Abstract
Estrogen-based hormone therapy (HT) in postmenopausal women may reduce the risk of Alzheimer’s disease (AD), although HT remains controversial. One key concern with HT is the potential of adverse outcomes such as breast and uterine cancer. A promising strategy to maximize HT benefits and minimize HT risks is the use of selective estrogen receptor modulators (SERMs) that exert tissue-specific estrogenic effects. To begin investigating the SERM approach in reducing the risk of AD, we investigated whether AD-like neuropathology in the 3xTg-AD mouse model of AD is regulated by the SERMs propylpyrazole triol (PPT) and diarylpropionitrile (DPN) that exhibit relative specificity for estrogen receptor-α and -β, respectively. Consistent with our previous observations, we found that ovariectomy-induced hormone depletion in adult female 3xTg-AD mice significantly increased accumulation of β-amyloid protein (Aβ) and decreased hippocampal-dependent behavioral performance. Treatment with 17β-estradiol (E2) prevented the ovariectomized-induced worsening of both pathologies. PPT treatment was similar to E2 in terms of reducing Aβ accumulation in hippocampus, subiculum, and amygdala but comparatively less effective in frontal cortex. In contrast, DPN did not significantly reduce Aβ accumulation in hippocampus and subiculum, was partially effective in frontal cortex, and nearly as effective as E2 in amygdala. Furthermore, PPT but not DPN mimicked the ability of E2 to improve behavioral performance. These findings provide initial evidence of beneficial actions of SERMs in a mouse model of AD and support continued investigation of SERMs as an alternative to estrogen-based HT in reducing the risk of AD in postmenopausal women.
HORMONE THERAPY (HT) has long been investigated as a therapeutic option for postmenopausal women to reduce the risk of developing several age-related disorders, including Alzheimer’s disease (AD). Prospective studies have demonstrated that HT use in postmenopausal women can significantly lower the risk of AD (1,2). Prolonged duration and or early initiation of HT appears to be important for reducing AD risk (2) because HT initiated several years after the onset of menopause can have adverse rather than beneficial effects on cognition (3). Despite its protective effect against AD, HT is also associated with adverse effects in estrogen-responsive tissues including breast and uterus (4). To minimize estrogen-related risks, progestins are typically included in HT. However, progestins can blunt beneficial actions of estrogens, including protective actions in brain that may be relevant to AD (5,6,7). These findings underscore the need to develop novel treatment strategies that realize the benefits but minimize the risks associated with long-term HT use.
One alternative approach to estrogen-based HT is the use of selective estrogen receptor agonists (SERMs), compounds that exert mixed agonist effects on estrogen receptors (ERs) in a tissue-specific manner. Ideally, SERMs used to maximize neural health would mimic beneficial estrogen actions in brain but exert negligible adverse effects on nonneural estrogen-responsive tissues. Two recently developed SERMs that show promise for mimicking neural benefits of estrogen are propylpyrazole triol (PPT) and diarylpropionitrile (DPN), which exhibit relative selectivity for ERα and ERβ, respectively (8,9). Both PPT and DPN have been shown to protect cultured neurons from toxic insults implicated in AD neurodegeneration (10,11) and reduce neuron loss in rodent models of stroke (12,13). Furthermore, PPT but not DPN increases synapse number in hippocampal neurons (14). Unknown are the effects of PPT and DPN on the development of AD-like neuropathology.
To begin evaluating the efficacy of SERMs in attenuating the development of AD-like changes, we used the 3xTg-AD mouse model of AD. Recently we found that 17β-estradiol (E2) treatment in female 3xTg-AD mice effectively prevents the acceleration of AD-like neuropathology and behavioral impairments caused by hormone depletion (7). In the present study, we compared the effects of E2, PPT, and DPN on β-amyloid protein (Aβ) accumulation and hippocampal-dependent memory performance in female 3xTg-AD mice.
Materials and Methods
Female 3xTg-AD mice were bred and maintained in accordance with National Institutes of Health (NIH) guidelines and a protocol approved by our Institutional Animal Care and Use Committee. At age 3 months, female 3xTg-AD mice were randomly assigned to the following treatment groups (n = 8 per group): sham ovariectomized (OVX), OVX, OVX+E2, OVX+PPT, and OVX+DPN. Mice were bilaterally OVX or sham OVX and immediately implanted with a sc, 90-d continuous-release drug delivery pellet containing 0.025 mg E2, 0.25 mg PPT, 0.25 mg DPN, or vehicle (Innovative Research of America, Sarasota, FL). The 0.025 mg dose of E2 was empirically determined based on a comparison of the effects of 0.25-, 0.025-, and 0.01-mg E2 pellets on uterine weight, a bioassay of estrogen action. PPT and DPN were delivered at a dose 10-fold higher than E2 based on their lower transcriptional activity than E2 (8,9) and demonstrated effectiveness at such doses in previous in vivo studies (15,16,17,18).
All mice were killed at age 6 months, which was 3 months after initiation of hormone treatment. Animals were behaviorally assessed on the morning the animals were killed. Mice were evaluated for 8 min for spontaneous alternation behavior (SAB) in a Y-maze, a hippocampal-dependent task of working memory, as previously described (7). SAB score was calculated as the proportion of alternations to the total number of alternation opportunities. Total arm entries were also counted as a measure of activity.
After behavioral assessment, mice were deeply anesthetized (100 mg/kg sodium pentobarbital), transcardially perfused with cold PBS, and killed by decapitation. Brains were rapidly collected and immersion fixed in 4% paraformaldehyde and 0.1 m PBS for 48 h and then stored in 4 C in PBS and 1% NaZ until use. To assess the efficacy of hormone treatments, uteri were dissected, blotted, and weighed.
Fixed hemibrains were blocked, sectioned (40 μm) exhaustively in the horizontal plane using a vibratome and then processed for immunohistochemistry using a standard protocol (7). Briefly, every eighth section (∼12 per brain) was immunostained using an antibody directed against Aβ (no. 71–5800 Aβ 1:300 dilution; Zymed, San Francisco, CA) and ABC Vector Elite immunohistochemistry and diaminobenzidine kits (Vector, Burlingame, CA). Levels of Aβ immunoreactivity were quantified in frontal cortex (layers 4–5), basolateral amygdala, subiculum, and hippocampus CA1 by immunohistochemistry load technique (7). In brief, high magnification fields (420 × 330 μm) from immunolabeled sections were captured and digitized. Using NIH Image software 1.61 (Bethesda, MD), digital gray-scale images were converted into binary-positive/negative data using a predetermined threshold limit that was held constant across all brain areas and samples. The percentage of positive pixels was quantified for each image and is termed immunoreactive load. Mean load values were calculated from analysis of two to three nonoverlapping, representative images per brain region from six to 12 sections (depending on brain region) per animal.
Raw data were analyzed by repeated-measures ANOVA with the Wilks’ λ-test followed by ANOVA with the Fisher least significant differences test for between group comparisons.
Results
E2, PPT, and DPN differentially regulate Aβ accumulation
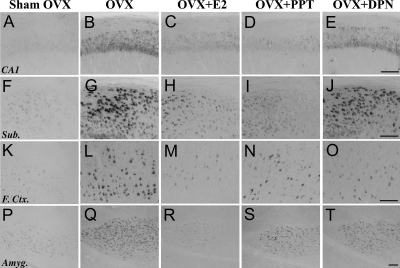
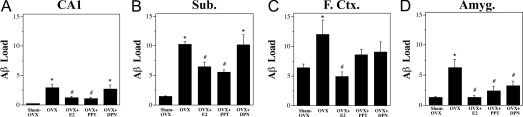
To investigate the effects of SERMs on the development of AD-like neuropathology, female 3xTg-AD mice were depleted of endogenous sex steroid hormones by OVX at age 3 months; treated immediately with E2, PPT, DPN, or vehicle; and then assessed 3 months later for Aβ accumulation in the following brain regions: hippocampus CA1, subiculum, frontal cortex, and amygdala. Repeated-measures ANOVA revealed a significant overall difference in Aβ load between brain regions [F (4,24) = 1.82, P < 0.0001] and a significant overall interaction of brain region by condition [F (3,22) = 17.42, P < 0.0001]. Consistent with our previous observations (7), we found that OVX resulted in significantly increased Aβ accumulation in all four brain regions and that E2 treatment largely prevented the increase (Figs. 1 and 2). If the studied SERMs also regulate Aβ, then treatment with PPT and or DPN should also prevent the increased Aβ accumulation caused by OVX. In hippocampus CA1 and subiculum, we found that PPT treatment was as efficacious as E2 in reducing Aβ load, whereas DPN treatment had no significant effect on Aβ load (Figs. 1 and 2). Both PPT and DPN had intermediate effects in frontal cortex with Aβ loads that were not significantly different from either the sham OVX or OVX groups. In amygdala, PPT and DPN were similar to E2 with Aβ loads that were significantly lower than the OVX group but not significantly different from the sham OVX and OVX+E2 groups (Figs. 1 and 2).
Figure 1.
Accumulation of Aβ is differentially regulated by E2 and the SERMs, PPT and DPN, across brain regions. Images show Aβ-immunoreactivity in hippocampus CA1 (A–E), subiculum (Sub; F–J), frontal cortex (F. Ctr.; K–O), and amygdala (Amyg; P–T) from female 3xTg-AD mice in sham OVX (A, F, K, and P), OVX (B, G, L, and Q), OVX+E2 (C, H, M, and R), OVX+PPT (D, I, N, and S), and OVX+DPN (E, J, O, and T) conditions. Scale bar, 100 μm.
Figure 2.
Quantitative comparison of E2, PPT, and DPN treatments on Aβ accumulation. Data show mean (±sem) Aβ loads for each treatment group in hippocampus CA1 (A), subiculum (Sub; B), frontal cortex (F. Ctr.; C) and amygdala (Amyg; D). Hormone and SERM treatments significantly altered Aβ load in the CA1 [F (4,29) = 7.26, P = 0.0004], subiculum [F (4,29) = 18.95, P < 0.0001], frontal cortex [F (4,30) = 3.80, P = 0.013], and amygdala [F (4,26) = 6.06, P = 0.0014]. Compared with sham OVX, the OVX group had a significantly higher Aβ load in all brain regions and E2 treatment significantly reduced Aβ load (A–D). In CA1 and subiculum (A and B), PPT treatment reduced Aβ load, whereas DPN treatment did not. Both PPT and DPN partially reduced Aβ loads in the frontal cortex (C). In amygdala (D), both PPT and DPN significantly decreased Aβ load. *, P < 0.05, compared with sham OVX group; #, P < 0.05, compared with OVX group.
E2 and PPT but not DPN rescue behavioral impairment
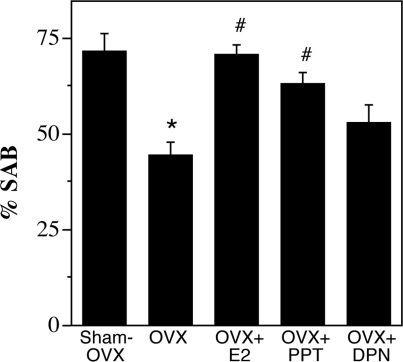
We have previously found that performance of female 3xTg-AD mice in hippocampal-dependent SAB is inversely associated with Aβ levels in hippocampus CA1 and subiculum but not directly affected by levels of sex steroid hormones in wild-type mice (7). To determine whether the effects of SERMs on Aβ accumulation extrapolate to behavior, we evaluated SAB across groups. Consistent with our prior findings in 3xTg-AD mice, we found that the OVX group had significantly impaired SAB performance in comparison with the sham OVX group, an effect that was prevented by E2 treatment (Fig. 3). Importantly, we observed that PPT but not DPN significantly improved SAB performance with the same efficacy as E2 (Fig. 3). The number of arm entries in the Y-maze did not differ significantly between groups [F (4,27) = 1.90; P = 0.14], suggesting that group differences in SAB performance were not associated with differences in activity levels.
Figure 3.
Assessment of working memory performance using the SAB in female 3xTg-AD mice. Data show mean (±sem) percentages of alternations in a Y-maze assessed during an 8-min trial on the day the animals were killed. Both E2 and PPT but not DPN treatment rescued the OVX-induced impairment in SAB, compared with sham OVX [F (4,27) = 9.22, P < 0.0001]. *, P < 0.05, compared with sham OVX group; #, P < 0.05, compared with OVX group.
Assays of hormone and SERM treatments
Uterine weight was measured at the time the animals were killed as a bioassay of hormone treatments. In comparison with the sham OVX group, uterine weights were significantly decreased in the OVX group and significantly elevated in the OVX+E2 group [sham OVX, 72.1 ± 4.1 mg; OVX, 16.1 ± 1.2 mg; and OVX+E2, 121 ± 13.6 mg; F (3,22) = 57.2, P < 0.001]. Uterine weight was not significantly increased by 3 months of treatment of OVX mice with PPT or DNP (30.7 ± 3.9 and 23.8 ± 2.0 mg, respectively). To further investigate the effects of SERMs on uterine weight, we performed a dose-response study using a small subset of wild-type mice (n = 2 mice/condition). PPT treatment in OVX mice had a transient uterotrophic effect, increasing uterine weight to approximately 50% that of sham OVX levels after 2 wk and peaking at approximately 75% of sham OVX levels after 2 months (data not shown). Conversely, DPN treatment had no uterotrophic effects because uterine weight remained at approximately 25% that of sham OVX after 1 wk, 2 wk, and 2 months (data not shown). To confirm neural efficacy of PPT and DPN treatments, sham OVX and OVX wild-type mice treated with vehicle, E2, PPT, or DPN were evaluated on the forced swim test, an assay of anxiety-related behavior. We observed that E2, PPT, and DPN treatments all significantly reduced the increase in anxiety behavior resulting from OVX (supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online Web site at http://endo.endojournals.org).
Discussion
In this study, we sought to investigate the efficacy of the SERMs, PPT and DPN, in comparison with E2 in regulating neuropathology in the 3xTg-AD mouse model of AD. Consistent with our recent findings (7), we observed that E2 treatment in OVX female 3xTg-AD mice significantly reduced both Aβ accumulation and working memory deficits. We observed that PPT was effective in lowering Aβ accumulation in most brain regions and attenuating impairments in working memory, whereas DPN had only modest protective effects. Notably, at the used doses, PPT exhibited only mild uterotrophic effects, whereas DPN exhibited none.
These results are consistent with several studies demonstrating a protective role of E2 in regulating pathological processes relevant to AD. For example, most previous studies in wild-type and transgenic rodents have demonstrated that OVX-induced hormone depletion increases, and E2 treatment reduces Aβ accumulation in brain (7,19,20,21). That E2 does not significantly regulate Aβ in some studies (22,23) may be explained by the recent observation that brain levels of E2 are critical to Aβ regulation and may not be fully depleted by OVX (24). Our results demonstrate for the first time that Aβ accumulation in brain can be effectively regulated by not only E2 but also SERMs, reinforcing the therapeutic potential of estrogenic drugs in preventing AD-related neuropathology.
In comparing the relative efficacies of the SERMs, PPT and DPN, to E2, we found brain region-specific relationships in which PPT was generally effective and DPN generally ineffective in reducing AD-like neuropathology. In hippocampus CA1 and subiculum, PPT was equivalent with E2 in terms of reducing OVX-induced Aβ accumulation, whereas DPN demonstrated no apparent benefit. In parallel, E2 and PPT but not DPN improved SAB performance, consistent with the hippocampal-dependent nature of the SAB task. Interestingly, all three compounds were similarly effective in reducing Aβ loads in the amygdala, but PPT and DPN were only partially effective, compared with E2, in the frontal cortex. That the two SERMs showed discrete, region-specific patterns of reducing Aβ loads suggests that E2 may differentially regulate Aβ accumulation through as yet undefined mechanisms that are dependent on ERα and ERβ. For example, because transcriptional studies have found that PPT is a preferential ERα agonist (9), it is tempting to speculate that the relatively stronger effects with PPT indicate a prominent ERα-dependent, genomic mechanism underlying E2 regulation of Aβ levels. However, this conclusion appears to be inconsistent with what is known about ER distribution in the brain. Both human and rodent studies have demonstrated a differential distribution of ERα and ERβ expression in brain regions affected in AD, including hippocampus, frontal cortex, and amygdala. In situ hybridization studies have demonstrated a higher density of ERβ-expressing cells than ERα-expressing cells in hippocampus (25) and layers 4–6 of cortex (26,27) and similarly high levels of ERβ- and ERα-expressing cells in amygdala (27). Further complicating the issue is that neither the mechanism(s) by which E2 reduces Aβ levels nor the relative contributions of ERα and ERβ have been clearly elucidated, although modulation of amyloid precursor protein processing may be involved. One possibility is that E2 regulation of Aβ largely involves nongenomic cell signaling rather than classic genomic pathways on which the ER subtype specificity of PPT and DPN is largely based. However the ER-dependent involvement in these signaling pathways remain unclear.
In addition to E2, the potential role of progesterone as a regulator of AD neuropathology must also be considered. Both progesterone and E2 are reduced as a consequence of menopause in women and OVX in experimental paradigms. In our paradigm, OVX-induced depletion of progesterone and E2 resulted in increased Aβ accumulation and diminished behavioral performance. However, E2 appears to be the most relevant hormone because E2 treatment alone was effective in fully restoring Aβ load and working memory performance to the levels seen in sham OVX mice. Also, using a similar experimental design, we recently found that progesterone treatment of OVX female 3xTg-AD mice neither reduced Aβ nor improved working memory (7). To the extent that such findings extrapolate to human treatment in which a progestin component is typically included in HT, they highlight the critical role of E2 in regulation of AD-like pathology and support the continued development of neuroactive SERMs such as PPT.
Clinically a primary concern regarding HT in postmenopausal women is the increased risk of breast and uterine cancer associated with prolonged treatment with estrogenic compounds. Therefore, development of SERMs that exert beneficial effects on the brain, bone, and/or cardiovascular system but minimal effects on estrogen-responsive, tumor susceptible tissue is of critical importance. Toward this end, recent clinical trials have demonstrated that various SERMs offer protection against cardiovascular disease and osteoporosis (reviewed in Refs. 28 and 29) and perhaps AD (30).
By demonstrating effective attenuation of Aβ accumulation and behavioral impairment by PPT in a mouse model of AD, our data confirm the potential of SERMs in protecting against AD neuropathology and support the continued development and investigation of neuroactive SERMs as an alternative strategy to HT in preventing and perhaps treating age-related neurodegenerative disorders.
Supplementary Material
Acknowledgments
The authors thank Dr. Wendy Mack for her assistance with statistical analyses.
Footnotes
This work was supported by National Institutes of Health (NIH) Grant AG026572. J.C.C. was supported by NIH Grant AG00093.
Disclosure Statement: The authors have nothing to disclose.
First Published Online February 14, 2008
Abbreviations: Aβ, β-Amyloid protein; AD, Alzheimer’s disease; DPN, 2,3-bis (4-hydroxyphenyl)propionitrile; E2, 17β-estradiol; ER, estrogen receptor; HT, hormone therapy; OVX, ovariectomy; PPT, propylpyrazole triol; SAB, spontaneous alternation behavior; SERM, selective ER modulator.
References
- Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, Bacal C, Lingle DD, Metter E 1997 A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology 48:1517–1521 [DOI] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JC 2002 Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA 288:2123–2129 [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J 2004 Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 291:2959–2968 [DOI] [PubMed] [Google Scholar]
- Warren MP 2004 A comparative review of the risks and benefits of hormone replacement therapy regimens. Am J Obstet Gynecol 190:1141–1167 [DOI] [PubMed] [Google Scholar]
- Rosario ER, Ramsden M, Pike CJ 2006 Progestins inhibit the neuroprotective effects of estrogen in rat hippocampus. Brain Res 1099:206–210 [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Nelson ME, Granholm AC 2004 Progesterone counteracts estrogen-induced increases in neurotrophins in the aged female rat brain. Neuroreport 15:2659–2663 [DOI] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ 2007 Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci 27:13357–13365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA 2001 Estrogen receptor-β potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem 44:4230–4251 [DOI] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA 2000 Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-α-selective agonists. J Med Chem 43:4934–4947 [DOI] [PubMed] [Google Scholar]
- Cordey M, Pike CJ 2005 Neuroprotective properties of selective estrogen receptor agonists in cultured neurons. Brain Res 1045:217–223 [DOI] [PubMed] [Google Scholar]
- Zhao L, Wu TW, Brinton RD 2004 Estrogen receptor subtypes α and beta contribute to neuroprotection and increased Bcl-2 expression in primary hippocampal neurons. Brain Res 1010:22–34 [DOI] [PubMed] [Google Scholar]
- Carswell HV, Macrae IM, Gallagher L, Harrop E, Horsburgh KJ 2004 Neuroprotection by a selective estrogen receptor β agonist in a mouse model of global ischemia. Am J Physiol Heart Circ Physiol 287:H1501–H1504 [DOI] [PubMed] [Google Scholar]
- Dai X, Chen L, Sokabe M 2007 Neurosteroid estradiol rescues ischemia-induced deficit in the long-term potentiation of rat hippocampal CA1 neurons. Neuropharmacology 52:1124–1138 [DOI] [PubMed] [Google Scholar]
- Jelks KB, Wylie R, Floyd CL, McAllister AK, Wise P 2007 Estradiol targets synaptic proteins to induce glutamatergic synapse formation in cultured hippocampal neurons: critical role of estrogen receptor-α. J Neurosci 27:6903–6913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HA, Katzenellenbogen JA, Katzenellenbogen BS 2002 Characterization of the biological roles of the estrogen receptors, ERα and ERβ, in estrogen target tissues in vivo through the use of an ERα-selective ligand. Endocrinology 143:4172–4177 [DOI] [PubMed] [Google Scholar]
- Le Saux M, Di Paolo T 2005 Chronic estrogenic drug treatment increases preproenkephalin mRNA levels in the rat striatum and nucleus accumbens. Psychoneuroendocrinology 30:251–260 [DOI] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WC, Handa RJ 2005 Novel actions of estrogen receptor-β on anxiety-related behaviors. Endocrinology 146:797–807 [DOI] [PubMed] [Google Scholar]
- Frasor J, Barnett DH, Danes JM, Hess R, Parlow AF, Katzenellenbogen BS 2003 Response-specific and ligand dose-dependent modulation of estrogen receptor (ER) α activity by ERβ in the uterus. Endocrinology 144:3159–3166 [DOI] [PubMed] [Google Scholar]
- Petanceska SS, Nagy V, Frail D, Gandy S 2000 Ovariectomy and 17β-estradiol modulate the levels of Alzheimer’s amyloid β peptides in brain. Exp Gerontol 35:1317–1325 [DOI] [PubMed] [Google Scholar]
- Zheng H, Xu H, Uljon SN, Gross R, Hardy K, Gaynor J, Lafrancois J, Simpkins J, Refolo LM, Petanceska S, Wang R, Duff K 2002 Modulation of A(β) peptides by estrogen in mouse models. J Neurochem 80:191–196 [DOI] [PubMed] [Google Scholar]
- Levin-Allerhand JA, Lominska CE, Wang J, Smith JD 2002 17α-estradiol and 17β-estradiol treatments are effective in lowering cerebral amyloid-β levels in AβPPSWE transgenic mice. J Alzheimers Dis 4:449–457 [DOI] [PubMed] [Google Scholar]
- Green PS, Bales K, Paul S, Bu G 2005 Estrogen therapy fails to alter amyloid deposition in the PDAPP model of Alzheimer’s disease. Endocrinology 146:2774–2781 [DOI] [PubMed] [Google Scholar]
- Heikkinen T, Kalesnykas G, Rissanen A, Tapiola T, Iivonen S, Wang J, Chaudhuri J, Tanila H, Miettinen R, Puolivali J 2004 Estrogen treatment improves spatial learning in APP + PS1 mice but does not affect β amyloid accumulation and plaque formation. Exp Neurol 187:105–117 [DOI] [PubMed] [Google Scholar]
- Yue X, Lu M, Lancaster T, Cao P, Honda S, Staufenbiel M, Harada N, Zhong Z, Shen Y, Li R 2005 Brain estrogen deficiency accelerates Aβ plaque formation in an Alzheimer’s disease animal model. Proc Natl Acad Sci USA 102:19198–19203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra RD, Sharma K, Nyakas C, Vij U 2005 Estrogen receptor α and β immunoreactive neurons in normal adult and aged female rat hippocampus: a qualitative and quantitative study. Brain Res 1056:22–35 [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE 2003 Immunolocalization of estrogen receptor β in the mouse brain: comparison with estrogen receptor α. Endocrinology 144:2055–2067 [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I 1997 Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol 388:507–525 [DOI] [PubMed] [Google Scholar]
- Cano A, Hermenegildo C, Oviedo P, Tarin JJ 2007 Selective estrogen receptor modulators and risk for coronary heart disease. Climacteric 10:97–111 [DOI] [PubMed] [Google Scholar]
- Gennari L, Merlotti D, Valleggi F, Martini G, Nuti R 2007 Selective estrogen receptor modulators for postmenopausal osteoporosis: current state of development. Drugs Aging 24:361–379 [DOI] [PubMed] [Google Scholar]
- Yaffe K, Krueger K, Cummings SR, Blackwell T, Henderson VW, Sarkar S, Ensrud K, Grady D 2005 Effect of raloxifene on prevention of dementia and cognitive impairment in older women: the Multiple Outcomes of Raloxifene Evaluation (MORE) randomized trial. Am J Psychiatry 162:683–690 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.