsky (University of Cambridge, Cambridge, UK) (22) provided plasmids expressing murine BAD proteins. The plasmids include wild-type BAD as well as BAD harboring a mutation of serine 112 to alanine, cloned into the pEGFP-N1 mammalian expression vector [termed green fluorescent protein (GFP)-BAD or GFP-mBADS112A, respectively]. JEG3 cells (10,000 cells/cm2) were plated 16 h before transfection. The modified calcium-phosphate coprecipitation method was used for transfection, as previously described (23) using 3 μg of plasmids. The culture medium was replaced 18 h after transfection, and cells were cultured in standard or hypoxic conditions for an additional 24 h before assessment of apoptosis or protein harvest.
Western immunoblotting
This analysis was performed as previously described (24) and modified as follows. Cells were lysed in buffer containing 10 mmol/liter HEPES, 7.5 mmol/liter MgCl2, 1% Triton X-100, 2 mmol/liter EGTA, 1 mmol/liter DTT, protease and phosphatase inhibitor cocktails (Sigma). Cells were scraped from dishes, and the slurry was incubated in ice for 10 min and then sonicated twice for 15 sec each time on ice. Protein aliquots of 15–30 μg/lane were electrophoresed at 100 V for 1–2 h in 10% sodium dodecyl sulfate-polyacrylamide gels, transferred onto polyvinylidene difluoride membranes (Millipore, Bedford, MA) for 1–2 h at 200–250 mA at 4 C, blocked with 5% skim milk for 1 h, and incubated overnight at 4 C with rabbit monoclonal antibodies (Cell Signaling Technology, Beverly, MA) to BAD or site-specific phosphorylated BADser112 (1:500), BADser136 (1:250), or BADser155 (1:500), the M30 antibody that detects the neoepitopes formed during apoptosis in the cytokeratin 18 intermediate filaments (cyt 18; 1:250; Roche, Indianapolis, IN) and antibody to actin (final 0.2 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA); washed twice for 10 min with Tris-buffered saline and 0.2% Tween 20; and incubated for 1 h with horseradish peroxidase-conjugated antirabbit IgG antibody (1:2000; Santa Cruz). The membranes were processed for chemiluminescence using a Western Lightning ECL kit (PerkinElmer, Boston, MA), and densitometry was performed by Epichemi-3 software (UVP BioImaging System, Upland, CA). The level of all proteins was normalized to the expression of actin.
Immunocytochemistry
Duplicate cultures (n = 4 for each paradigm) were fixed in ice-cold methanol for 20 min and blocked with 1% BSA and 0.1% Tween 20 in PBS for 30 min. Apoptosis was assessed by immunostaining of cultured cells with the M30 antibody (1:100; Roche), as previously described (25). Controls included omission of the primary antibody or addition of nonspecific immune serum instead of the primary antibody. Cell nuclei were counterstained with Topro3 (Molecular Probes, Eugene, OR), rinsed in PBS, mounted with Vectashield (Vector, Burlingame, CA), and viewed under a Nikon T800 confocal microscope equipped with epifluorescence optics, a digital image capture system (Image Content Technology, New Britain, CT) and MagnaFire software (Optronics, Goleta, GA). Images of five random fields at ×400 were captured, and the percentage of cells expressing cyt 18 among all nuclei stained by 4′,6′-diamino-2-phenylindole was used as a measure of apoptosis.
Fluorescence resonance energy transfer (FRET)
Cells were fixed in 2% paraformaldehyde/PBS for 15 min on culture dishes and permeabilized with 0.5% Triton X-100 in PBS for 5 min before blocking in PBS with 3% BSA at room temperature. Dishes were incubated with rabbit monoclonal anti-BAD (1:100) and murine monoclonal anti-GFP (IgGk1 clones: 7.1 and 13.1, 1:100; Roche) for 4 h at 4 C and subsequently incubated with donkey antirabbit secondary antibody (1:200) conjugated with Alexa 546 or donkey antimouse secondary antibody (1:200) conjugated with Alexa 488. Images in five fields were evaluated for background fluorescence in specimens incubated with nonspecific immune serum. Background fluorescence from this control was subtracted from the expected yellow signal that resulted from the red and green colocalization fluorescence of the two proteins. A FRET signal was also determined. The FRET signal was expected if there was a molecular interaction of the fluorophores for BAD and GFP, as analyzed in each field using Autoquant software (Autoquant Imaging, Inc., Troy, NY) with FRET plug-in and after subtracting background fluorescence from inherent spectral overlap in the fluorophores in the microscope detector and filter system.
Coimmunoprecipitation (IP)
JEG3 cells were cultured for 48 h in standard or hypoxic conditions, and cells were lysed in cold co-IP lysis buffer [50 mm Tris-HCl (pH 7.6), 150 mm NaCl, 2 mm EDTA, 0.5% Triton X-100, and 1% Nonidet P-40] containing a protease inhibitor cocktail (Sigma, St. Louis, MO). The lysate was centrifuged and protein concentration determined (Bio-Rad, Hercules, CA). Crude lysates were precleared with protein A Sepharose beads (Sigma) and incubated with anti-BAD antibodies (1:100) at 4 C overnight. Immune complexes were precipitated with protein A-Sepharose beads followed by two washes in IP buffer [50 mm Tris-HCl (pH 7.6), 2 mm EDTA containing either 500 or 150 mm NaCl]. The pellet was resuspended in 2× Laemmli sample buffer and proteins were resolved by SDS-PAGE and immunoblotted for 14-3-3 or Bcl-XL (both from Cell Signaling) as above.
Statistics
The number of primary cultures is listed in each figure legend and was three or more for all experiments. Data are expressed as mean ± sd. Comparisons were analyzed using ANOVA (Primer of biostatistics, McGraw-Hill, New York, NY) with Bonferroni correction for multiple comparisons or by t test. P < 0.05 was considered significant.
Results
EGF enhances phosphorylation of BADser112 in cultured human trophoblasts
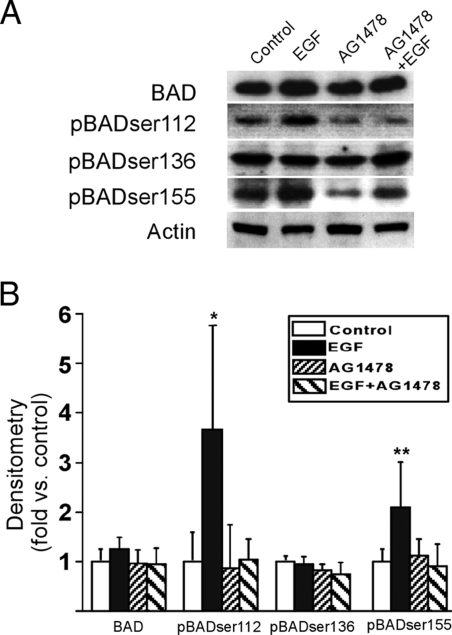
Cytotrophoblasts cultured in standard conditions for 24 h expressed BAD and the three phosphorylated isoforms, BADser112, BADser136, and BADser155 (Fig. 1). Compared with 24-h cells exposed to vehicle only, EGF enhanced the expression of phosphorylated BADser112 and phosphorylated BADser155, but EGF had no effect on BAD or BADser136 (Fig. 1). The effect of EGF on BADser112, but not BADser155, was specific for the EGF receptor because the increased expression of phosphorylated BADser112 was diminished when trophoblasts were coincubated with EGF and the EGF receptor antagonist AG1478, compared with vehicle-treated control cells (Fig. 1). These data indicate that EGF stimulation of EGF receptor in trophoblasts selectively enhances the phosphorylation of BADser112.
Figure 1.
The influence of EGF on the expression of BAD and phosphorylated BAD in cultured human trophoblasts. A, A representative immunoblot of four isoforms of BAD in trophoblasts cultured in standard conditions with vehicle (control) or 100 ng/ml EGF, the EGF receptor tyrosine kinase inhibitor AG1478 (10 μm), or both. B, Densitometric analysis of the six primary cultures examined. Vehicle control vs. EGF exposure for phosphorylated BADser112 (P < 0.01) and phosphorylated BADser155 (P < 0.05) was significant (ANOVA with Bonferroni correction).
Hypoxia modulates phosphorylation of BAD
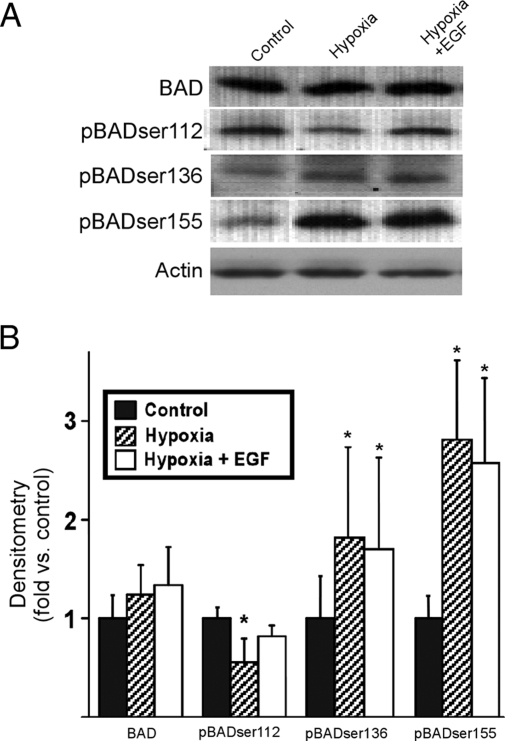
We have previously shown that hypoxia enhances apoptosis in cytotrophoblasts, and EGF abrogates this effect (15). We questioned whether hypoxia regulated the phosphorylation of BAD. Trophoblasts cultured for 24 h in standard or hypoxic conditions expressed similar levels of BAD, and these levels were not influenced by exposure to hypoxia or in the presence of EGF (Fig. 2). Importantly, hypoxia diminished the expression of phosphorylated BADser112 yet increased the expression of phosphorylated BADser136 and BADser155 (Fig. 2). Moreover, the addition of EGF to trophoblasts during exposure to hypoxia restored phosphorylated BADser112 to a level not significantly different from that of vehicle-exposed control cells in standard conditions (Fig. 2). EGF had no effect on hypoxia-induced phosphorylation of BADser136 or BADser155.
Figure 2.
The influence of hypoxia and EGF on the expression of BAD and phosphorylated BAD. The upper panel (A) is an immunoblot of the isoforms of BAD and actin in trophoblasts cultured in a FiO2 = 20% with vehicle (control) or FiO2 less than 1% (hypoxia) in the presence or absence of 100 ng/ml EGF. The lower panel (B) is the densitometric analysis of isoforms (n = 6) normalized to actin. *, P < 0.05 vs. control (ANOVA with Bonferroni correction).
Transfected GFP-BAD physically interacts with endogenous BAD in JEG3 cells
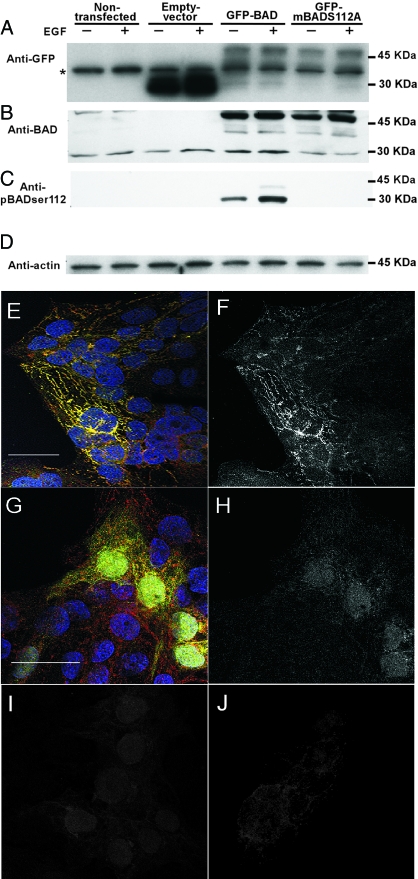
We next sought a trophoblast cell line for transfection of expression plasmids of BAD to establish the role of the phosphorylated BADser112 in hypoxia-induced apoptosis. We chose JEG3 cells because this trophoblast cell line expressed BAD and the three phosphorylated forms of BAD, albeit at low levels. Thirty minutes of exposure to EGF enhanced phosphorylation of BADser112 in JEG3 cells, which also exhibited enhanced apoptosis when exposed to hypoxic conditions (data not shown). We thus transfected JEG3 cells with vector alone, GFP-tagged wild-type BAD (GFP-BAD), or GFP-BAD mutated from serine to alanine at the 112 site (GFP-mBADS112A). Cell lysates were screened by Western analysis to identify endogenous BAD and GFP-BAD protein (Fig. 3, A–D). JEG3 cells expressed endogenous BAD and those transfected with the vector expressed GFP. JEG3 transfected with GFP-BAD, with or without the mutation, expressed the fusion protein at the expected molecular weight (Fig. 3, A–C). In the absence of EGF, the level of phosphorylated BADser112 was below the level of detection in nontransfected JEG3. JEG3 transfected with wild-type GFP-BAD yielded detectable phosphorylated BADser112, and this expression was increased in cells exposed to EGF (Fig. 3C). As expected, no phosphorylated BADser112 was detectable in cells transfected with GFP-mBADS112A, either in the absence or presence of EGF. Using confocal fluorescence microscopy, we colocalized endogenous BAD and GFP-BAD by identification of a yellow signal that resulted from the spectral overlap of red and green fluorescence, emitted by the fluorophores for the anti-BAD and anti-GFP (Fig. 3 E). Using FRET analysis, we also detected direct interaction between endogenous BAD and the GFP-BAD fusion protein (Fig. 3F). In contrast, GFP-expressing control cells showed separate red and green fluorescence that did not colocalize, and no specific FRET signal was detected (Fig. 3, G–J).
Figure 3.
The expression and interaction of BAD and GFP-BAD in transfected JEG3 cells. Lysates of JEG3 cells stably transfected with vector alone, GFP-BAD, or GFP-mBADS112A were screened to identify GFP (A), endogenous BAD (23 kDa; B), GFP-BAD (46 kDa; B), phosphorylated BADser112 (pBAD-ser112; C), and actin (D). An asterisk (A) indicates a nonspecific band present in all paradigms using the anti-GFP antibody. The level of phosphorylated BADser112 was below the level of detection in the JEG3 and vector control cells examined in the blot of C. E, A confocal fluorescence microscopy image of JEG3 cells transfected with GFP-BAD and immunostained for both endogenous BAD (anti-BAD primary antibody with secondary antibody conjugated to Alexa 546, red fluorescence) and GFP-BAD fusion protein (anti-GFP primary antibody with secondary antibody conjugated to Alexa 488, green fluorescence). A punctate yellow signal resulted in transfected cells, consistent with preferential localization in mitochondria. This yellow signal resulted from the spectral overlap of the red and green fluorescence, indicating endogenous BAD and the GFP-BAD fusion protein were in the same subcellular compartment. A FRET signal was also detected in this field (F) to confirm that there was not only colocalization, but also a molecular interaction, of endogenous BAD and the GFP-BAD fusion protein. Control images of JEG3 cells transfected with GFP-only vector (G) showed punctate red and diffuse green fluorescence throughout transfected cells, with no punctate yellow signal, indicating no specific subcellular colocalization. There was no specific FRET signal detected in these cultures (H). Controls for the FRET analysis are shown for the spectral overlap of the fluorophores for the anti-BAD labeling alone (I) and anti-GFP labeling alone (J). This spectral overlap fluorescence was subtracted from the FRET-calculated images as shown in F and H (bar, 10 μm).
EGF does not attenuate apoptosis in JEG3 trophoblasts overexpressing GFP-mBADS112A
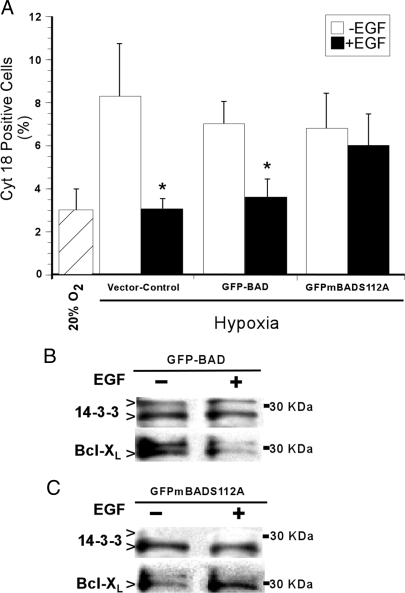
We used immunocytochemical analysis for cyt 18 as a measure of apoptosis. JEG3 cells transfected with the empty vector and grown in standard culture conditions (FiO2 = 20%) showed cyt 18 levels of 3.0% ± 0.7 (Fig. 4 A), not different from the 3.7% ± 0.9% in GFP-BAD transfected cells or 2.9% ± 0.7% in GFP-mBADS112A transfected cells, also grown in an FiO2 = 20%. Hypoxia increased the number of cells expressing cyt 18 by 2- to 3-fold in all three transfected JEG3 paradigms (Fig. 4A). Whereas EGF significantly reduced the level of cyt 18 expression in hypoxic control cells and hypoxic cells transfected with GFP-BAD, EGF had no effect on the number of cells expressing cyt 18 in hypoxic JEG3 cells expressing GFP-mBADS112A protein (Fig. 4A). These data establish that the BADser112 site is critical for the protective effect of EGF against hypoxia-induced apoptosis in trophoblasts.
Figure 4.
Apoptosis and BAD interaction with 14-3-3 and Bcl-XL in JEG3 cells. Apoptosis was assessed (A; n = 3) by immunocytochemical detection of cyt 18 filament neoepitopes in transfected JEG3 cells in the presence or absence of EGF in a FiO2 = 20% or hypoxia (FiO2 < 1%). *, P < 0.05 vs. no EGF, t test. Cell lysates from JEG3 cells ± 100 ng/ml EGF, transfected with either wild-type GFP-BAD (B) or GFP-mBADS112A (C), were immunoprecipitated with an antibody to BAD before immunoblotting for 14-3-3 proteins and Bcl-XL (27 kDa). The upper band of the 14-3-3 proteins detected showed interaction with BAD in the wild-type transfectants (B), but this interaction was not detected in the mutant BAD transfectants (C). The other band detected by the 14-3-3 antibody showed interaction with BAD in both transfectants.
We conducted coimmunoprecipitation experiments to examine the action of phosphorylated BADser112 in hypoxia-induced apoptosis. JEG3 cells transfected with wild-type GFP-BAD (Fig. 4B) or GFP-mBADS112A (Fig. 4C) were exposed to vehicle or EGF, and lysates were immunoprecipitated with an antibody to BAD before immunoblotting for 14-3-3 proteins or Bcl-XL. In the absence of EGF, transfectants with wild-type GFP-BAD exhibited BAD associated with both 14-3-3 protein and Bcl-XL (Fig. 4B). Importantly, EGF reduced GFP-BAD association with Bcl-XL with a minimal increase of BAD with 14-3-3 proteins (Fig. 4B). Conversely, JEG3 transfectants with GFP-mBADS112A showed no detectable interaction of BAD with one of the two 14-3-3 protein isoforms present and increased interaction with Bcl-XL in the presence of EGF (Fig. 4C).
EGF signals phosphorylation of BADser112 through p38
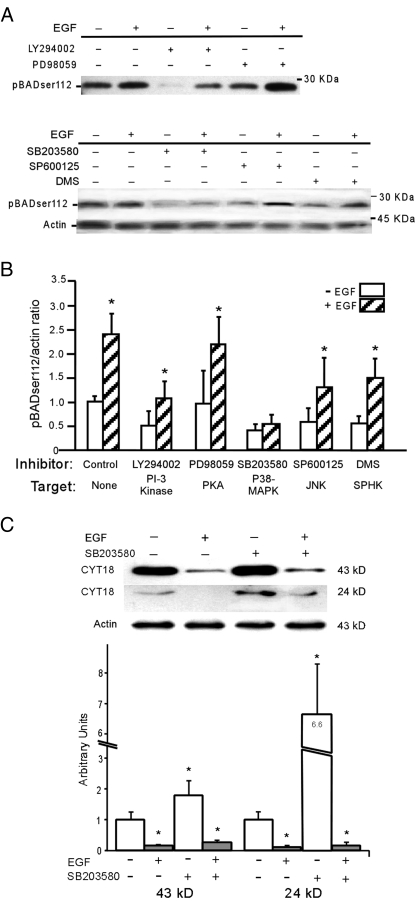
EGF signals through multiple cascades in human trophoblasts (16). We used selective kinase inhibitors to dissect the specific EGF signaling pathway(s) that modulate phosphorylation of BADser112. We found that the EGF-dependent, enhanced expression of phosphorylated BADser112 was abrogated exclusively by SB203580, a selective inhibitor of p38 (Fig. 5, A and B). None of the other inhibitors affected the increased level of expression of phosphorylated BADser112 in response to EGF (Fig. 5), although LY294002 inhibited basal levels of phosphorylated BADser112. We established that each inhibitor was active by verifying reduced phosphorylation of the known protein targets listed on the x-axis of Fig. 5B for each pathway, compared with control (data not shown).
Figure 5.
The effect of kinase inhibitors on the expression of phosphorylated (p) BADser112. Primary trophoblasts were exposed for 1.5 h [PI-3 kinase and protein kinase A (PKA) inhibitors; n = 3] or 24 h [p38, JNK, and sphingosine-1-PO4 kinase (SPHK) inhibitors; n = 6] in the absence or presence of 100 ng/ml EGF, with or without the following inhibitors (kinase listed in parentheses): 10 μm LY 294002 (PI-3 kinase); 20 μm PD98509 (ERK 1/2); 10 μm SB203580 (p38 MAPK); 10 μm SP600125 (JNK); and 20 μm dimethylsphingosine (DMS; SPHK). Proteins were immunoblotted for expression of phosphorylated BADser112, normalized to actin. A, A representative blot. B, Densitometric analysis, normalized to actin. *, P < 0.05 for control vs. EGF (t test). C, An immunoblot and densitometry of the Western analysis of lysates of primary trophoblasts (n = 3) exposed 24 h to vehicle or the p38 inhibitor SB203580. Expression by arbitrary units of both the 24- and 43-kDa peptides that expose a neoepitope in cyt 18 filaments when cleaved by caspase-3 (normalized to actin). *, P < 0.01 vehicle vs. EGF or p38 inhibitor (ANOVA with Bonferroni).
We next questioned whether inhibition of the p38 pathway in primary trophoblasts would itself enhance apoptosis. As predicted, we found that cultures exposed to the p38 inhibitor SB203580, compared with vehicle control cells, showed an increased level of apoptosis, as determined by a higher expression of both the 43- and the 24-kDa proteins with the neoepitopes of cyt 18 (Fig. 5C). Addition of EGF protected trophoblasts from the effects of the p38 inhibitor. Collectively these data indicate that p38 mediates EGF-stimulated phosphorylation of BADser112 at the time point studied and consequently an EGF-dependent reduction in apoptosis in hypoxic trophoblasts.
Discussion
The data show that EGF increases phosphorylation of BADser112 through the EGF receptor, with no effect on expression of BAD or phosphorylated BADser136 and an effect on BADser155 that is unlikely transduced through the EGF receptor. Hypoxia enhances apoptosis and diminishes expression of phosphorylated BADser112. Importantly, EGF reduces hypoxia-induced apoptosis and rescues phosphorylation of BADser112 in hypoxic trophoblasts to yield expression levels similar to, but not higher than, control nonhypoxic cells. The absence of a higher than control level of BADser112 after EGF treatment suggests that EGF protection from apoptosis is incomplete, a finding consistent with our previous data (15). EGF also reduces hypoxia-induced apoptosis in JEG3 cells transfected with wild-type GFP-BAD, but EGF has no effect on hypoxic trophoblasts transfected with GFP-BAD mutated at the serine 112 site, underscoring the key role for this BAD phosphorylation site in the effect of EGF on trophoblasts. EGF reduces the association of phosphorylated BADser112 with Bcl-XL, thereby limiting the proapoptotic interaction of BAD with Bcl-XL. Whereas EGF activates multiple signaling cascades in trophoblasts (16), the p38 pathway transduces EGF-dependent phosphorylation of BADser112 in trophoblasts under the conditions of our experiments. Moreover, a p38 inhibitor enhances trophoblast apoptosis, verifying a role for p38 in modulating cell death. We concluded that EGF selectively enhances phosphorylation of BADser112 through the p38 pathway as one mechanism for EGF protection against apoptosis in human trophoblasts.
Placentas of pregnancies complicated by preeclampsia and some forms of IUGR are characterized by underperfusion and exhibit signs of villous hypoxia (26,27). Placentas from women with these clinical entities also exhibit enhanced apoptosis (2,3,28). Our studies (15) and those of others (29) show that extreme hypoxia with pO2 less than 15 mm Hg causes apoptosis in cultured term trophoblasts, an effect abrogated by EGF (15). EGF also hinders cytokine-induced apoptosis (13,16,30) and apoptosis stimulated by reactive oxygen species (31) in cultured human trophoblasts. EGF therefore not only regulates development and function of the normal placenta (6,7) but also plays a key role in protecting the trophoblast from the death-inducing effects of multiple exogenous stimuli. Ours is the first study to identify a mechanism for the protective effect of EGF on hypoxia-induced apoptosis in human trophoblasts, by selective phosphorylation of BADser112.
Whereas native BAD functions as a latent death effector, phosphorylated BAD participates in normal mitochondrial physiology (32,33). When homeostasis is disrupted, unphosphorylated BAD heterodimerizes with Bcl-XL, resulting in enhanced apoptotic cell death because the antiapoptotic function of Bcl-XL is neutralized (34). We and others (35,36) show that phosphorylated BADser112 coimunoprecipitates with 14-3-3 proteins that reside in the cytoplasm, and this protein-protein interaction sequesters BAD from heterodimer formation with Bcl-XL in mitochondria.
The signaling pathways that selectively phosphorylate BAD are multiple and cell type specific. For example, UV radiation activates survival pathways in epidermal cells by phosphorylation of BADser112 via ERK, JNK, and p38 MAP kinases (37). Additionally, EGF limits apoptosis in prostate cancer cells by activation of the Ras/MEK module, which enhances expression of phosphorylated BADser112, and Rac/Pac1 signals, which yields higher levels of phosphorylated BADser136 (38). Fibroblasts depend on activation of MAPK because the MEK inhibitor blocks EGF-induced phosphorylation of BADser112 but not phosphorylation of BADser136. The PI-3 kinase is an alternative source for phosphorylation of selected serine sites on BAD (39). These studies guided our inhibitor experiments to target pathways that might play a role in selective phosphorylation of BAD.
The effects of p38 signaling on differentiation of trophoblasts is established in studies of p38-null mice (40,41) and human trophoblasts (17,42) with cross talk between p38 and peroxisomal proliferator-activated receptor-γ signaling modulating differentiation (43). Guilbert and colleagues (16) showed that EGF stimulates phosphorylation and activation of p38, but this effect did not promote resistance to TNF-α/interferon-γ-induced apoptosis. Protection from cytokine-induced injury was instead mediated through multiple other signaling pathways, including the PI-3 kinase/AKT, ERK kinase, JNK kinase, and sphingosine kinase 1 (16). That study (16) also reported that inhibition of the p38 pathway had no effect on apoptosis. This contrasts with our finding that p38 inhibition enhances cell death in human trophoblasts. The differing results are likely due to the longer exposure to the p38 inhibitor before cell death assay (i.e. 12 vs. 24 h) and our assessment of apoptosis using the cleavage of cyt 18 filaments as a measure instead of the less specific terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling assay. Our results also contrast with the role of activated p38 to mediate enhanced apoptosis in Fas-induced death of CD8+ T cells (44) or increase TNF-α-induced apoptosis in endothelial cells, in which p38 activation reduces phosphorylation of BADser112 and enhances cell death (45). Collectively these data allow us to speculate that the effect of EGF activation on hypoxia-induced apoptosis in human trophoblasts may play a role in the placental responses to hypoxic injury in vivo. We suggest that the lower activity for the p38 pathway in placentas of women with preeclampsia (46) may contribute to the enhanced trophoblast apoptosis characteristic of villous trophoblasts in preeclampsia (3,5). Further studies are needed to verify this speculation.
Acknowledgments
We thank Linda Dioneda for assistance in manuscript preparation and Dr. A. Tolkovsky (University of Cambridge, Cambridge, UK) for generously providing the GFP-BAD expression plasmids.
Footnotes
This work was supported by National Institutes of Health Grants R01 HD29190 (to D.M.N.) and R01 HD45675 (to Y.S.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online February 14, 2008
Abbreviations: BAD, Bcl-2-associated death promoter; cyt 18, cytokeratin 18 intermediate filaments; EGF, epidermal growth factor; FRET, fluorescence resonance energy transfer; GFP, green fluorescent protein; IP, immunoprecipitation; IUGR, intrauterine growth restriction; JNK, c-Jun N-terminal kinase; MEK, MAPK kinase; PI-3 kinase, phosphatidylinositol 3 kinase.
References
- Huppertz B, Frank HG, Kingdom JC, Reister F, Kaufmann P 1998 Villous cytotrophoblast regulation of the syncytial apoptotic cascade in the human placenta. Histochem Cell Biol 110:495–508 [DOI] [PubMed] [Google Scholar]
- Smith SC, Baker PN, Symonds EM 1997 Increased placental apoptosis in intrauterine growth restriction. Am J Obstet Gynecol 177:1395–1401 [DOI] [PubMed] [Google Scholar]
- Allaire AD, Ballenger KA, Wells SR, McMahon MJ, Lessey BA 2000 Placental apoptosis in preeclampsia. Obstet Gynecol 96:271–276 [DOI] [PubMed] [Google Scholar]
- Leung DN, Smith SC, To KF, Sahota DS, Baker PN 2001 Increased placental apoptosis in pregnancies complicated by preeclampsia. Am J Obstet Gynecol 184:1249–1250 [DOI] [PubMed] [Google Scholar]
- Crocker IP, Cooper S, Ong SC, Baker PN 2003 Differences in apoptotic susceptibility of cytotrophoblasts and syncytiotrophoblasts in normal pregnancy to those complicated with preeclampsia and intrauterine growth restriction. Am J Pathol 162:637–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei Y, Tsutsumi O, Yamakawa A, Oka Y, Taketani Y, Imaki J 1999 Maternal epidermal growth factor deficiency causes fetal hypoglycemia and intrauterine growth retardation in mice: possible involvement of placental glucose transporter GLUT3 expression. Endocrinology 140:4236–4243 [DOI] [PubMed] [Google Scholar]
- Sibilia M, Steinbach JP, Stingl L, Aguzzi A, Wagner EF 1998 A strain-independent postnatal neurodegeneration in mice lacking the EGF receptor. EMBO J 17:719–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandina G, Lanzone A, Scambia G, Caruso A, Panici PB, Mancuso S 1995 Epidermal growth factor receptors in placentae and fetal membranes from hypertension-complicated pregnancies. Hum Reprod 10:1845–1849 [DOI] [PubMed] [Google Scholar]
- Fondacci C, Alsat E, Gabriel R, Blot P, Nessmann C, Evain-Brion D 1994 Alterations of human placental epidermal growth factor receptor in intrauterine growth retardation. J Clin Invest 93:1149–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann GE, Drews MR, Scott Jr RT, Navot D, Heller D, Deligdisch L 1992 Epidermal growth factor and its receptor in human implantation trophoblast: immunohistochemical evidence for autocrine/paracrine function. J Clin Endocrinol Metab 74:981–988 [DOI] [PubMed] [Google Scholar]
- Reiter JL, Maihle NJ 2003 Characterization and expression of novel 60-kDa and 110-kDa EGFR isoforms in human placenta. Ann NY Acad Sci 995:39–47 [DOI] [PubMed] [Google Scholar]
- Tseng JJ, Hsu SL, Wen MC, Ho ES, Chou MM 2004 Expression of epidermal growth factor receptor and c-erbB-2 oncoprotein in trophoblast populations of placenta accreta. Am J Obstet Gynecol 191:2106–2113 [DOI] [PubMed] [Google Scholar]
- Garcia-Lloret MI, Yui J, Winkler-Lowen B, Guilbert LJ 1996 Epidermal growth factor inhibits cytokine-induced apoptosis of primary human trophoblasts. J Cell Physiol 167:324–332 [DOI] [PubMed] [Google Scholar]
- Payne SG, Brindley DN, Guilbert LJ 1999 Epidermal growth factor inhibits ceramide-induced apoptosis and lowers ceramide levels in primary placental trophoblasts. J Cell Physiol 180:263–270 [DOI] [PubMed] [Google Scholar]
- Levy R, Smith SD, Chandler K, Sadovsky Y, Nelson DM 2000 Apoptosis in human cultured trophoblasts is enhanced by hypoxia and diminished by epidermal growth factor. Am J Physiol Cell Physiol 278:C982–C988 [DOI] [PubMed] [Google Scholar]
- Johnstone ED, Mackova M, Das S, Payne SG, Lowen B, Sibley CP, Chan G, Guilbert LJ 2005 Multiple anti-apoptotic pathways stimulated by EGF in cytotrophoblasts. Placenta 26:548–555 [DOI] [PubMed] [Google Scholar]
- Johnstone ED, Sibley CP, Lowen B, Guilbert LJ 2005 Epidermal growth factor stimulation of trophoblast differentiation requires MAPK11/14 (p38 MAP kinase) activation. Biol Reprod 73:1282–1288 [DOI] [PubMed] [Google Scholar]
- Reed JC, Doctor K, Rojas A, Zapata JM, Stehlik C, Fiorentino L, Damiano J, Roth W, Matsuzawa S, Newman R, Takayama S, Marusawa H, Xu F, Salvesen G, Godzik A 2003 Comparative analysis of apoptosis and inflammation genes of mice and humans. Genome Res 13:1376–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss 3rd JF 1986 Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology 118:1567–1582 [DOI] [PubMed] [Google Scholar]
- Nelson DM, Johnson RD, Smith SD, Anteby EY, Sadovsky Y 1999 Hypoxia limits differentiation and up-regulates expression and activity of prostaglandin H synthase 2 in cultured trophoblast from term human placenta. Am J Obstet Gynecol 180:896–902 [DOI] [PubMed] [Google Scholar]
- Guilbert LJ, Winkler-Lowen B, Sherburne R, Rote NS, Li H, Morrish DW 2002 Preparation and functional characterization of villous cytotrophoblasts free of syncytial fragments. Placenta 23:175–183 [DOI] [PubMed] [Google Scholar]
- Virdee K, Parone PA, Tolkovsky AM 2000 Phosphorylation of the pro-apoptotic protein BAD on serine 155, a novel site, contributes to cell survival. Curr Biol 10:1151–1154 [DOI] [PubMed] [Google Scholar]
- Mouillet JF, Sonnenberg-Hirche C, Yan X, Sadovsky Y 2004 p300 regulates the synergy of steroidogenic factor-1 and early growth response-1 in activating luteinizing hormone-beta subunit gene. J Biol Chem 279:7832–7839 [DOI] [PubMed] [Google Scholar]
- Hu C, Smith SD, Pang L, Sadovsky Y, Nelson DM 2006 Enhanced basal apoptosis in cultured term human cytotrophoblasts is associated with a higher expression and physical interaction of p53 and Bak. Placenta 27:978–983 [DOI] [PubMed] [Google Scholar]
- Kamudhamas A, Pang L, Smith SD, Sadovsky Y, Nelson DM 2004 Homocysteine thiolactone induces apoptosis in cultured human trophoblasts: a mechanism for homocysteine-mediated placental dysfunction? Am J Obstet Gynecol 191:563–571 [DOI] [PubMed] [Google Scholar]
- Kingdom JC, Kaufmann P 1997 Oxygen and placental villous development: origins of fetal hypoxia. Placenta 18:613–621; discussion 623–626 [DOI] [PubMed] [Google Scholar]
- Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, Post M, Caniggia I 2005 Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab 90:4299–4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker IP, Barratt S, Kaur M, Baker PN 2001 The in vitro characterization of induced apoptosis in placental cytotrophoblasts and syncytiotrophoblasts. Placenta 22:822–830 [DOI] [PubMed] [Google Scholar]
- Kilani RT, Mackova M, Davidge ST, Guilbert LJ 2003 Effect of oxygen levels in villous trophoblast apoptosis. Placenta 24:826–834 [DOI] [PubMed] [Google Scholar]
- Smith S, Francis R, Guilbert L, Baker PN 2002 Growth factor rescue of cytokine mediated trophoblast apoptosis. Placenta 23:322–330 [DOI] [PubMed] [Google Scholar]
- Moll SJ, Jones CJ, Crocker IP, Baker PN, Heazell AE 2007 Epidermal growth factor rescues trophoblast apoptosis induced by reactive oxygen species. Apoptosis 12:1611–1622 [DOI] [PubMed] [Google Scholar]
- Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, Datta SR, Greenberg ME, Licklider LJ, Lowell BB, Gygi SP, Korsmeyer SJ 2003 BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature 424:952–956 [DOI] [PubMed] [Google Scholar]
- Seo SY, Chen YB, Ivanovska I, Ranger AM, Hong SJ, Dawson VL, Korsmeyer SJ, Bellows DS, Fannjiang Y, Hardwick JM 2004 BAD is a pro-survival factor prior to activation of its pro-apoptotic function. J Biol Chem 279:42240–42249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha J, Harada H, Osipov K, Jockel J, Waksman G, Korsmeyer SJ 1997 BH3 domain of BAD is required for heterodimerization with BCL-XL and pro-apoptotic activity. J Biol Chem 272:24101–24104 [DOI] [PubMed] [Google Scholar]
- Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ 1996 Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L). Cell 87:619–628 [DOI] [PubMed] [Google Scholar]
- Zhou XM, Liu Y, Payne G, Lutz RJ, Chittenden T 2000 Growth factors inactivate the cell death promoter BAD by phosphorylation of its BH3 domain on Ser155. J Biol Chem 275:25046–25051 [DOI] [PubMed] [Google Scholar]
- She QB, Ma WY, Zhong S, Dong Z 2002 Activation of JNK1, RSK2, and MSK1 is involved in serine 112 phosphorylation of Bad by ultraviolet B radiation. J Biol Chem 277:24039–24048 [DOI] [PubMed] [Google Scholar]
- Sastry KS, Karpova Y, Kulik G 2006 Epidermal growth factor protects prostate cancer cells from apoptosis by inducing BAD phosphorylation via redundant signaling pathways. J Biol Chem 281:27367–27377 [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME 1997 Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231–241 [DOI] [PubMed] [Google Scholar]
- Mudgett JS, Ding J, Guh-Siesel L, Chartrain NA, Yang L, Gopal S, Shen MM 2000 Essential role for p38α mitogen-activated protein kinase in placental angiogenesis. Proc Natl Acad Sci USA 97:10454–10459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RH, Porras A, Alonso G, Jones M, Vintersten K, Panelli S, Valladares A, Perez L, Klein R, Nebreda AR 2000 Essential role of p38α MAP kinase in placental but not embryonic cardiovascular development. Mol Cell 6:109–116 [PubMed] [Google Scholar]
- Daoud G, Amyot M, Rassart E, Masse A, Simoneau L, Lafond J 2005 ERK1/2 and p38 regulate trophoblasts differentiation in human term placenta. J Physiol 566:409–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild RL, Sonnenberg-Hirche CM, Schaiff WT, Bildirici I, Nelson DM, Sadovsky Y 2006 The kinase p38 regulates peroxisome proliferator activated receptor-γ in human trophoblasts. Placenta 27:191–199 [DOI] [PubMed] [Google Scholar]
- Farley N, Pedraza-Alva G, Serrano-Gomez D, Nagaleekar V, Aronshtam A, Krahl T, Thornton T, Rincon M 2006 p38 mitogen-activated protein kinase mediates the Fas-induced mitochondrial death pathway in CD8+ T cells. Mol Cell Biol 26:2118–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grethe S, Porn-Ares MI 2006 p38 MAPK regulates phosphorylation of Bad via PP2A-dependent suppression of the MEK1/2-ERK1/2 survival pathway in TNF-α induced endothelial apoptosis. Cell Signal 18:531–540 [DOI] [PubMed] [Google Scholar]
- Webster RP, Brockman D, Myatt L 2006 Nitration of p38 MAPK in the placenta: association of nitration with reduced catalytic activity of p38 MAPK in pre-eclampsia. Mol Hum Reprod 12:677–685 [DOI] [PubMed] [Google Scholar]