Abstract
Protein kinase C-ζ, a downstream effector of phosphatidylinositol 3-kinase (PI3K), phosphorylates insulin receptor substrate (IRS)-1 on serine residues impairing activation of PI3K in response to insulin. Because IRS-1 is upstream from PI3K, this represents a negative feedback mechanism that may contribute to signal specificity in insulin action. To determine whether similar feedback pathways exist for other IRS isoforms, we evaluated IRS-2, -3, and -4 as substrates for PKC-ζ. In an in vitro kinase assay, purified recombinant PKC-ζ phosphorylated IRS-1, -3 and -4 but not IRS-2. Similar results were obtained with an immune-complex kinase assay demonstrating that wild-type, but not kinase-deficient mutant PKC-ζ, phosphorylated IRS-1, -3, and -4 but not IRS-2. We evaluated functional consequences of serine phosphorylation of IRS isoforms by PKC-ζ in NIH-3T3IR cells cotransfected with epitope-tagged IRS proteins and either PKC-ζ or empty vector control. Insulin-stimulated IRS tyrosine phosphorylation was impaired by overepxression of PKC-ζ for IRS-1, -3, and -4 but not IRS-2. Significant insulin-stimulated increases in PI3K activity was coimmunoprecipitated with all IRS isoforms. In cells overexpressing PKC-ζ there was marked inhibition of insulin-stimulated PI3K activity associated with IRS-1, -3 and -4 but not IRS-2. That is, PI3K activity associated with IRS-2 in response to insulin was similar in control cells and cells overexpressing PKC-ζ. We conclude that IRS-3 and -4 are novel substrates for PKC-ζ that may participate in a negative feedback pathway for insulin signaling similar to IRS-1. The inability of PKC-ζ to phosphorylate IRS-2 may help determine specific functional roles for IRS-2.
BIOLOGICAL ACTIONS OF insulin are initiated when insulin binds to specific cell surface insulin receptors leading to receptor autophosphorylation that enhances the intrinsic tyrosine kinase activity of the receptor (1,2). The next step in insulin signaling involves tyrosine phosphorylation of intracellular substrates including the insulin receptor substrate (IRS) family IRS-1, -2, -3, and -4. Tyrosine phosphorylated motifs on these IRS isoforms serve as binding sites for the SH2 domains contained in adaptor proteins such as the p85 regulatory subunit of phosphatidylinositol 3-kinase (PI3K). Downstream from PI3K, a series of serine kinases such as phosphoinositide-dependent kinase-1, Akt, and protein kinase C (PKC)-ζ are activated. This propagates insulin signaling to downstream effectors leading to biological actions of insulin including increased glucose transport, glycogen synthesis, and protein synthesis (3). One mechanism that adds complexity to insulin signaling and that may contribute to signal specificity is the presence or absence of feedback pathways (4). We previously reported that PKC-ζ can phosphorylate IRS-1 on serine residues in vitro as well as in intact cells (5). Using coimmunoprecipitation, we showed that the association between IRS-1 and PKC-ζ increases after insulin stimulation. In addition, serine phosphorylation of IRS-1 by PKC-ζ impairs insulin-stimulated tyrosine phosphorylation of IRS-1, resulting in decreased IRS-1 associated PI3K activity (5). Thus, this represents a negative feedback pathway for insulin signaling involving IRS-1 and its downstream effector PKC-ζ. Others have made similar observations (6). More recently a number of other groups have identified PKC-ζ serine phosphorylation sites on rat IRS-1 at Ser318, Ser498, Ser570, and Ser612 (7,8,9). IRS isoforms share a number of overlapping features including pleckstrin homology and phosphotyrosine binding domains as well as multiple phosphotyrosine docking sites for SH2-domain containing proteins that lead to overlapping signaling pathways and functions (10). Nevertheless, each IRS isoform also has distinct nonredundant biological actions. This is demonstrated by differences in phenotypes among knockout mice for the various IRS isoforms (11,12,13,14,15,16,17). To determine whether all IRS isoforms are substrates for PKC-ζ and participate in similar feedback pathways, we evaluated IRS-2, -3, and -4 as potential substrates for PKC-ζ. Interestingly, we found that IRS-3 and -4 are novel substrates for PKC-ζ. However, IRS-2 is not. Our results may help to explain some of the specificity arising from complex signaling networks and feedback pathways in insulin signaling.
Materials and Methods
Reagents
Reagents were obtained from the following sources: monoclonal anti-myc antibody from Covance Research Products (Denver, PA); monoclonal antiphosphotyrosine antibody (4G10), polyclonal anti-p85, anti-IRS-1, anti-IRS-2, anti-IRS-3, and anti-IRS-4 from Millipore (Billerica, MA); recombinant PKC-ζ from PanVera Corp. (Madison, WI); rabbit polyclonal anti-HA antibody from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); and protein A- and protein G-agarose beads and LipofectAMINE Plus from Invitrogen (Carlsbad, CA).
Expression plasmids
pCIS2.
This is the parental expression vector with a cytomegalovirus promoter/enhancer (18).
pCIS2-IRS1-HA.
cDNA for human IRS-1 with a C-terminal HA-epitope tag was subcloned into pCIS2 expression vector as described (5,19).
pCIS2-IRS2.
cDNA for mouse IRS-2 was subcloned into pCIS2 as described (20).
pCIS2-IRS3-myc.
cDNA for mouse IRS-3 with a C-terminal myc-epitope tag was subcloned into pCIS2 as described (21).
pCIS2-IRS4-myc.
cDNA for mouse IRS-4 with a C-terminal myc-epitope tag was subcloned into pCIS2 as described (21).
PKCζ-WT.
This is an expression vector for rat wild-type PKC-ζ with an N-terminal HA-epitope tag in the pcDNA3 expression vector (5,22).
PKCζ kinase dead (KD).
This is the kinase-inactive point mutant of rat PKC-ζ (L281W) with N-terminal HA-epitope tag in pcDNA3 expression vector (5,22).
Cell culture and transfection
NIH-3T3 fibroblasts stably overexpressing human insulin receptors (NIH-3T3IR) and COS-7 cells were maintained in DMEM containing 10% fetal bovine serum, l-glutamine (2 mm), penicillin (100 U/ml), and streptomycin (100 μg/ml) in a humidified atmosphere with 5% CO2 at 37 C. COS-7 cells were transiently transfected with PKCζ-WT or PKCζ KD and/or IRS-1, -2, -3, and -4 constructs using LipofectAMINE Plus according to the manufacturer’s instructions. NIH-3T3IR cells were transiently transfected with PKC-ζ or empty vector and/or IRS-1, -2, -3, and -4 constructs using LipofectAMINE Plus according to the manufacturer’s instructions.
In vitro PKC-ζ kinase assays
In vitro kinase assays using purified PKC-ζ as the kinase and recombinant IRS isoforms immunoprecipitated from cell lysates of transfected COS-7 cells as substrates were carried out at 30 C for 30 min in kinase assay buffer containing 50 mm Tris-HCl (pH 7.4), 10 mm MgCl2, 50 μm ATP, 2.5 μCi of [γ-32P]ATP/assay, and 4 μg of phosphatidylserine. The reactions were stopped by adding Laemmli sample buffer and boiling for 10 min. Samples were subjected to 7.5% SDS-PAGE, and phosphorylated IRS-1, -2, -3 or -4 and autophosphorylated PKC-ζ were detected using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA). In addition, gel contents were transferred to nitrocellulose and immunoblotted with antibodies against IRS-1, -2, -3, and -4, respectively. Finally, the activity of PKC-ζ in each assay was independently verified using peptide-ε as a substrate. For assays using purified PKC-ζ and IRS isoform proteins, 0.5 μg of IRS isoforms and 0.1 μg of PKC-ζ (specific activity of 1410 nmol of phosphate transferred to peptide-ε substrate per minute per milligram of protein) were used. Immune-complex in vitro kinase assays were performed in the presence of [γ-32P]ATP as described above using wild-type or KD HA-tagged recombinant PKC-ζ immunoprecipitated from lysates of transfected COS-7 cells and recombinant IRS isoform proteins immunoprecipitated from another group of transfected cells.
Functional assessment of IRS-1, -2, -3, and -4
NIH-3T3IR cells transiently cotransfected with IRS1-HA, IRS-2, IRS3-myc, IRS4-myc, and either a control vector or PKC-ζ were serum starved overnight and then stimulated with insulin (100 nm) for 0, 2, or 60 min. Cell lysates (300–500 μg of total protein) were subjected to immunoprecipitation with anti-HA, anti-IRS-2, and anti-myc antibodies as above. Samples were separated by 7.5% SDS-PAGE and gel contents were transferred to nitrocellulose. Membranes were subsequently immunoblotted with phosphotyrosine antibody, anti-p85 antibody or anti-IRS-1, -2, -3, or -4 antibodies. To assess PI3K activity associated with IRS-1, -2, -3, or -4, anti-HA, anti-IRS-2, or anti-myc immunoprecipitates were washed once with PBS containing 1% Nonidet P-40 and 100 μm Na3VO4; twice with 100 mm Tris-HCl (pH 7.5) containing 500 mm LiCl2 and 100 mm Na3VO4; and once with 10 mm Tris-HCl (pH 7.5) containing 100 mm NaCl, 1 mm EDTA, and 100 mm Na3VO4. For each reaction, 10 μg of phosphatidylinositol (Sigma, St. Louis, MO) sonicated in 10 μl of PI3K reaction buffer [20 mm Tris-HCl (pH 7.5), 100 mm NaCl, 0.3 mm EGTA], and 10 μCi of [γ-32P]ATP in 40 μl of PI3K reaction buffer were added along with MgCl2 at a final concentration of 10 mm. The phosphorylation reaction was started by adding 50 μl of the substrate solution with 50 μl of the immune complex. After incubation for 20 min at 30 C, the reaction was stopped by adding 100 μl of 0.1 N HCl and 200 μl of CHCl3/CH3OH (1:1). The organic phase containing the phosphorylated phospholipid products was extracted and applied to a silica gel thin-layer chromatography plate (Whatman, Florham Park, NJ) coated with 1% potassium oxalate. Thin-layer chromatography plates were developed in CHCl3/CH3OH/H2O/NH4OH (60:47:11.3:2), dried, and visualized by autoradiography. Assays were quantified by PhosphorImager (Molecular Dynamics) and normalized for the amount of IRS-1 recovered in anti-HA immunoprecipitates.
Results
IRS-1, -3, and -4 but not IRS-2 are substrates for PKC-ζ in vitro
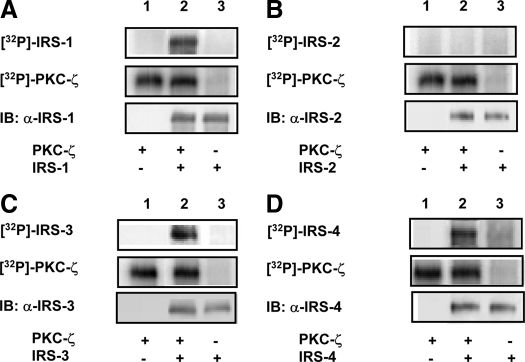
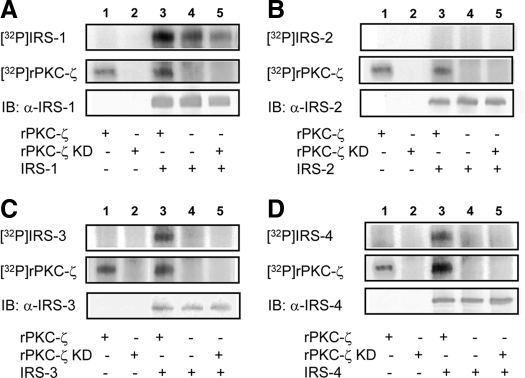
In vitro kinase assays using recombinant IRS isoforms immunoprecipitated from lysates of transfected cells and purified PKC-ζ were performed to determine which IRS isoforms are capable of functioning as substrates for PKC-ζ. As we previously reported, IRS-1 functioned as a substrate for PKC-ζ in vitro (Fig. 1A) (5). Similarly, IRS-3 and -4 were also phosphorylated by purified PKC-ζ in vitro (Fig. 1, C and D). By contrast, IRS-2 was not phosphorylated in the presence or absence of PKC-ζ (Fig. 1B). To confirm these results, we next performed immune complex kinase assays in vitro using either recombinant wild-type PKC-ζ (rPKC-ζ) or kinase-inactive PKC-ζ (rPKC-ζ KD) immunoprecipitated from lysates of transfected cells. IRS-1, -3, and -4 were phosphorylated by rPKC-ζ but not rPKC-ζ KD (Fig. 2, A, C, and D). By contrast, IRS-2 was not phosphorylated in the presence of either rPKC-ζ or rPKC-ζ KD (Fig. 2B). Thus, IRS-3, and -4 are novel substrates for PKC-ζ similar to IRS-1, whereas IRS-2 does not undergo phosphorylation by PKC-ζ in vitro.
Figure 1.
Purified PKC-ζ phosphorylates IRS-1, -3, and -4 but not IRS-2 in vitro. Kinase assays were performed using purified PKC-ζ as the kinase, [γ-32P]ATP, and recombinant IRS isoforms immunoprecipitated from cell lysates of transfected COS-7 cells as substrates. Top and middle panels, Autoradiograms demonstrating phosphorylated IRS isoforms and autophosphorylation of PKC-ζ, respectively, where present. Bottom panels, Immunoblotting controls confirming the presence or absence of IRS substrates in each of the in vitro kinase reactions. Representative autoradiograms and immunoblots are shown for experiments that were repeated independently five times. A, HA-tagged IRS-1 immunoprecipitated using an anti-HA antibody. B, IRS-2 immunoprecipitated with anti-IRS-2 antibody. C, Myc-tagged IRS-3 immunoprecipitated using an anti-myc antibody. D, Myc-tagged IRS-4 immunoprecipitated using an anti-myc antibody.
Figure 2.
Wild-type PKC-ζ phosphorylates IRS-1, -3, and -4 but not IRS-2 in immune-complex kinase assays. Immune-complex in vitro kinase assays were performed using wild-type or KD HA-tagged recombinant PKC-ζ immunoprecipitated from lysates of transfected COS-7 cells. Recombinant IRS proteins immunoprecipitated from another group of transfected cells was used as substrate in the presence of [γ-32P]ATP. Top and middle panels, Autoradiograms demonstrating phosphorylated IRS isoforms and autophosphorylation of PKC-ζ, respectively, where present. Bottom panels, Immunoblotting controls confirming the presence or absence of IRS substrates in each of the in vitro kinase reactions. Representative autoradiograms and immunoblots are shown for experiments that were repeated independently five times. A, HA-tagged IRS-1 immunoprecipitated using an anti-HA antibody. B, IRS-2 immunoprecipitated with anti-IRS-2 antibody. C, Myc-tagged IRS-3 immunoprecipitated using an anti-myc antibody. D, Myc-tagged IRS-4 immunoprecipitated using an anti-myc antibody.
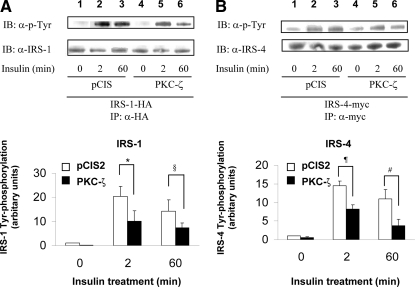
Overexpression of PKC-ζ impairs insulin-stimulated tyrosine phosphorylation of IRS-1, IRS-3, and IRS-4, but not IRS-2
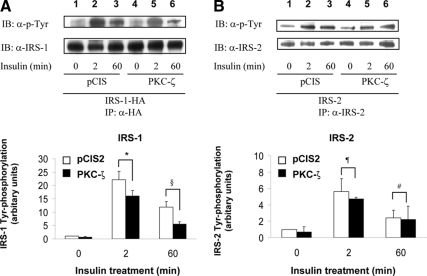
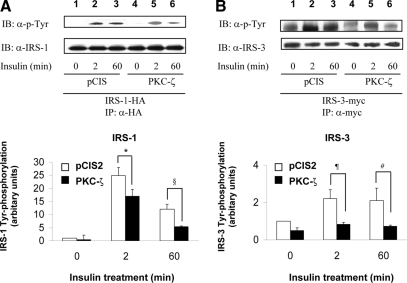
We previously demonstrated that phosphorylation of IRS-1 by PKC-ζ determines a negative feedback pathway resulting in diminished tyrosine phosphorylation of IRS-1 in response to insulin stimulation (5). To assess whether phosphorylation of IRS-3 and -4 by PKC-ζ has a similar function, we examined the time course of tyrosine phosphorylation of IRS-1, -2, -3, and -4 after insulin stimulation in NIH-3T3IR cells transiently cotransfected with various IRS isoforms and either the empty expression vector (pCIS2) or wild-type PKC-ζ. As previously reported, tyrosine phosphorylation of IRS-1 after 2 and 60 min of insulin treatment was significantly diminished by overexpression of wild-type PKC-ζ (Figs. 3A, 4A, and 5A) (5). Tyrosine phosphorylation of IRS-2 in response to insulin at 0, 2, and 60 min was not significantly altered by overexpression of PKC-ζ (when comparing the same time points in cells transfected with the control vector) (Fig. 3B). However, similar to IRS-1, tyrosine phosphorylation of IRS-3 and -4 after 2 and 60 min of insulin treatment was significantly diminished by overexpression of wild-type PKC-ζ (Figs. 4B and 5B) Thus, the ability of PKC-ζ to phosphorylate IRS-1, -3, and -4 in vitro (Figs. 1 and 2) corresponds to our results demonstrating that overexpression of PKC-ζ impairs insulin-stimulated tyrosine phosphorylation of IRS-1, -3, and -4 in transfected cells. Likewise, the inability of PKC-ζ to phosphorylate IRS-2 in vitro (Figs. 1B and 2B) is consistent with our observation that overexpression of PKC-ζ does not significantly alter insulin-stimulated tyrosine phosphorylation of IRS-2 in transfected cells.
Figure 3.
Overexpression of PKC-ζ inhibits insulin-stimulated tyrosine phosphorylation of IRS-1 but not IRS-2. NIH-3T3IR cells were transiently cotransfected with HA-tagged IRS-1 or IRS-2 and either an empty control vector or PKC-ζ. Transfected cells were treated with 100 nm insulin for 0, 2, or 60 min. A, Cell lysates were immunoprecipitated (IP) with anti-HA antibody and subsequently immunoblotted (IB) with either antiphosphotyrosine (top panel) or anti-IRS-1 antibody (bottom panel). Results from five independent experiments were quantified by scanning densitometry and expressed as mean ± sem of tyrosine phosphorylated IRS protein normalized to total IRS protein. Overexpression of PKC-ζ significantly reduced insulin-stimulated tyrosine phosphorylation of IRS-1 at 2 and 60 min when compared with control cells (*, P = 0.04; §, P = 0.02). B, Cell lysates were immunoprecipitated with anti-IRS-2 antibody and subsequently immunoblotted with antiphosphotyrosine (top panel) or anti-IRS-2 antibody (bottom panel). Results from five independent experiments were quantified by scanning densitometry and expressed as mean ± sem of tyrosine phosphorylated IRS protein normalized to total IRS protein. Overexpression of PKC-ζ did not significantly reduce insulin-stimulated tyrosine phosphorylation of IRS-2 at 2 and 60 min when compared with control cells (¶, P = 0.32; #, P = 0.47).
Figure 4.
Overexpression of PKC-ζ inhibits insulin-stimulated tyrosine phosphorylation of IRS-3. NIH-3T3IR cells were transiently cotransfected with HA-tagged IRS-1 or myc-tagged IRS-3 and either an empty control vector or PKC-ζ. Transfected cells were treated with 100 nm insulin for 0, 2, or 60 min. A, Experiments were as described in legend to Fig. 3A. B, Cell lysates were immunoprecipitated (IP) with anti-myc antibody and subsequently immunoblotted (IB) with antiphosphotyrosine (top panel) or anti-IRS-3 antibody (bottom panel). Results from five independent experiments were quantified by scanning densitometry and expressed as mean ± sem of tyrosine phosphorylated IRS protein normalized to total IRS protein. Overexpression of PKC-ζ significantly reduced insulin-stimulated tyrosine phosphorylation of both IRS-1 and IRS-3 at 2 and 60 min when compared with control cells (*, P = 0.03; §, P = 0.03; ¶, P = 0.03; #, P = 0.04).
Figure 5.
Overexpression of PKC-ζ inhibits insulin-stimulated tyrosine phosphorylation of IRS-4. NIH-3T3IR cells were transiently cotransfected with HA-tagged IRS-1 or myc-tagged IRS-4 and either an empty control vector or PKC-ζ. Transfected cells were treated with 100 nm insulin for 0, 2, or 60 min. A, Experiments were as described in Fig. 3A. B, Cell lysates were immunoprecipitated (IP) with anti-myc antibody and subsequently immunoblotted (IB) with antiphosphotyrosine (top panel) or anti-IRS-4 antibody (bottom panel). Results from five independent experiments were quantified by scanning densitometry and expressed as mean ± sem of tyrosine-phosphorylated IRS protein normalized to total IRS protein. Overexpression of PKC-ζ significantly reduced insulin-stimulated tyrosine phosphorylation of both IRS-1 and IRS-4 at 2 and 60 min when compared with control cells (*, P = 0.04; §, P = 0.04; ¶, P = 0.01; #, P = 0.04).
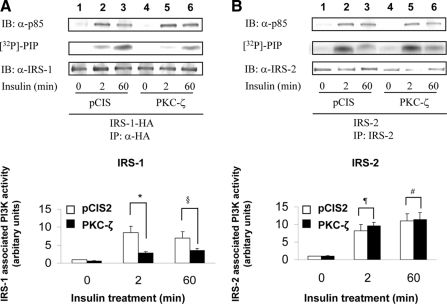
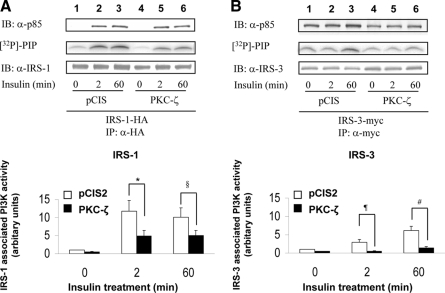
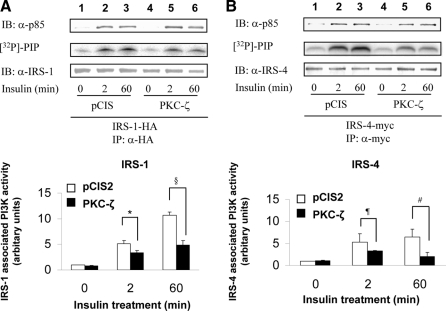
Overexpression of PKC-ζ impairs insulin-stimulated PI3K activity associated with IRS-1, -3, and -4 but not IRS-2
Tyrosine phosphorylated IRS isoforms bind and activate PI3K to then activate downstream kinases including PKC-ζ. Therefore, we next examined the ability of IRS isoforms to bind and activate PI3K after insulin stimulation in NIH-3T3IR cells transiently cotransfected with various IRS isoforms and either a control vector (pCIS2) or wild-type PKC-ζ. As previously reported, insulin-stimulated PI3K activity associated with IRS-1 after 2 and 60 min of insulin treatment was significantly diminished by overexpression of wild-type PKC-ζ (Figs. 6A, 7A, and 8A), even though the amount of p85 associated with IRS-1 was not substantially altered (5). PI3K activity and p85 associated with IRS-2 in response to insulin at 0, 2, and 60 min was not significantly altered by overexpression of PKC-ζ (when comparing the same time points in cells transfected with the control vector) (Fig. 6B). However, similar to IRS-1, PI3K activity associated with IRS-3 and -4 after 2 and 60 min of insulin treatment was significantly diminished by overexpression of wild-type PKC-ζ even though the amount of p85 associated with IRS-3 and -4 was not altered (Figs. 7B and 8B). Thus, consistent with diminished insulin-stimulated tyrosine phosphorylation of IRS-1, -3, and -4 in cells overexpressing PKC-ζ (Figs. 4 and 5), PI3K activity associated with IRS-1, -3, and -4 is diminished by overexpression of PKC-ζ. Likewise, the inability of overexpression of PKC-ζ to impair insulin-stimulated tyrosine phosphorylation of IRS-2 in transfected cells (Fig. 3B) is consistent with our observation that overexpression of PKC-ζ does not significantly alter insulin-stimulated activity of PI3K associated with IRS-2. Taken together, our results suggest that IRS-3 and IRS-4 (but not IRS-2) are novel substrates for PKC-ζ that participate in negative feedback pathways in insulin signaling similar to that involving IRS-1 and PKC-ζ.
Figure 6.
Overexpression of PKC-ζ impairs insulin-stimulated activation of PI3K associated with IRS-1 but not IRS-2. NIH-3T3IR cells were transiently cotransfected with HA-tagged IRS-1 or IRS-2 and either an empty control vector or PKC-ζ. Transfected cells were treated with 100 nm insulin for 0, 2, or 60 min. A, Cell lysates were immunoprecipitated (IP) with anti-HA antibody and subsequently immunoblotted (IB) with either anti-p85 antibody (top panel) or anti-IRS-1 antibody (bottom panel). IRS-1-associated PI3K activity was determined in parallel using a lipid kinase assay measuring [32P]-phosphatidylinositol phosphate (PIP) product (middle panel). Results from five independent lipid kinase experiments were quantified by PhosphorImager and expressed as mean ± sem. Overexpression of PKC-ζ significantly reduced insulin-stimulated PI 3-kinase activity associated with IRS-1 at 2 and 60 min when compared with control cells (*, P = 0.02; §, P = 0.04). B, Cell lysates were immunoprecipitated (IP) with anti-IRS-2 antibody and subsequently immunoblotted with either anti-p85 antibody (top panel) or anti-IRS-2 antibody (bottom panel). IRS-2-associated PI3K activity was determined in parallel using a lipid kinase assay measuring [32P]-phosphatidylinositol phosphate (PIP) product (middle panel). Results from five independent lipid kinase experiments were quantified by PhosphorImager and expressed as mean ± sem. Overexpression of PKC-ζ did not significantly reduce insulin-stimulated PI3K activity associated with IRS-2 at 2 and 60 min when compared with control cells (¶, P = 0.26; #, P = 0.42). Moreover, there were no significant differences between levels of IRS-2-associated PI3K activity when the 2-min point was compared with the 60-min point for either control cells or cells overexpressing PKC-ζ.
Figure 7.
Overexpression of PKC-ζ impairs insulin-stimulated activation of PI3K associated with IRS-3. NIH-3T3IR cells were transiently cotransfected with HA-tagged IRS-1 or myc-tagged IRS-3 and either an empty control vector or PKC-ζ. Transfected cells were treated with 100 nm insulin for 0, 2, or 60 min. A, Experiments were as described in Fig. 6A. B, Cell lysates were immunoprecipitated (IP) with anti-myc antibody and subsequently immunoblotted (IB) with either anti-p85 antibody (top panel) or anti-IRS-3 antibody (bottom panel). IRS-3-associated PI3K activity was determined in parallel using a lipid kinase assay measuring [32P]-phosphatidylinositol phosphate (PIP) product (middle panel). Results from five independent lipid kinase experiments were quantified by PhosphorImager and expressed as mean ± sem. Overexpression of PKC-ζ significantly reduced insulin-stimulated PI3K activity associated with both IRS-1 and IRS-3 at 2 and 60 min when compared with control cells (*, P = 0.03; §, P = 0.04; ¶, P = 0.03; #, P = 0.04).
Figure 8.
Overexpression of PKC-ζ impairs insulin-stimulated activation of PI3K associated with IRS-4. NIH-3T3IR cells were transiently cotransfected with HA-tagged IRS-1 or myc-tagged IRS-4 and either an empty control vector or PKC-ζ. Transfected cells were treated with 100 nm insulin for 0, 2, or 60 min. A, Experiments were as described in Fig. 6A. B, Cell lysates were immunoprecipitated (IP) with anti-myc antibody and subsequently immunoblotted (IB) with either anti-p85 antibody (top panel) or anti-IRS-4 antibody (bottom panel). IRS-4-associated PI3K activity was determined in parallel using a lipid kinase assay measuring [32P]-phosphatidylinositol phosphate (PIP) product (middle panel). Results from five independent lipid kinase experiments were quantified by PhosphorImager and expressed as mean ± sem. Overexpression of PKC-ζ significantly reduced insulin-stimulated PI3K activity associated with both IRS-1 and IRS-4 at 2 and 60 min when compared with control cells (*, P = 0.04; §, P = 0.02; ¶, P = 0.03; #, P = 0.04).
Discussion
A highly complex signaling network determines specificity of cellular and physiological actions of insulin in various insulin target tissues (1,2,3). IRS proteins play key roles in determining specificity of insulin action for several reasons. As an early step in insulin signaling, IRS proteins are among the primary substrates for the insulin receptor tyrosine kinase. Tyrosine phosphorylated IRS proteins serve as docking molecules for multiple downstream effectors that create an important primary node and scaffold for subsequent signaling networks (3). Moreover, there are multiple Ser/Thr phosphorylation sites on IRS proteins that contribute to modulating IRS function. For example, kinases from heterologous signaling pathways (e.g. angiotensin II receptor, innate immune signaling pathways, etc.) may cross talk with insulin signaling pathways by phosphorylating IRS-1 on serine residues to cause insulin resistance (23,24,25,26,27,28,29). In addition, a number of kinases downstream from IRS-1 in canonical insulin signaling pathways including glycogen synthase kinase-3, Akt, and PKC-ζ phosphorylate IRS-1 on serine residues to create feedback pathways that influence IRS-1 function and insulin signaling (5,30,31). In general, serine phosphorylation of IRS-1 by downstream kinases impairs IRS-1 tyrosine phosphorylation, resulting in reduced binding and activation of downstream effectors such as PI3K (29,32). This represents negative feedback regulation. However, positive feedback regulation in response to serine phosphorylation of IRS-1 on specific sites has also been reported (31,33,34,35) Feedback pathways contribute to signal complexity and may help to determine specificity in insulin signaling (4). The presence or absence or specific feedback pathways involving IRS proteins may help to explain some of the mechanisms underlying distinct functions of IRS isoforms.
IRS-1, -3, and -4 but not IRS-2 are substrates for PKC-ζ
A primary finding of our present study is that IRS-1, -3, and -4 can serve as direct substrates for PKC-ζ. By contrast, under similar conditions, we were unable to detect phosphorylation of IRS-2 by PKC-ζ. Thus, we have identified IRS-3 and -4 as novel substrates for PKC-ζ. Because our experiments relied on immune-complex kinase assays using IRS proteins immunoprecipitated from transfected mammalian cells, it is possible that a coimmunoprecipitated factor participated in the phosphorylation of IRS-1, -3, and -4. However, this seems unlikely because a kinase-inactive form of PKC-ζ was unable to phosphorylate immunoprecipitated IRS proteins under similar conditions.
We and others previously reported that serine phosphorylation of IRS-1 by PKC-ζ impairs insulin-stimulated tyrosine phosphorylation of IRS-1 and that this negative feedback pathway results in decreased IRS-1 associated PI3K activity and insulin resistance (5,6). Subsequently, a number of other groups have identified specific PKC-ζ serine phosphorylation sites on rat IRS-1 including Ser318 (7,9) as well as Ser498, Ser570, and Ser612 (8). We performed a database search to align regions of IRS-2, -3, and -4 that are homologous to regions containing these previously identified IRS-1 serine phosphorylation sites (data not shown). However, we were unable to identify any sequences in these homologous regions that might explain why IRS-3 and -4 are phosphorylated by PKC-ζ, whereas IRS-2 is not. This suggests that there may be additional important regulatory PKC-ζ serine phosphorylation sites on IRS-1, -3, and -4 that have yet to be identified.
Functional consequences of phosphorylation of IRS-3 and -4 by PKC-ζ
Similar to our previous findings with IRS-1 (5), overexpression of PKC-ζ impairs insulin-stimulated tyrosine phosphorylation of both IRS-3 and -4 in intact cells. Moreover, this is associated with impaired insulin-stimulated PI3K activity associated with IRS-3 and -4. Interestingly, as we reported previously with IRS-1 (5), we were unable to detect significant changes in insulin-stimulated binding of p85 to any of the IRS isoforms in the presence of PKC-ζ overexpression. This may be due to the fact that subtle changes in p85 binding difficult to detect by immunoprecipitation may have significant functional consequences for PI3K activity. Indeed, the tandem SH2 domains of p85 must be occupied simultaneously for full activation of PI3K (36,37,38,39,40). It is possible that serine phosphorylation of IRS-1, -3 and -4 by PKC-ζ may be sufficient to affect the geometry of interactions between the tandem SH2 domains in p85 and IRS isoforms to cause functional impairment without a detectable change in p85 binding as assessed by immunoprecipitation. Taken together, our data suggest that IRS-3 and -4 are novel substrates for PKC-ζ that participate in a negative feedback loop for PI3K-dependent insulin signaling similar to that previously identified for IRS-1.
PKC-ζ does not create a negative feedback loop with IRS-2
By contrast, with IRS-1, -3, and -4, in cells overexpressing PKC-ζ, insulin-stimulated tyrosine phosphorylation of IRS-2, p85 binding, and PI3K activity associated with IRS-2 were similar to that observed in control cells transfected with empty vector. These data are consistent with our initial findings that IRS-2 is unable to serve as a substrate for PKC-ζ. Thus, with respect to IRS-2, PKC-ζ does not appear to determine a negative feedback loop for PI3K-dependent signaling. This may have significant implications for the specificity of IRS-2 functions in insulin signaling.
In a previous study, coimmunoprecipitation between IRS-2 and PKC-ζ was reported in a pancreatic cancer cell line (AsPC-1) (41). In this same study, overexpression of a constitutively active PKC-ζ mutant decreased the binding of p85 to IRS-2 in the absence of insulin. Based on these experiments, the authors concluded that there is a negative feedback loop involving PKC-ζ and IRS-2 in these cells. However, this study did not directly demonstrate that IRS-2 is a substrate for PKC-ζ and also did not perform any experiments to show impairment in insulin signaling. Thus, their conclusion regarding a negative feedback loop between PKC-ζ and IRS-2 is not well supported by their data, and their results may be specific to a particular cancer cell line.
IRS-3 and -4 have a highly restricted tissue distribution (42,43). Indeed, in humans, there is no IRS-3 homolog (44). In rat, IRS-3 is expressed predominantly in adipocytes, whereas in mice, mRNA for IRS-3 can also be detected by Northern blotting in liver, lung, kidney, and heart (43). In most human tissues, mRNA for IRS-4 is undetectable by Northern blotting. Using RT-PCR, mRNA for IRS-4 can be detected in human prostate and ovary as well as mouse skeletal muscle, brain, heart, kidney, and liver (42,45,46). However, the physiological relevance of this very low level expression of IRS-4 mRNA is unknown. By contrast, IRS-1 and IRS-2 are widely expressed, especially in tissues that are important for metabolic and vascular homeostasis including muscle, fat, liver, brain, heart, vascular endothelium, and pancreatic β-cells (45,46). The relative expression levels of IRS-1 and IRS-2 differ in various tissues. However, this is unlikely to completely explain the distinct functions of IRS-1 and IRS-2, given their high degree of homology. Results from targeted disruption of IRS genes in mice provide important information about functional differences and nonredundancy between IRS-1 and -2 (11,12,13,15). IRS-1 homozygous knockout (IRS-1−/−) mice have impaired intrauterine growth, resistance to insulin and insulin-like growth factor I (IGF-I), and glucose intolerance (11,12). By contrast, knockout mice lacking IRS-2 (IRS-2−/−) exhibit nearly normal birth size and body weight with insulin resistance and abnormal glucose tolerance that later develops into frank diabetes as a result of reduced β-cell mass (13). IRS-1 plays a major role in insulin-stimulated glucose uptake in skeletal muscle (15,47), whereas IRS-2 plays an important function to regulate hepatic insulin action as well as pancreatic β-cell development and survival (14,15). It is possible that the inability of PKC-ζ to phosphorylate IRS-2 and create a negative feedback loop (unlike with IRS-1) is one mechanism that contributes to differences between IRS-1 and IRS-2 function in various insulin target tissues.
In summary, we found that IRS-3 and -4 are novel substrates for PKC-ζ that participate in negative feedback pathways for insulin signaling similar to that previously reported for IRS-1. By contrast, the inability of PKC-ζ to phosphorylate IRS-2 precludes a feedback loop between PKC-ζ and IRS-2 that may help to determine specific functional roles for IRS-2.
Footnotes
This work was supported by in part by the Intramural Research Program, National Center for Complementary and Alternative Medicine, National Institutes of Health, and a mentor-based postdoctoral fellowship award from the American Diabetes Association (to M.J.Q.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online January 17, 2008
Abbreviations: IRS, Insulin receptor substrate; KD, kinase dead; PI3K, phosphatidylinositol 3-kinase; PKC, protein kinase C; rPKC, recombinant wild-type PKC.
References
- Nystrom FH, Quon MJ 1999 Insulin signalling: metabolic pathways and mechanisms for specificity. Cell Signal 11:563–574 [DOI] [PubMed] [Google Scholar]
- Ver MR, Chen H, Quon MJ 2005 Insulin signaling pathways regulating translocation of GLUT4. Curr Med Chem Immunol Endocrinol Metab Agents 5:159–165 [Google Scholar]
- Taniguchi CM, Emanuelli B, Kahn CR 2006 Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 7:85–96 [DOI] [PubMed] [Google Scholar]
- Sedaghat AR, Sherman A, Quon MJ 2002 A mathematical model of metabolic insulin signaling pathways. Am J Physiol Endocrinol Metab 283:E1084–E1101 [DOI] [PubMed] [Google Scholar]
- Ravichandran LV, Esposito DL, Chen J, Quon MJ 2001 Protein kinase C-ζ phosphorylates insulin receptor substrate-1 and impairs its ability to activate phosphatidylinositol 3-kinase in response to insulin. J Biol Chem 276:3543–3549 [DOI] [PubMed] [Google Scholar]
- Liu YF, Paz K, Herschkovitz A, Alt A, Tennenbaum T, Sampson SR, Ohba M, Kuroki T, LeRoith D, Zick Y 2001 Insulin stimulates PKCζ-mediated phosphorylation of insulin receptor substrate-1 (IRS-1). A self-attenuated mechanism to negatively regulate the function of IRS proteins. J Biol Chem 276:14459–14465 [DOI] [PubMed] [Google Scholar]
- Beck A, Moeschel K, Deeg M, Haring HU, Voelter W, Schleicher ED, Lehmann R 2003 Identification of an in vitro insulin receptor substrate-1 phosphorylation site by negative-ion muLC/ES-API-CID-MS hybrid scan technique. J Am Soc Mass Spectrom 14:401–405 [DOI] [PubMed] [Google Scholar]
- Sommerfeld MR, Metzger S, Stosik M, Tennagels N, Eckel J 2004 In vitro phosphorylation of insulin receptor substrate 1 by protein kinase C-ζ: functional analysis and identification of novel phosphorylation sites. Biochemistry 43:5888–5901 [DOI] [PubMed] [Google Scholar]
- Moeschel K, Beck A, Weigert C, Lammers R, Kalbacher H, Voelter W, Schleicher ED, Haring HU, Lehmann R 2004 Protein kinase C-ζ-induced phosphorylation of Ser318 in insulin receptor substrate-1 (IRS-1) attenuates the interaction with the insulin receptor and the tyrosine phosphorylation of IRS-1. J Biol Chem 279:25157–25163 [DOI] [PubMed] [Google Scholar]
- White MF 1998 The IRS-signalling system: a network of docking proteins that mediate insulin action. Mol Cell Biochem 182:3–11 [PubMed] [Google Scholar]
- Araki E, Lipes MA, Patti ME, Bruning JC, Haag 3rd B, Johnson RS, Kahn CR 1994 Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature 372:186–190 [DOI] [PubMed] [Google Scholar]
- Tamemoto H, Kadowaki T, Tobe K, Yagi T, Sakura H, Hayakawa T, Terauchi Y, Ueki K, Kaburagi Y, Satoh S, Sekihara H, Yoshioka S, Horikoshi H, Furata Y, Ikawa Y, Kasuza M, Yazaki Y, Aizawa S 1994 Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature 372:182–186 [DOI] [PubMed] [Google Scholar]
- Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, Bonner-Weir S, White MF 1998 Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391:900–904 [DOI] [PubMed] [Google Scholar]
- Kubota N, Tobe K, Terauchi Y, Eto K, Yamauchi T, Suzuki R, Tsubamoto Y, Komeda K, Nakano R, Miki H, Satoh S, Sekihara H, Sciacchitano S, Lesniak M, Aizawa S, Nagai R, Kimura S, Akanuma Y, Taylor SI, Kadowaki T 2000 Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory β-cell hyperplasia. Diabetes 49:1880–1889 [DOI] [PubMed] [Google Scholar]
- Previs SF, Withers DJ, Ren JM, White MF, Shulman GI 2000 Contrasting effects of IRS-1 versus IRS-2 gene disruption on carbohydrate and lipid metabolism in vivo. J Biol Chem 275:38990–38994 [DOI] [PubMed] [Google Scholar]
- Liu SC, Wang Q, Lienhard GE, Keller SR 1999 Insulin receptor substrate 3 is not essential for growth or glucose homeostasis. J Biol Chem 274:18093–18099 [DOI] [PubMed] [Google Scholar]
- Fantin VR, Wang Q, Lienhard GE, Keller SR 2000 Mice lacking insulin receptor substrate 4 exhibit mild defects in growth, reproduction, and glucose homeostasis. Am J Physiol Endocrinol Metab 278:E127–E133 [DOI] [PubMed] [Google Scholar]
- Quon MJ, Zarnowski MJ, Guerre-Millo M, de la Luz Sierra M, Taylor SI, Cushman SW 1993 Transfection of DNA into isolated rat adipose cells by electroporation: evaluation of promoter activity in transfected adipose cells which are highly responsive to insulin after one day in culture. Biochem Biophys Res Commun 194:338–346 [DOI] [PubMed] [Google Scholar]
- Quon MJ, Butte AJ, Zarnowski MJ, Sesti G, Cushman SW, Taylor SI 1994 Insulin receptor substrate 1 mediates the stimulatory effect of insulin on GLUT4 translocation in transfected rat adipose cells. J Biol Chem 269:27920–27924 [PubMed] [Google Scholar]
- Zhou L, Chen H, Lin CH, Cong LN, McGibbon MA, Sciacchitano S, Lesniak MA, Quon MJ, Taylor SI 1997 Insulin receptor substrate-2 (IRS-2) can mediate the action of insulin to stimulate translocation of GLUT4 to the cell surface in rat adipose cells. J Biol Chem 272:29829–29833 [DOI] [PubMed] [Google Scholar]
- Zhou L, Chen H, Xu P, Cong LN, Sciacchitano S, Li Y, Graham D, Jacobs AR, Taylor SI, Quon MJ 1999 Action of insulin receptor substrate-3 (IRS-3) and IRS-4 to stimulate translocation of GLUT4 in rat adipose cells. Mol Endocrinol 13:505–514 [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay G, Standaert ML, Zhao L, Yu B, Avignon A, Galloway L, Karnam P, Moscat J, Farese RV 1997 Activation of protein kinase C (α, β, and ζ) by insulin in 3T3/L1 cells. Transfection studies suggest a role for PKC-ζ in glucose transport. J Biol Chem 272:2551–2558 [DOI] [PubMed] [Google Scholar]
- Andreozzi F, Laratta E, Sciacqua A, Perticone F, Sesti G 2004 Angiotensin II impairs the insulin signaling pathway promoting production of nitric oxide by inducing phosphorylation of insulin receptor substrate-1 on Ser312 and Ser616 in human umbilical vein endothelial cells. Circ Res 94:1211–1218 [DOI] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS 2006 TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116:3015–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J 2002 Serine phosphorylation of insulin receptor substrate 1 by inhibitor κB kinase complex. J Biol Chem 277:48115–48121 [DOI] [PubMed] [Google Scholar]
- Gao Z, Zhang X, Zuberi A, Hwang D, Quon MJ, Lefevre M, Ye J 2004 Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Mol Endocrinol 18:2024–2034 [DOI] [PubMed] [Google Scholar]
- Gao Z, Zuberi A, Quon MJ, Dong Z, Ye J 2003 Aspirin inhibits serine phosphorylation of insulin receptor substrate 1 in tumor necrosis factor-treated cells through targeting multiple serine kinases. J Biol Chem 278:24944–24950 [DOI] [PubMed] [Google Scholar]
- Kim JA, Yeh DC, Ver M, Li Y, Carranza A, Conrads TP, Veenstra TD, Harrington MA, Quon MJ 2005 Phosphorylation of Ser24 in the pleckstrin homology domain of insulin receptor substrate-1 by mouse Pelle-like kinase/interleukin-1 receptor-associated kinase: cross-talk between inflammatory signaling and insulin signaling that may contribute to insulin resistance. J Biol Chem 280:23173–23183 [DOI] [PubMed] [Google Scholar]
- Paz K, Hemi R, LeRoith D, Karasik A, Elhanany E, Kanety H, Zick Y 1997 A molecular basis for insulin resistance. Elevated serine/threonine phosphorylation of IRS-1 and IRS-2 inhibits their binding to the juxtamembrane region of the insulin receptor and impairs their ability to undergo insulin-induced tyrosine phosphorylation. J Biol Chem 272:29911–29918 [DOI] [PubMed] [Google Scholar]
- Eldar-Finkelman H, Krebs EG 1997 Phosphorylation of insulin receptor substrate 1 by glycogen synthase kinase 3 impairs insulin action. Proc Natl Acad Sci USA 94:9660–9664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz K, Liu YF, Shorer H, Hemi R, LeRoith D, Quon MJ, Kanety H, Seger R, Zick Y 1999 Phosphorylation of insulin receptor substrate-1 (IRS-1) by protein kinase B positively regulates IRS-1 function. J Biol Chem 274:28816–28822 [DOI] [PubMed] [Google Scholar]
- Zick Y 2001 Insulin resistance: a phosphorylation-based uncoupling of insulin signaling. Trends Cell Biol 11:437–441 [DOI] [PubMed] [Google Scholar]
- Luo M, Langlais P, Yi Z, Lefort N, De Filippis EA, Hwang H, Christ-Roberts CY, Mandarino LJ 2007 Phosphorylation of human insulin receptor substrate-1 at Serine 629 plays a positive role in insulin signaling. Endocrinology 148:4895–4905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud J, Leshan R, Lee YH, White MF 2004 Nutrient-dependent and insulin-stimulated phosphorylation of insulin receptor substrate-1 on serine 302 correlates with increased insulin signaling. J Biol Chem 279:3447–3454 [DOI] [PubMed] [Google Scholar]
- Furukawa N, Ongusaha P, Jahng WJ, Araki K, Choi CS, Kim HJ, Lee YH, Kaibuchi K, Kahn BB, Masuzaki H, Kim JK, Lee SW, Kim YB 2005 Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell Metab 2:119–129 [DOI] [PubMed] [Google Scholar]
- Backer JM, Myers Jr MG, Shoelson SE, Chin DJ, Sun XJ, Miralpeix M, Hu P, Margolis B, Skolnik EY, Schlessinger J, White MF 1992 Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J 11:3469–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rordorf-Nikolic T, Van Horn DJ, Chen D, White MF, Backer JM 1995 Regulation of phosphatidylinositol 3′-kinase by tyrosyl phosphoproteins. Full activation requires occupancy of both SH2 domains in the 85-kDa regulatory subunit. J Biol Chem 270:3662–3666 [DOI] [PubMed] [Google Scholar]
- Ottinger EA, Botfield MC, Shoelson SE 1998 Tandem SH2 domains confer high specificity in tyrosine kinase signaling. J Biol Chem 273:729–735 [DOI] [PubMed] [Google Scholar]
- Herbst JJ, Andrews G, Contillo L, Lamphere L, Gardner J, Lienhard GE, Gibbs EM 1994 Potent activation of phosphatidylinositol 3′-kinase by simple phosphotyrosine peptides derived from insulin receptor substrate 1 containing two YMXM motifs for binding SH2 domains. Biochemistry 33:9376–9381 [DOI] [PubMed] [Google Scholar]
- Esposito DL, Li Y, Cama A, Quon MJ 2001 Tyr(612) and Tyr(632) in human insulin receptor substrate-1 are important for full activation of insulin-stimulated phosphatidylinositol 3-kinase activity and translocation of GLUT4 in adipose cells. Endocrinology 142:2833–2840 [DOI] [PubMed] [Google Scholar]
- Neid M, Datta K, Stephan S, Khanna I, Pal S, Shaw L, White M, Mukhopadhyay D 2004 Role of insulin receptor substrates and protein kinase C-ζ in vascular permeability factor/vascular endothelial growth factor expression in pancreatic cancer cells. J Biol Chem 279:3941–3948 [DOI] [PubMed] [Google Scholar]
- Fantin VR, Lavan BE, Wang Q, Jenkins NA, Gilbert DJ, Copeland NG, Keller SR, Lienhard GE 1999 Cloning, tissue expression, and chromosomal location of the mouse insulin receptor substrate 4 gene. Endocrinology 140:1329–1337 [DOI] [PubMed] [Google Scholar]
- Sciacchitano S, Taylor SI 1997 Cloning, tissue expression, and chromosomal localization of the mouse IRS-3 gene. Endocrinology 138:4931–4940 [DOI] [PubMed] [Google Scholar]
- Bjornholm M, He AR, Attersand A, Lake S, Liu SC, Lienhard GE, Taylor S, Arner P, Zierath JR 2002 Absence of functional insulin receptor substrate-3 (IRS-3) gene in humans. Diabetologia 45:1697–1702 [DOI] [PubMed] [Google Scholar]
- White MF 2002 IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab 283:E413–E422 [DOI] [PubMed] [Google Scholar]
- Sesti G, Federici M, Hribal ML, Lauro D, Sbraccia P, Lauro R 2001 Defects of the insulin receptor substrate (IRS) system in human metabolic disorders. FASEB J 15:2099–2111 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Tobe K, Tamemoto H, Ueki K, Kaburagi Y, Yamamoto-Honda R, Takahashi Y, Yoshizawa F, Aizawa S, Akanuma Y, Sonenberg N, Yazaki Y, Kadowaki T 1996 Insulin signalling and insulin actions in the muscles and livers of insulin-resistant, insulin receptor substrate 1-deficient mice. Mol Cell Biol 16:3074–3084 [DOI] [PMC free article] [PubMed] [Google Scholar]