Abstract
Sex differences in gonadal function are driven by either cyclical (females) or tonic (males) hypothalamic GnRH1 release and, subsequently, gonadotrophin (LH and FSH) secretion from the pituitary. This sex difference seems to depend on the perinatal actions of gonadal hormones on the hypothalamus. We used α-fetoprotein (AFP) knockout mice (Afp−/−) to study the mechanisms by which estrogens affect the sexual differentiation of the GnRH1 system. Afp−/− mice lack the protective actions of AFP against estrogens circulating during embryonic development, leading to infertility probably due to a hypothalamic dysfunction. Therefore, we first determined whether Afp−/− females are capable of showing a steroid-induced preovulatory LH surge by FOS/GnRH1 immunohistochemistry and RIA of plasma LH levels. Because the KISS1/GPR54 system is a key upstream regulator of the GnRH1 system as well as being sexually dimorphic, we also analyzed whether Kisspeptin-10 neurons were activated in Afp−/− mice after treatment with estradiol and progesterone. We found that the GnRH1 and Kisspeptin-10 neuronal systems are defeminized in Afp−/− females because they did not show either steroid-induced LH surges or significant FOS/GnRH1 double labeling. Furthermore, Kisspeptin-10 immunoreactivity and neural activation, measured by the number of double-labeled FOS/Kisspeptin-10 cells, were lower in Afp−/− females, suggesting a down-regulation of GnRH1 function. Thus, the sex difference in the ability to show preovulatory LH surges depends on the prenatal actions of estrogens in the male hypothalamus and, thus, is lost in Afp−/− females because they lack AFP to protect them against the defeminizing effects of estrogens during prenatal development.
GnRH1 IS THE key hormone that provokes the release of LH and FSH from the pituitary gonadotrophs. Moreover, the GnRH1 system presents sex differences in their function, i.e. triggering a cyclical gonadal function in females and a distinct tonic gonadal function in males.
Both GnRH1 and gonadotrophin hormones are regulated by steroid hormones, in particular estrogens, acting at the level of the hypothalamus and pituitary, respectively. For most of the estrous cycle, estrogens exert a negative feedback on the hypothalamus-pituitary axis. By contrast, on the afternoon of proestrus, estrogens exert a positive feedback that produces the release of GnRH1 from the median eminence and, subsequently, LH from the pituitary. Both are essential for the induction of ovulation (1,2). Thus, ovarian estradiol (E2) secreted by the developing follicles from metestrus through early proestrus sensitizes the pituitary to become more responsive to GnRH1 (3). In males this hypothalamic-pituitary positive feedback does not occur, and, therefore, there is no ovulation in castrated males that were implanted with ovarian tissue (4). This sex difference presumably depends on the perinatal actions of testosterone and/or E2 on the hypothalamus (4,5) because male rats that were treated neonatally with the aromatase inhibitor 1,4,6-androstatriene-3,17-dione (ATD) showed cyclical gonadal function when grafted with ovarian tissue (4).
We recently showed that female mice carrying a targeted mutation in the α-fetoprotein (AFP) gene, Afp−/−, are infertile (6,7). AFP is a major plasma protein that circulates in high concentrations during embryonic development and plays an important role in the sexual differentiation of the rodent brain due to its estrogen-binding capacity (8). The fertility, as well as sexual behavior, of Afp−/− females could be rescued by blocking estrogen production (and, thus, reducing the excessive levels of estrogens derived from the mother and male siblings) during prenatal development by administration of ATD (6,9). These results indicate that AFP serves to protect the developing female rodent brain from the defeminizing effects of estrogens during prenatal development and have resolved a long-running debate on the role of this fetal protein in brain sexual differentiation.
Afp−/− females are clearly not hypogonadal because their ovaries are normal size, and they have normal, wild-type levels of circulating E2. Furthermore, after an artificial induction of ovulation with gonadotropins, Afp−/− and wild-type females exhibited similar numbers of blastocysts in the uterus, indicating that their normal anovulatory state is not due to an ovarian defect (6,7). Thus, the abnormal sexual cycle found in Afp−/− females reflects a failure to enter into the proestrus phase of the cycle during which the LH surge occurs. Furthermore, they have decreased, male-like numbers of tyrosine hydroxylase-expressing neurons in the anteroventral periventricular nucleus (AVPe) of the preoptic area, a brain region implicated in the positive feedback actions of E2 on the GnRH1 system (10), and they show a transcriptional down-regulation of several genes included in the GnRH1 pathway, including the Gnrh1 gene itself and its receptor (6).
Thus, these results indicate that the ability to show steroid-induced preovulatory GnRH1 and LH surges may be defeminized in female Afp−/− mice because they are not protected from prenatal estrogens by AFP. Therefore, in the present study, we first determined whether Afp−/− mice are capable of showing a steroid-induced GnRH1 and LH surge. We used the expression of FOS, an immediate early gene product that serves as a neuronal activation marker (11), to determine whether hypothalamic GnRH1 neurons were activated by hormonal replacement treatments with either E2 or progesterone (P). Because we found that Afp−/− females did not show steroid-induced preovulatory GnRH1 and LH surges, we next determined whether upstream signals from GnRH1, such as Kisspeptin, were also altered in Afp−/− females. Kisspeptin is the peptide product of the Kiss1 gene and the endogenous agonist of the G protein-coupled receptor 54 (GPR54). Recent evidence suggests that the KISS1/GPR54 system is a key upstream regulator of the GnRH1 system. Mutations or targeted deletions of the GPR54 gene point to this gene as the key regulator for the initiation of puberty (12). Additional studies have revealed that KISS1/GPR54 signaling may serve an important regulatory function in the neuroendocrine reproductive axis after puberty as well (13,14). Furthermore, it has been demonstrated that peripheral as well as intracerebroventricular administration of the active Kisspeptin fragment, Kisspeptin-10, stimulates the hypothalamo-pituitary-gonadal axis and increases plasma gonadotrophins in both male and female adult rats (15,16,17,18,19). In fact, Kisspeptin has been the most potent activator of GnRH1 neuron firing yet to be discovered (20), acting through the GPR54 in these neurons. Most importantly, a greater number of Kisspeptin-10-immunoreactive neurons are present in the AVPe and periventricular hypothalamic nucleus (Pe) of female as opposed to male mice and rats (21), supporting the hypothesis that Kisspeptin-10 neurons may play a specific role in stimulating the preovulatory LH surge.
Materials and Methods
Experimental animals
All breeding and genotyping were performed at the Université Libre de Bruxelles, Institut de Biologie et Médecine Moléculaires, Gosselies, Belgium. In the present studies, Afp−/− of the CD1 strain (Afp KO1, allele Afptm1Ibmm) were used as experimental subjects (7). At the time of the study, these mice had been backcrossed for at least 20 generations. All mice were housed in groups of the same sex, genotype, and treatment under a reversed light-dark cycle (14-h light, 10-h dark cycle; 2300 h lights on and 1300 h lights off). Food and water were always available ad libitum. All experiments were conducted in accordance with the guidelines set forth by the National Institutes of Health Guiding Principles for the Care and Use of Research Animals, and were approved by the Ethical Committees for Animal Use of the University de Liège and of the Université Libre de Bruxelles, Institut de Biologie et Médecine Moléculaires.
Surgery and hormone feedback
In adulthood, all subjects were gonadectomized under general anesthesia using a mixture of ketamine and medetomidine (80 and 1 mg/kg, respectively, ip). Mice were injected with atipamezole (4 mg/kg sc) at the end of the surgery to antagonize the medetomidine-induced effects and accelerating their recovery.
At the time of gonadectomies, males and females were implanted sc with a 5-mm long SILASTIC brand capsule (inner diameter: 1 mm; outer diameter: 2 mm; Dow Corning, Corp., Midland, MI) containing crystalline 17β-E2 diluted in sesame oil (SO) (1 μg/20 g body weight). Animals were divided into three groups. Group 1 was treated sequentially with estradiol benzoate (EB) and P. Group 2 was treated only with EB, whereas group 3 received no additional hormone treatments and served as a control. Thus, 1 wk after surgery, a single injection of EB (1 μg/20 g body weight/0.05 ml SO) was administered sc to each animal in groups 1 and 2 at 0900 h (d 1). Then on d 2, 500 μg P in 0.05 ml SO was administered to each animal in group 1 at 0900 h, whereas none of the animals from group 2 was injected. The same day at 1300 h, when lights went off, all animals were anesthetized with ketamine/medetomidine, and a blood sample was taken by heart puncture to perform the plasma LH content analysis by RIA, before being perfused with paraformaldehyde. The EB plus P treatment has reliably induced preovulatory LH surges in mice (22,23). We have used P as additional hormone because it has been demonstrated that in ovariectomized, E2 primed rodents, LH surges comparable to those observed in intact proestrus females were produced by injecting both EB and P (2), and that the activation of the P receptors by P during proestrus was necessary for showing increased LH surges (24).
The total number of animals in this study was: 11 Afp+/+ female controls; eight Afp+/+ EB plus P-treated females; seven Afp+/+ EB-treated females; four Afp−/− female controls; eight Afp−/− EB plus P-treated females; five Afp−/− EB-treated females; five Afp+/+ male controls; six Afp+/+ EB plus P-treated males; three Afp+/+ EB-treated males; four Afp−/− EB plus P-treated males; and six Afp−/− EB-treated males.
Immunohistochemistry
To determine the distribution of FOS, GnRH1, and Kisspeptin-10 in Afp+/+ and Afp−/− mice, animals were perfused with 4% paraformaldehyde in 0.1 m PBS. Brains were postfixed for 2 h and then transferred to 30% sucrose until they sank. They were then frozen on dry ice and stored at −80 C until used for immunohistochemistry. Cryostat (Leica CM1510 S; Leica Microsystems GmbH, Wetzlar, Germany) brain sections were cut from the rostral telencephalon to the posterior hypothalamus making three sets of 30-μm sections.
Primary antibodies were specific to FOS polyclonal antibody (pAb) (1:2,000 sc-52 rabbit c-fos Ab; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), GnRH1 pAb (1:10,000 LR-1 rabbit Ab, donated by Dr. R. Benoit, Montreal General Hospital, Montreal, Canada), and Kisspeptin-10 [Kisspeptin-10 pAb; Kisspeptin-10 is a polypeptide derived from the KiSS-1 product; 1:20,000 rabbit Ab, donated by Dr. A. Caraty (Unité Mixte de Recherche Physiologie de la Reproduction, Institut National de la Recherche Agronomique, Centre National de la Recherche Scientifique, Université de Tours, Nouzilly, France) (25)].
The specificity of the Kisspeptin-10 Ab to detect Kisspeptin has been validated recently (26). In the latter study, a clear Kisspeptin-10 immunoreaction was shown in wild-type animals, but no immunoreactivity was observed in mice lacking a functional Kiss1 gene. In addition, several experiments have been conducted to test the specificity of this Ab (25). They investigated a possible cross-reactivity with peptides of similar size and/or known to be present in the same hypothalamic structures. No cross-reaction could be observed with any of the eight hypothalamic peptides tested, including GnRH1, galanin, neuropeptide Y, specificity protein, α-MSH, somatostatin, CRH, and prolactin releasing peptide. Moreover, we have tested the specificity of the Ab by preabsorbing for 2 h at room temperature 1 μg Ab with 2 μg of the polypeptide YNWNSFGLRY-NH2 corresponding to amino acid residues 43–52 of mouse metastin (Kisspeptin-10) immunogen used for raising this Ab. This control has been done in adjacent sections and shows no labeling with the preabsorbed Ab, as can be seen in Fig. 1H.
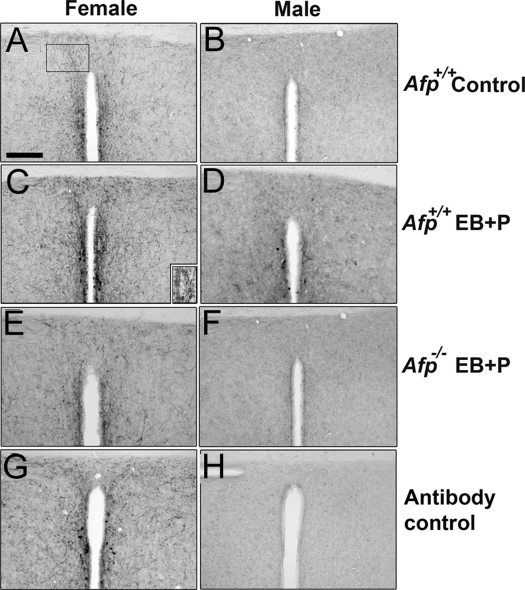
Figure 1.
Kisspeptin-10 distribution in the Afp−/− brain. All photomicrographs depict detailed photomicrographs of Kisspeptin-10 immunoreactivity in the ADP and Pe. A, An untreated Afp+/+ female. B, An untreated Afp+/+ male. C, A EB plus P-treated Afp+/+ female. D, A EB plus P-treated Afp+/+ male. E, A EB plus P-treated Afp−/− female. F, A EB plus P-treated Afp−/− male. G, A positive immunoreaction using the anti-Kisspeptin-10 Ab. H, A specific negative control after having incubated an adjacent section with the YNWNSFGLRY-NH2 preabsorbed Ab. Scale bar in A represents 250 μm and is applicable to all photomicrographs. Labeled areas in A represent the area of counting the immunoreactive fibers. In C we represent a detailed photomicrograph of a group of Kisspeptin-10 neurons with a round shape and short neurites, found on the ventral part of the Pe.
For the double-labeling immunohistochemistry, sections were first washed in 0.1 m PBS (pH 7.4), then the peroxidase activity was blocked in 1:4 methanol-PBS solution with 30% H2O2. Sections were permeabilized in PBS-0.1% Triton X-100 (PBST) and then saturated in 5% normal goat serum (NGS) in NGS-PBST. Immediately after this step, sections were incubated in diluted FOS Ab overnight (1 μg/ml NGS-PBST). On the following day, sections were washed in PBST and incubated in a goat antirabbit biotinylated secondary Ab [product reference no. B0432 (Dako Corp., Carpinteria, CA); 0.75 μg/ml PBST]. Sections were then washed in PBST and incubated in the Vectastain Elite ABC Kit (product reference no. PK6100; Vector Laboratories, Burlingame, CA). After development with the 3,3′-diaminobenzidine (DAB) Substrate Kit (SK-4100; Vector Laboratories) in a black precipitate (DAB plus Ni2+), sections were washed thoroughly in PBS, refixed in 4% paraformaldehyde, and the residual peroxidase activity was blocked in 1:4 methanol-PBS solution with 30% H2O2. Sections were then permeabilized and blocked in 5% NGS-PBST and incubated in the correspondent diluted primary Ab from 24 h (GnRH1 Ab) to 72 h (Kisspeptin-10 Ab). Similar secondary Ab and ABC incubation steps were then performed. The developing reaction used in this step was a DAB brown precipitate using the same kit. Once the immunohistochemical reactions were completed, sections were mounted in Eukitt (Sigma-Aldrich, St. Louis, MO) after being air-dried. Photomicrograph bars represent 25 μm in Fig. 2, 250 μm in Fig. 1, and 200 μm in Fig. 3.
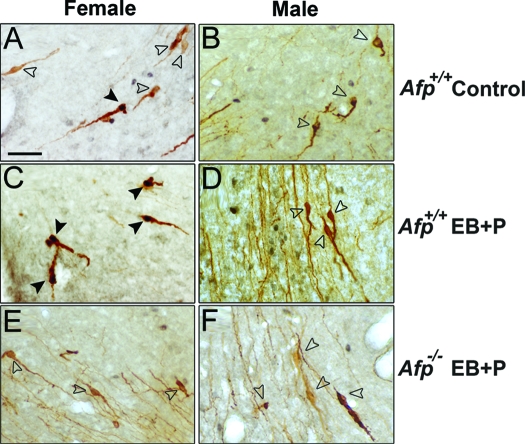
Figure 2.
Qualitative analysis of FOS activation in GnRH1 cells present in the AVPe. A, A detailed photomicrograph of FOS activation in GnRH1 neurons of an untreated Afp+/+ female. B, A detailed photomicrograph of the absence of FOS activation in the GnRH1 neurons of an untreated Afp+/+ male. C, A detailed photomicrograph of FOS activation in GnRH1 neurons of a treated Afp+/+ female. D, A detailed photomicrograph of the absence of FOS activation in GnRH1 neurons of a treated Afp+/+ male. E, A detailed photomicrograph of the absence of FOS activation in GnRH1 neurons of a treated Afp−/− female. F, A detailed photomicrograph of the absence of FOS activation in GnRH1 neurons of a treated Afp−/− male. In all pictures GnRH1 immunoreactive neurons are stained with DAB (brown precipitate) and FOS with DAB plus nickel (black precipitate). Empty arrowheads show nonactivated FOS/GnRH1 neurons. Black arrowheads show activated FOS/GnRH1 neurons. Scale bar in A represents 25 μm and is applicable to all photomicrographs.
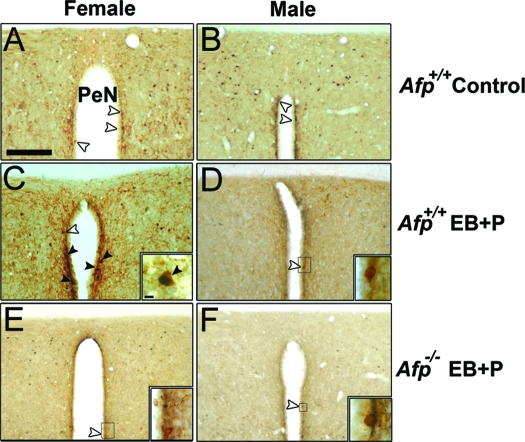
Figure 3.
Analysis of FOS activation in Kisspeptin-10 neurons present in the Pe. A, A detailed photomicrograph of the absence of FOS activation in Kisspeptin-10 neurons of an untreated Afp+/+ female. B, A detailed photomicrograph of the absence of FOS activation in the Kisspeptin-10 neurons of an untreated Afp+/+ male. C, A detailed photomicrograph of FOS activation in the Kisspeptin-10 neurons of a treated Afp+/+ female. A photomicrograph of a double-labeled neuron is represented in the right-bottom corner of this panel. D, A detailed photomicrograph of the absence of FOS activation in Kisspeptin-10 neurons of a treated Afp+/+ male. A photomicrograph of an unlabeled neuron is represented in the right-bottom corner of this panel. E, A detailed photomicrograph of the absence of FOS activation in Kisspeptin-10 neurons of a treated Afp−/− female. A photomicrograph of an unlabeled neuron is represented in the right-bottom corner of this panel. F, A detailed photomicrograph of the absence of FOS activation in Kisspeptin-10 neurons of a treated Afp−/− male. A photomicrograph of an unlabeled neuron is represented in the right-bottom corner of this panel. In all pictures Kisspeptin-10 immunoreactive neurons are stained with DAB (brown precipitate) and FOS with DAB plus nickel (black precipitate). Empty arrowheads show nonactivated FOS/Kisspeptin-10 neurons. Black arrowheads show activated FOS/Kisspeptin-10 expressing neurons. Scale bar in A represents 200 μm, and it is applicable to all photomicrographs. The detailed photomicrograph of the selected area in the right corner represents 25 μm except in E, where it represents 50 μm.
The majority of GnRH neurons expressing c-fos were located within the preoptic area, more specifically between the region comprising the AVPe and the organum vasculosum laminae terminalis (interaural 4.42–4.30 mm and bregma 0.62–0.50 mm approximately according to Ref. 27). Double-labeled FOS/GnRH neurons were only rarely detected in the more rostral areas, such as the medial septum, and, therefore, were not included in our analysis. For the Kisspeptin-10, the counting areas were limited to the anteromedial part of the Pe, where Kisspeptin-10 neurons were found to be activated, i.e. showing FOS/Kisspeptin-10 double labeling. Finally, we also analyzed any differences in Kisspeptin-10 immunoreactivity between different genotypes and treatments (EB plus P vs. control) to determine whether Afp−/− females were defeminized in comparison with Afp+/+ females. Therefore, we analyzed the area occupied by immunoreactive Kisspeptin-10 fibers that are found in the anterior part of anterior commissure, at the anterodorsal preoptic nucleus (ADP). Kisspeptin-10 expression was analyzed by Scion Image (National Institutes of Health, Bethesda, MD).
RIA of plasma LH levels
Serum LH levels were determined in a volume of 100 μl using a double Ab method and a RIA kit (mLHRia), kindly supplied by the National Institutes of Health (Dr. A. F. Parlow, National Institute of Diabetes and Digestive and Kidney Diseases, National Hormone and Peptide Program, Torrance, CA). Rat LH-I-10 (AFP-11536B) was labeled with 125I by the chloramine-T method, and the hormone concentration was expressed using the mouse LH reference preparation (AFP-5306A) as standard. Intraassay and interassay coefficients were less than 7 and 10%, respectively. The sensitivity of the assay was 4 pg/100 μl.
Statistics
All data were analyzed using either one variable or repeated measures ANOVA. When appropriate, all ANOVAs were followed by post hoc comparisons with the Bonferroni test. Only significant (P < 0.05) effects detected are mentioned in detail in Results.
Results
Absence of a steroid-induced LH surge in Afp−/− mice
Afp−/− female mice did not show a preovulatory LH surge in response to treatment with EB plus P, whereas Afp+/+ females clearly did when treated with EB plus P (Fig. 4A). In addition, neither Afp+/+ nor Afp−/− males showed any significant increases in plasma LH levels after treatment with EB plus P, thereby further demonstrating the existence of clear sex differences in the ability of mice to show steroid-induced LH surges. Thus, Afp−/− females are clearly defeminized with regard to their ability to show preovulatory LH surges, confirming that their anovulatory state is due to the absence of appropriate signaling from the hypothalamus (6,7).
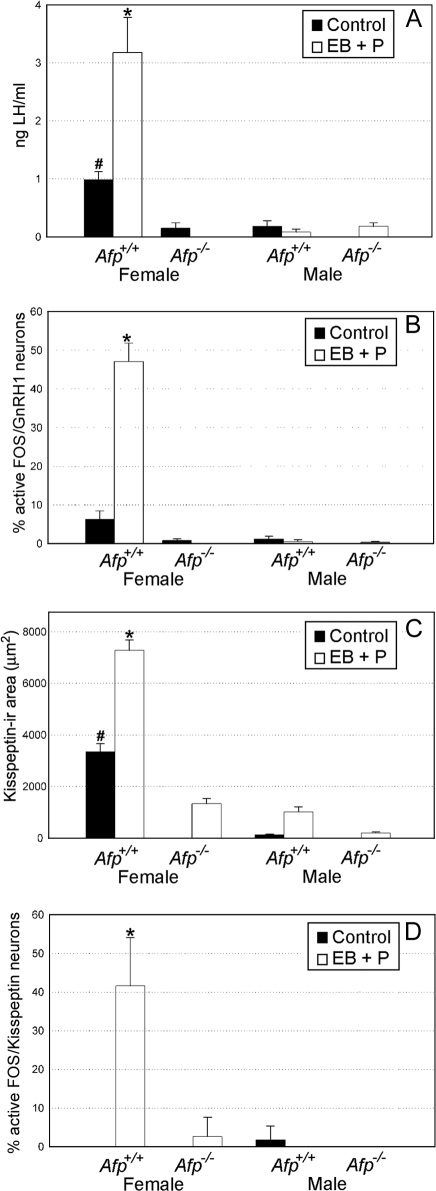
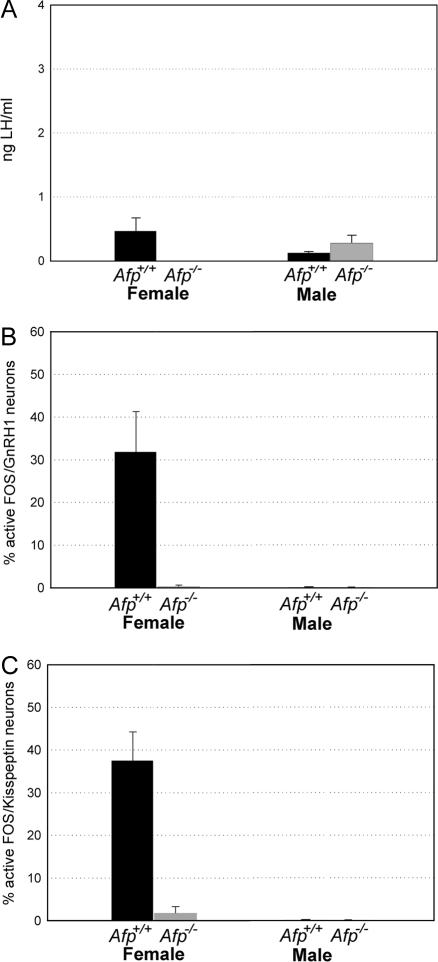
Figure 4.
Quantitative analysis of the ability to show a steroid-induced preovulatory LH surge. A, Plasma LH levels in ng/ml (mean ± sem). B, The percentage of FOS-activated GnRH1 cells (mean ± sem) in the AVPe. C, The area occupied by Kisspeptin-10 immunoreactive fibers (mean ± sem) present in the ADP nucleus. D, The percentage of FOS-activated Kisspeptin-10 cells (mean ± sem) in the Pe. All experiments were conducted in Afp−/− and Afp+/+ mice of both sexes. Control treatments consisted of a sc SILASTIC brand capsule containing crystalline 17β-E2 (1 wk before hormonal induction). EB plus P treatments consisted of a sc SILASTIC brand capsule containing crystalline 17β-E2 and sequential treatment with EB (d 1) and P (d 2). *, P < 0.05 compared with all other groups. #, P < 0.05 compared with all other groups.
ANOVA on plasma LH levels showed significant effects of sex (F1,39 = 29.48; P < 0.001), genotype (F1,39 = 33.17; P < 0.001), treatment (F1,39 = 8.90; P = 0.0018), and a significant triple sex X genotype X treatment interaction (F1,39 = 13.55; P < 0.001). Post hoc analysis of the significant interaction showed that plasma LH levels were significantly higher in treated Afp+/+ females compared with treated Afp−/− females as well as with Afp+/+ and Afp−/− males. Furthermore, plasma LH levels were also higher in nontreated Afp+/+ females compared with all other groups (Afp−/− females, Afp+/+ and Afp−/− males) (Fig. 4A).
Accordingly, female Afp−/− mice, as well as Afp+/+ and Afp−/− males, did not show any significant FOS activation in hypothalamic GnRH1 neurons, whereas in Afp+/+ females, approximately 50% of GnRH1 neurons showed FOS expression after EB plus P treatment (Figs. 2 and 4B). ANOVA on the number of FOS/GnRH1 double-labeled neurons showed a significant effect of sex (F1, 38 = 45.51; P < 0.001), genotype (F1,38 = 48.72; P < 0.001), treatment (F1, 38 = 28.51; P < 0.001), and a significant triple sex X genotype X treatment interaction (F1, 38 = 28.13; P < 0.001). Post hoc analysis of the significant interaction showed that only Afp+/+ females treated with EB plus P showed a significant induction of FOS in GnRH1 neurons. The mean percentages (±sem) of FOS double-labeled/GnRH1 neurons of every group studied are presented in Table 1. This percentage was calculated by dividing the number of FOS double-labeled/GnRH1 neurons by the total number of GnRH1 neurons analyzed in the region of interest and then multiplied by 100. The statistical analysis of the numbers of GnRH1 immunoreactive neurons did not show any significant differences between genotypes or hormone treatments as well as no significant interaction between genotypes or hormone treatments (raw data are included in Table 2).
Table 1.
Mean percentages (±sems) of FOS double-labeled GnRH1 and Kisspeptin-10 (Kp10) neurons analyzed per animal group after different treatments in wild-type and knockout subjects
| Analysis treatment | Female Afp+/+
|
Female Afp−/−
|
Male Afp+/+
|
Male Afp−/−
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FOS/Kp10 | FOS/GnRH1 | No. | FOS/Kp10 | FOS/GnRH1 | No. | FOS/Kp10 | FOS/GnRH1 | No. | FOS/Kp10 | FOS/GnRH1 | No. | |
| Control | 0 | 6.3 ± 2.2 | 11 | 0 | 0.8 ± 0.4 | 4 | 1.81 ± 3.6 | 0.2 ± 0.1 | 5 | |||
| EB | 37.5 ± 7.9 | 31.8 ± 9.5 | 7 | 1.8 ± 1.81 | 0 | 5 | 0 | 0 | 3 | 0 | 0 | 4 |
| EB + P | 41.6 ± 12.5 | 47.1 ± 4.8 | 8 | 2.6 ± 5.02 | 0 | 8 | 0 | 0.1 ± 0.1 | 6 | 0 | 0.1 ± 0.6 | 6 |
Analysis refers to sex, genotype, and immunochemical analysis (FOS/Kp10 and FOS/GnRH1). Treatment refers to: Control, EB, and EB+P.
Table 2.
Kisspeptin-10 (Kp10) and GnRH1 neurons detected in each genotype (male-female; −/− vs. +/+) after control or EB plus P treatments
| Analysis treatment | Female Afp+/+
|
Female Afp−/−
|
Male Afp+/+
|
Male Afp−/−
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kp10 | GnRH1 | No. | Kp10 | GnRH1 | No. | Kp10 | GnRH1 | No. | Kp10 | GnRH1 | No. | |
| Control | 59.1 (650) | 52.2 (574) | 11 | 12.8 (51) | 55.0 (220) | 4 | 4.0 (20) | 53.6 (268) | 5 | |||
| EB + P | 64.2 (514) | 48.9 (391) | 8 | 11.6 (93) | 63.9 (511) | 8 | 5.3 (32) | 54.3 (326) | 6 | 5.5 (33) | 49.2 (295) | 6 |
The values represent the average cell number per mouse (total number of cells in the brain sections of study) and the number (No.) of animals studied. Analysis refers to sex, genotype, and immunohistochemical analysis (Kp10 and GnRH1 neurons). Treatment refers to: Control and EB+P.
Down-regulation of Kisspeptin-10 in Afp−/− females
The area of Kisspeptin-10 immunoreactivity was clearly defeminized in Afp−/− females, i.e. they showed male-typical Kisspeptin-10 immunoreactivity in the ADP and Pe (Figs. 1 and 4C). Furthermore, no significant FOS double labeling could be discerned in Pe Kisspeptin-10 neurons in Afp−/− females (2.6% of Kisspeptin-10 neurons were double labeled for FOS after EB+P treatment) or in Afp+/+ and Afp−/− males (no double-labeled FOS/Kisspeptin-10 neurons were found), whereas Afp+/+ females showed a significant induction of FOS in Kisspeptin-10-immunoreactive neurons (41.6% of Kisspeptin-10 neurons were double labeled with FOS after EB plus P treatment; Fig. 4C).
This was confirmed by statistical analysis with ANOVA demonstrating clear sex differences in the area occupied by Kisspeptin-10 immunoreactive fibers in the ADP with females showing higher levels of immunoreactivity than males (F1,99 = 125.80; P < 0.0001). Moreover, Kisspeptin-10 immunoreactivity present in the same area showed significant differences between Afp+/+ and Afp−/− mice (F1,99 = 117.19; P < 0.0001), and between control vs. EB plus P treated (F1,99 = 44.82; P < 0.0001). In addition, we observed a significant triple sex X genotype X treatment interaction (F1, 99 = 4.03; P < 0.05). The post hoc analysis showed that Afp+/+ females treated with EB plus P showed higher levels of Kisspeptin-10 immunoreactivity compared with control Afp+/+ females, whereas EB plus P-treated Afp−/− females showed even lower levels than the control Afp+/+ females. Post hoc analyses on the male data also showed significant differences between control and treated Afp+/+ males, but levels in males were always significantly lower than those found in Afp+/+ females.
Quantitative analysis of FOS/Kisspeptin-10 double-labeling results showed that only EB plus P-treated Afp+/+ females showed double-labeled neurons in the Pe (Fig. 3). Thus, in Afp+/+ females around 40% of Kisspeptin-10 immunoreactive neurons in the Pe showed FOS immunoreactivity, whereas female Afp−/− mice, as well as Afp+/+ and Afp−/− males, did not show any significant FOS activation in Pe Kisspeptin-10 immunoreactive neurons (Fig. 4D). This was confirmed by ANOVA on the number of FOS/Kisspeptin-10 double-labeled neurons showing a significant effect of sex (F1,27 = 28.24; P < 0.001), genotype (F1,27 = 26.06; P < 0.001), and treatment (F1,27 = 28.24; P < 0.001), and a significant triple sex X genotype X treatment interaction (F1,27 = 34.17; P < 0.001). The mean percentages (±sem) of FOS double-labeled /Kisspeptin-10 neurons of every group studied are presented in Table 1. This percentage was calculated by dividing the number of FOS double-labeled/Kisspeptin-10 neurons by the total number of Kisspeptin-10 neurons analyzed in the area of interest and then multiplied by 100.
Effect of estrogens on the activation of the Kisspeptin-10 and GnRH1 system
To study the role of estrogen alone in the activation of the GnRH1 and Kisspeptin-10 systems, we have treated an additional group of Afp+/+ and Afp−/− males and females (group 2) with a single injection of EB 24 h before the LH, GnRH1, and Kisspeptin-10 analysis. The results clearly showed that treatment with E2 is sufficient to induce an activation of the GnRH1 system as measured by the percentage of FOS/GnRH1 double labeling (31.82 ± 9.46%; Fig. 5B) as well as the Kisspeptin-10 system (percentage of FOS/Kisspeptin-10 double labeling: 37.53 ± 6.71%; Fig. 5C) in Afp+/+ females compared with all the other groups. These results are very similar to what has been observed in animals treated with EB plus P. By contrast, treatment with EB alone did not induce an LH surge in Afp+/+ females (0.47 ± 0.21 ng/ml; Fig. 5A). LH levels were very similar in all four groups. The mean percentages (±sem) of FOS double-labeled/GnRH1 neurons and FOS double-labeled/Kisspeptin-10 neurons are presented in Table 1.
Figure 5.
Quantitative analysis of the ability to show an EB-induced preovulatory LH surge. A, Plasma LH levels in ng/ml (mean ± sem). B, The percentage of FOS-activated GnRH1 cells (mean ± sem) in the AVPe. C, The percentage of FOS-activated Kisspeptin-10 cells (mean ± sem) in the Pe. All experiments were conducted in Afp−/− and Afp+/+ mice of both sexes. EB treatments consisted of a sc SILASTIC brand capsule containing crystalline 17β-E2 and sequential treatment with EB (d 1) and terminating the animals on d 2.
Discussion
In the present study, we showed that the infertility of Afp−/− females is linked to the absence of appropriate GnRH1 signaling and subsequent pituitary LH secretion that is required to induce ovulation. Thus, female Afp−/− mice cannot show preovulatory LH surges in response to estrogen and P treatment. Interestingly, the absence of positive feedback actions of estrogen on the GnRH1 neuronal system might be due to the absence of adequate Kisspeptin-10 signaling because the amount of Kisspeptin-10 immunoreactivity and activation was severely diminished in Afp−/− females. After treatment with estrogen and P, Afp−/− females actually showed male-typical levels of Kisspeptin-10 immunoreactivity (Fig. 1). A recent study in Kiss1-null mice supports this hypothesis because it was shown that Kiss1-null mice did not become sexually mature, whereas females did not have estrous cycles and had persistently low levels of plasma LH (26). Thus, whereas estrogens clearly activated Kisspeptin-10 neurons in Afp+/+ females, confirming previous studies (28,29) indicating that Pe Kisspeptin-10 neurons are an important upstream signal in GnRH1 signaling, this activation was completely absent in Afp−/− females as well as in males (either Afp+/+ or Afp−/−). These results suggest that aspects of GnRH1 functioning have been defeminized in Afp−/− females, presumably because they are no longer protected from estrogens during prenatal life due to the absence of AFP (5,7). Thus, sex differences in the ability to show preovulatory LH surges most likely result from the perinatal actions in the male of estrogens on the Kiss1 and GnRH1 neuronal systems.
De Mees et al. (6) have recently shown that GnRH1 expression is down-regulated in Afp−/− females. Interestingly, prenatal treatment of Afp−/− female mice with the aromatase inhibitor, ATD, rescued their fertility and restored an almost normal level of expression of different pituitary factors found in fertile heterozygous females (6). However, there was evidence indicating that AFP has a dose-dependent effect because it was observed that the expression level of the GnRH1 receptor of heterozygous females was intermediate between values of those of Afp−/− and Afp+/+ females. Thus, the absence of any significant steroid-induced activation of the GnRH1 system in Afp−/− females may be explained by either the lack of adequate Kisspetin-10 signaling to the GnRH1 system (see below), initiation of an autocrine control of GnRH1 neurons by ultrashort feedback mechanisms (30), or even the release of pulsatile suboptimal levels of GnRH1 that could elicit an inadequate pituitary response at the level of the GnRH1r expression.
In Afp+/+ females the estrogen-induced LH peak is preceded by activation of FOS/GnRH1 expressing cells in the AVPe but also by the presence of activated FOS/Kisspeptin-10 neurons in the same periventricular area. Our results support the idea that Kisspeptin-10 signaling in the preoptic region is regulated by sex steroids and thereby mediating the positive feedback action of estrogen on GnRH1 neurons. Kisspeptin signaling through its receptor (GPR54) might synchronize GnRH1 release into the median eminence and ultimately tuning the hypothalamic control of reproduction. Our data also suggest that in the presence of estrogens, Kisspeptin-10 neurons in the Pe may stimulate GnRH1 neurons present in the same adjacent area, thereby favoring the preovulatory LH surge. This hypothesis is supported by the previous observation of an inhibition of the proestrus LH surge and estrous cyclicity by centrally administered metastin (Kisspeptin) antibodies in the female rat (31). Furthermore, it has already been shown that GnRH1 neurons within the preoptic area do not express estrogen receptor (ER)α, but only ERβ, and that ovulation is driven by positive feedback actions of estrogens upon ERα-expressing neurons projecting to GnRH neurons existing in the Pe and median preoptic nucleus in addition to the AVPe (22). The mechanism by which Kisspeptin is up-regulated by estrogens likely involves the activation of the ERα present in Kisspeptin neurons in the periventricular preoptic region (19,25,32,33). In fact, recent evidence suggests that only Kiss1 neurons expressing the ERα in this area are stimulated by E2, indicating that they may participate in the positive feedback regulation of GnRH1 secretion (33), whereas Kiss1 neurons in the arcuate nucleus are inhibited by E2 and, thus, may play a role in the negative feedback regulation of GnRH1 secretion. Our results suggest that there is an activation of hypothalamic Kisspeptin neurons by estrogen that facilitates GnRH1 release, thereby leading to a preovulatory LH surge. Finally, although there is no clear evidence of sex differences in the GnRH1 neural population, it has been clearly established that Kisspeptin neurons and their innervation are sexually differentiated in both the rat and mouse (21,34). In fact, Kauffman et al. (21) showed that a single injection with testosterone on the day of birth decreased Kiss1 mRNA levels to male typical levels in the AVPe of female rats, suggesting a defeminizing role of testosterone on the Kiss1 system in female rats. In the present study, we showed that Afp−/− females are defeminized so that they have fewer Kisspeptin-10 neurons as well as fewer Kisspeptin-10 immunoreactive fibers in the preoptic region, and the Kisspeptin-10 neurons present in the Pe lose their ability to be activated by estrogens, suggesting a role for fetal estrogens (in contrast to neonatal androgen) in the sexual differentiation of the Kiss1 system. By contrast, it is unclear whether the Kisspeptin-10 population in the arcuate nucleus is affected in Afp−/− females because no significant Kisspeptin-10 immunoreactivity was observed in this nucleus, either in control (implanted with a low dose of E2), EB, or EB plus P primed animals. These observations are in agreement with previous studies conducted by Smith et al. (33) who found that E2 treatment inhibited Kiss1 expression in the mouse arcuate nucleus. However, neither a sex difference nor an influence of neonatal androgen treatment on Kiss1 expression in the arcuate nucleus was observed in the study by Kauffman et al. (21), suggesting that the Kiss1 system in the arcuate nucleus is not organized perinatally by sex steroid hormones.
Although Afp−/− males are fertile and seem to have a normal male-typical phenotype, the possibility cannot be excluded that some aspects of brain sexual differentiation have been affected in these males. Presumably, Afp−/− males are overexposed to estrogens prenatally because they are exposed to estrogens derived from neural aromatization of testosterone as normal males are, but they are also exposed to estrogens coming from external sources such as their mother or their brothers because they lack AFP. To date, no clear effects of overexposure to estrogens have been reported for the development of the male brain other than that transgenic male mice expressing human aromatase and, thus, having an increased estrogen to androgen ratio show a multitude of structural and functional alterations in the reproductive organs such as cryptorchidism, Leydig cell hyperplasia, disrupted spermatogenesis, and infertility (20). However, no data are available for the brain for these transgenic mice. In the present study, we determined whether aspects of hypothalamic functioning, such as the Kisspeptin-10 and GnRH1 system, were hypermasculinized in Afp−/− males. However, Afp−/− males did not show any significant differences at the level of either Kisspeptin-10 content, FOS/Kisspeptin-10 activation, FOS/GnRH1 activation, or LH secretion compared with Afp+/+ males, suggesting no particular protective role for AFP in the development of the male brain.
In summary, the present study shows that sex differences in the ability to show preovulatory LH surges and, thus, cyclical gonadal function may reflect the perinatal actions of estrogens on the hypothalamic Kiss1 and GnRH1 neuronal systems. This ability is normally absent in males due to prenatal estrogens coming from aromatization of testicular testosterone; in Afp−/− females it is lost due to the absence of AFP, and as a consequence, females are no longer protected against estrogens circulating during prenatal development. The use of the Afp−/− model should further unravel the sex differences of the Kiss1 and GnRH1 systems and their differential sexual functions in vertebrates. The Afp−/− model makes a better model to analyze the role of fetal estrogens in brain sexual differentiation because it is a natural model for prenatal estrogen exposure in females. So there are no particular toxic effects as has been shown when diethylstilbestrol has been used to expose fetuses to estrogens for instance (for example, see Ref. 35). Finally, the role of prenatal estrogens in the sexual differentiation of the GnRH1 and Kiss1 system should be confirmed by analyzing the ability to show steroid-induced LH surges and, accordingly, significant FOS activation of these systems in male aromatase knockout (ArKO) mice. ArKO mice carry a mutation in the aromatase gene and, thus, are incapable of converting androgens to estrogens. Thus, if estrogens are the critical hormone in defeminizing the GnRH1 and Kiss1 systems, then ArKO males should not be defeminized.
Acknowledgments
We thank Dr. Michael Baum for reading and commenting on the manuscript. We also thank Ms. Arlette Gerard and Professor Bourguignon for their contribution to the LH RIA.
Footnotes
This work was supported by grants from Fonds National de la Recherche Scientifique (Mandat d’Impulsion Scientifique F.4502.07) and the National Institute of Child Health and Human Development (HD044897) (to J.B.), and the Fonds de la Recherche Fondamental Collective (2.4603.06) (to C.S.). J.B. is a Research Associate of the Fonds National de la Recherche Scientifique. D.G.M. and C.D.M. are Postdoctoral Researchers of the Fonds National de la Recherche Scientifique. C.S. is a Research Director of the Fonds National de la Recherche Scientifique.
Disclosure Statement: The authors have nothing to disclose.
First Published Online January 17, 2008
Abbreviations: Ab, Antibody; ADP, anterodorsal preoptic nucleus; AFP, α-fetoprotein; ArKO, aromatase knockout; ATD, 1,4,6-androstatriene-3,17-dione; AVPe, anteroventral periventricular nucleus; DAB, 3,3′-diaminobenzidine; E2, estradiol; EB, estradiol benzoate; ER, estrogen receptor; GPR54, G protein-coupled receptor 54; NGS, normal goat serum; P, progesterone; pAb, polyclonal antibody; PBST, PBS-0.1% Triton X-100; Pe, periventricular hypothalamic nucleus; SO, sesame oil.
References
- Petersen SL, Ottem EN, Carpenter CD 2003 Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol Reprod 69:1771–1778 [DOI] [PubMed] [Google Scholar]
- Bronson FH, Vom Saal FS 1979 Control of the preovulatory release of luteinizing hormone by steroids in the mouse. Endocrinology 104:1247–1255 [DOI] [PubMed] [Google Scholar]
- Herbison AE 1998 Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev 19:302–330 [DOI] [PubMed] [Google Scholar]
- Vreeburg JT, van der Vaart PD, van der Schoot P 1977 Prevention of central defeminization but not masculinization in male rats by inhibition neonatally of oestrogen biosynthesis. J Endocrinol 74:375–382 [DOI] [PubMed] [Google Scholar]
- Neill JD 1972 Sexual differences in the hypothalamic regulation of prolactin secretion. Endocrinology 90:1154–1159 [DOI] [PubMed] [Google Scholar]
- De Mees C, Laes JF, Bakker J, Smitz J, Hennuy B, Van Vooren P, Gabant P, Szpirer J, Szpirer C 2006 α-Fetoprotein controls female fertility and prenatal development of the gonadotropin-releasing hormone pathway through an antiestrogenic action. Mol Cell Biol 26:2012–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabant P, Forrester L, Nichols J, Van Reeth T, De Mees C, Pajack B, Watt A, Smitz J, Alexandre H, Szpirer C, Szpirer J 2002 α-Fetoprotein, the major fetal serum protein, is not essential for embryonic development but is required for female fertility. Proc Natl Acad Sci USA 99:12865–12870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynaud JP 1973 Influence of rat estradiol binding plasma protein (EBP) on uterotrophic activity. Steroids 21:249–258 [DOI] [PubMed] [Google Scholar]
- Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C 2006 α-Fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci 9:220–226 [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E 1982 Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology 34:395–404 [DOI] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR, Curran T 1988 Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science 240:1328–1331 [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno Jr JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley Jr WF, Aparicio SA, Colledge WH 2003 The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- Plant TM 2006 The role of KiSS-1 in the regulation of puberty in higher primates. Eur J Endocrinol 155(Suppl 1):S11–S16 [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE 2005 Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsch ML, Clifton DK, Steiner RA 2006 Kisspepeptin-GPR54 signaling in the neuroendocrine reproductive axis. Mol Cell Endocrinol 254- 255:91–96 [DOI] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA 2004 Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80:264–272 [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA 2004 A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077 [DOI] [PubMed] [Google Scholar]
- Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR 2004 Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol 16:850–858 [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Barreiro ML, Casanueva FF, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M 2005 Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology 146:1689–1697 [DOI] [PubMed] [Google Scholar]
- Li C, Chen P, Smith MS, 1999 Morphological evidence for direct interaction between arcuate nucleus neuropeptide Y (NPY) neurons and gonadotropin-releasing hormone neurons and the possible involvement of NPY Y1 receptors. Endocrinology 140:5382–5390 [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M 2007 Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 148:1774–1783 [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE 2006 Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 52:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendren G 2001 Subsets of gonadotropin-releasing hormone (GnRH) neurons are activated during a steroid-induced luteinizing hormone surge and mating in mice: a combined retrograde tracing double immunohistochemical study. Brain Res 918:74–79 [DOI] [PubMed] [Google Scholar]
- Nequin LG, Alvarez J, Schwartz NB 1979 Measurement of serum steroid and gonadotropin levels and uterine and ovarian variables throughout 4 day and 5 day estrous cycles in the rat. Biol Reprod 20:659–670 [DOI] [PubMed] [Google Scholar]
- Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A 2006 Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor α. Neurosci Lett 401:225–230 [DOI] [PubMed] [Google Scholar]
- d’Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, Aparicio SA, Colledge WH 2007 Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA 104:10714–10719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ 2001 The mouse brain in stereotaxic coordinates. 2nd ed. San Diego: Academic Press [Google Scholar]
- Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda KI 2007 Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 53:367–378 [DOI] [PubMed] [Google Scholar]
- Kuohung W, Kaiser UB 2006 GPR54 and KiSS-1: role in the regulation of puberty and reproduction. Rev Endocr Metab Disord 7:257–263 [DOI] [PubMed] [Google Scholar]
- Xu C, Xu XZ, Nunemaker CS, Moenter SM 2004 Dose-dependent switch in response of gonadotropin-releasing hormone (GnRH) neurons to GnRH mediated through the type I GnRH receptor. Endocrinology 145:728–735 [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K 2005 Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 146:4431–4436 [DOI] [PubMed] [Google Scholar]
- Dufourny L, Skinner DC 2002 Progesterone receptor, estrogen receptor α, and the type II glucocorticoid receptor are coexpressed in the same neurons of the ovine preoptic area and arcuate nucleus: a triple immunolabeling study. Biol Reprod 67:1605–1612 [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA 2005 Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE 2006 Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marselos M, Tomatis L 1992 Diethylstilboestrol: II, pharmacology, toxicology and carcinogenicity in experimental animals. Eur J Cancer 29A:149–155 [DOI] [PubMed] [Google Scholar]