Abstract
Obesity is associated with cognitive impairments. Long-term mechanisms for this association include consequences of hyperglycemia, dyslipidemia, or other factors comprising metabolic syndrome X. We found that hypertriglyceridemia, the main dyslipidemia of metabolic syndrome X, is in part responsible for the leptin resistance seen in obesity. Here we determined whether triglycerides have an immediate and direct effect on cognition. Obese mice showed impaired acquisition in three different cognitive paradigms: the active avoidance T-maze, the Morris water maze, and a food reward lever press. These impairments were not attributable to differences in foot shock sensitivity, swim speed, swimming distance, or voluntary milk consumption. Impaired cognition in obese mice was improved by selectively lowering triglycerides with gemfibrozil. Injection into the brain of the triglyceride triolein, but not of the free fatty acid palmitate, impaired acquisition in normal body weight mice. Triolein or milk (97% of fats are triglycerides), but not skim milk (no triglycerides), impaired maintenance of the N-methyl-d-aspartate component of the hippocampal long-term synaptic potential. Measures of oxidative stress in whole brain were reduced by gemfibrozil. We conclude that triglycerides mediate cognitive impairment as seen in obesity, possibly by impairing maintenance of the N-methyl-d-aspartate component of hippocampal long-term potentiation, and that lowering triglycerides can reverse the cognitive impairment and improve oxidative stress in the brain.
OBESITY IS EPIDEMIC within the Western world. Currently, 30% of adults over 20 yr of age and 16% of young persons aged 6–19 yr in the United States are obese. Studies in humans have found an association between obesity and poor cognitive performance (1,2,3). The mechanism(s) by which obesity results in cognitive impairment are uncertain. Postulated mechanisms include the effects of hyperglycemia, hyperinsulinemia, and vascular damage to the central nervous system (4). Some studies have found a correlation between lipid levels and cognitive function, whereas other studies have not (5,6,7). An association between lipids and cognitive function is usually explained as based on dyslipidemia being a risk factor for stroke or cerebrovascular hypoperfusion.
Recently we have shown that triglycerides can impair the transport of leptin across the blood-brain barrier (8), which may account in part for the peripheral leptin resistance seen in obesity and in starvation. Here we determined whether triglycerides might also account for the cognitive impairments associated with obesity. We tested mice in two different hippocampal reference learning and memory tasks and a third nonhippocampal-dependent response memory rewarded bar press task. These tasks were chosen because they represent different types of memory (episodic/procedural, hippocampal/nonhippocampal, reward/aversion avoidance) and are considered to require different brain circuitries (9,10). We found that cognitive impairments could be produced in mice with diet-induced obesity, reversed pharmacologically by lowering triglycerides, and induced by direct injection of triglycerides into the brain. Triglycerides impaired the N-methyl-d-aspartate (NMDA)-mediated maintenance of hippocampal long-term synaptic potentiation. Lowering triglyceride levels in diet-induced obese mice decreased oxidative stress in the central nervous system, providing a possible mechanism for the deleterious effects of triglycerides. These findings show that triglycerides are likely one mechanism by which obesity can induce cognitive impairments.
Materials and Methods
Subjects
Except where indicated [triolein and long-term potentiation (LTP) studies], all subjects were 12-month-old CD-1 male mice obtained from our breeding colony. The housing facility is located at the St. Louis Veterans Affairs and is fully Association Assessment and Accreditation of Laboratory Animal Care accredited. All procedures were approved by the St. Louis Veterans Affairs Animal Care Committee. The colony is tested regularly to ensure it is virus and pathogen free. Mice were randomized at 8 wk of age to either breeder chow (obese mice; Teklad Mouse Breeder Diet 8626, contains 10% fat; Harlan/Teklad, Madison, WI) or regular chow (normal mice; Lab Diet 5001, contains 5% fat; PMI Nutrition, Brentwood, MO). A mouse-fed breeder chow was classified as obese if it weighed 30% more than the average mouse that had been raised on regular chow. Food and water were available on an ad libitum basis and the rooms had a 12 h light, 12-h dark cycle with lights on at 0600 h. Behavioral testing was performed between 0800 and 1500 h.
Mice that were given triolein or used in the LTP study were 8- to 10-wk-old male mice from our breeding colony. They were maintained after weaning on regular chow.
Drugs
Gemfibrozil was purchased from Sigma (St. Louis, MO). Gemfibrozil was dissolved in vegetable oil and fed (1 g/kg in a volume of 1 ml/kg) to the mice twice daily for 21 consecutive days with acquisition tested at d 14 and retention at d 21. For this treatment, mice were randomized to gemfibrozil and nongemfibrozil groups with the control group fed the vegetable oil vehicle. The vegetable oil with or without the gemfibrozil was drawn up into a pipette that was inserted into the mouth behind the molars and about 10 μl at a time placed into the oropharynx so as to induce swallowing. Triolein and palmitate were purchased from Sigma. Triolein was dissolved in a phosphatidylcholine and chloroform solution as previously described (8).
Surgery and drug administration
Surgery.
Forty-eight hours before testing the effects of triolein, the mice were prepared for intracerebroventricular (ICV) injection by drilling a hole through the skull. Mice were anesthetized with 2,2,2 tribromoethanol (240 mg/kg, ip; Aldrich Chemical Co., Inc., Milwaukee, WI). A unilateral hole was drilled 0.5 mm posterior to and 1.0 mm to the right of the bregma without penetrating the meninges or entering the brain. The scalp flap was placed back into position and the mice were placed in cages with clean bedding. The procedure lasted about 5 min per mouse and anesthesia lasted for approximately 30 min. Appearance, color, condition of fur, posture, and respirations of the mice were monitored for the rest of the day to ensure no ill effects from the surgery.
Drug administration.
Immediately before training, the mice were placed under light anesthesia with isoflurane (Webster Veterinary, Sterling, MA) and positioned in a stereotaxic apparatus. Anesthesia was maintained with isoflurane by nose cone and a 30-gauge blunt-end needle inserted through the previously drilled hole into the lateral ventricle of the brain to a depth of 2.0 mm. A volume of 2.0 μl of saline or triolein at doses of 36, 180, or 360 μg or palmitate at 360 μg was infused into the lateral ventricle over 30 sec. After infusion, mice were removed from the stereotaxic instrument, the scalp closed with Vetbond (3M Animal Care Products, St. Paul, MN), and the mice returned to their cages. The ICV procedure took approximately 2 min and the period of anesthesia lasted about 5 min. Mice were monitored as above for the rest of the day to ensure no ill effects of the injected compound or the infusion procedure.
Acquisition and retention testing in mice
T-maze foot shock avoidance
The T-maze is a hippocampal-dependent reference learning task in which the animal must integrate multiple cues in a novel environment to learn a new task (9). The T-maze consisted of a black plastic alley with a start box at one end and two goal boxes at the other. The methodology has been previously described in detail (9,10) and is briefly described here. The start box located at the bottom of the start alley was separated from the alley by a plastic guillotine door, which prevented movement down the alley until training began. An electrifiable stainless steel rod floor ran throughout the maze to deliver a scrambled foot shock.
Mice were not permitted to explore the maze before training. A block of training trials began when a mouse was placed into the start box. The guillotine door was raised and a buzzer sounded simultaneously; 5 sec later foot shock was applied. The goal box that was entered on the first trial was designated incorrect and the foot shock was continued until the mouse entered the other goal box, which in all subsequent trials was designated as correct for that particular mouse. At the end of each trial, the mouse was returned to its home cage until the next trial. The intertrial interval was 30 sec with a foot shock intensity of 0.35 mA. The buzzer intensity was 55 dB. Mice were trained until they made one avoidance. Long-term retention was tested 1 wk later by continuing training until the mice achieved the criterion of making five avoidances in six consecutive trials. The number of trials to make one avoidance was the measure of acquisition. The number of trials needed to reach criterion was the measure of retention.
Foot shock startle response
Differences in sensitivity to shock could produce artifact in the T-maze foot shock avoidance. We used foot shock startle to determine whether there were differences in sensitivity to foot shock between the obese and normal mice. An automated SR-Lab startle response system (San Diego Instruments, San Diego, CA) was used to measure sensitivity to foot shock stimuli by eliciting a startle response. The higher the stimulus intensity needed to elicit a startle, the less sensitive a subject is to foot shock stimulus. The SR-LAB contained an animal enclosure, which consisted of a clear acrylic cylinder 15 cm long and 5 cm in diameter, which was attached to a flat acrylic square. This apparatus rested with four legs on a response transducer used to detect vertical movement. A foot shock grid was located along the length of the animal enclosure and was remotely controlled by an eight-bit programmable shocker. A computer connected to the SR-LAB preformed stimulus presentation and data collection.
Mice were placed in the testing apparatus for a 1- to 2-min acclimation period with no background noise presented throughout the testing period. Each trial consisted of a series of ascending and descending foot shock trials of 0.00, 0.06, 0.09, 0.11, 0.13, 0.16, 0.17, 0.19, 0.20 and 0.22 mA. Every second or third trial was a no-shock control trial. A total of 72 trials were presented. Foot shock duration was 20 msec. Sampling for foot shock startle was initiated concurrently with stimulus presentation and continued for a total sample time of 250 msec. The percent of mice with a positive startle response at each foot shock intensity was determined.
Acquisition of lever press for milk reinforcement
Lever press is an appetitive bar-pressing task that challenges response memory. In this task, mice learn to press a lever to receive a reward. Because mice do not readily consume a novel substance, they were first habituated to the milk (one part evaporated milk and two parts water) by giving them access to it in their home cages for 3 consecutive nights. During the 3 nights of habituation, food and water were removed to encourage drinking but returned the next morning. By the end of the third night of habituation, all mice were drinking at least 20 ml of milk per night. The amount of milk drunk by each mouse was recorded and compared for differences between obese and normal mice. To further determine whether both obese and normal mice were appropriately consuming the milk, the mice were food deprived overnight, the milk reintroduced the next morning, and the amount of milk drunk during the first hour recorded.
To measure learning, mice were placed into a fully automated chamber. Pressing a lever on one wall of the chamber caused a light and dipper containing 100 μl of milk to rise into a reward compartment located on the wall opposite the lever. The reward compartment was 4 cm high and 3.1 cm across with a depth of 3.7 cm. Photo sensors are located in the reward compartment to determine whether the mouse claimed the reward. On d 1, mice had 11 sec to run to the reward compartment to claim the reward, after which access was denied. The reward had to be claimed to count as a rewarded lever press. On d 2–6, a shorter time period of 6 sec was used to avoid a possible ceiling effect in the number of lever presses made. Mice were given one 40-min training session on each of 6 d (Monday, Wednesday, Friday over a 2 wk period) with data automatically recorded by computer. The measure of acquisition was the number of rewarded lever presses.
Spatial water maze tasks
The water maze is a visuospatial hippocampal task in which the mouse must learn the location of a hidden platform using random cues throughout the room. The apparatus consisted of a black, circular tank 67.5 cm in diameter and 30 cm in height. The tank was filled with water (21 C) to a depth of 12.25 cm, submerging a 4- × 11.25-cm circular plexiglas platform to a depth of 1.5 cm. Thus, resting on this escape platform would mean the mouse was still in the water but no longer having to swim. Powdered milk was added to make the water opaque and so hide the platform. The maze was located in a room containing many visual cues. Thus, mice would have to learn where the escape platform was by placing it in relation to these external visual cues. Four starting positions (north, south, east, west) were equally spaced around the perimeter of the tank, dividing the pool into four equal quadrants.
Each trial was recorded with a Polytrak (San Diego Instruments, San Diego, CA) recording device that collects the data on a computer. Mice were given two habituation sessions that consisted of four trials each over 2 consecutive days. The hidden platform was randomly placed throughout the tank during habituation. Training began the day after the second habituation session. Each mouse received one session of four trials per day for 5 d. The platform remained in the same quadrant for the entire 5 d of training. The measures of learning were the latency and distance to reach the platform. Swim speed was also calculated.
A probe test served as a further cognitive measure. At the end of the fifth day, each mouse received a 1-min probe test during which no platform was present. The probe trial took place 1 h after the last platform trial and so assessed short-term retention in the mice. The amount of time spent in the quadrant that previously contained the platform was recorded.
The cued platform test was used to determine whether differences in swimming ability between obese and normal mice could have acted as a confounder in the swim tests. A flag on a pole 10 cm high was placed on the submerged platform. The platform was moved about the tank for each trial to remove any spatial element of the task. The cued platform test had four trials per session with one session per day for 2 d.
Statistical analysis
Results were expressed as means and reported with their ses. Student’s t test was used when only two means were compared, one-way ANOVA when more than two groups were compared with a single independent variable, and two-way ANOVA when there were two independent variables. The ANOVAs were followed by Dunnett’s or Tukey’s posttest analysis as indicated. Statistical significance was taken to be P < 0.05. T-maze, the water mazes, foot shock startle, and lever press were run on the same mice. Separate groups of mice were used for the triglyceride ICV, gemfibrozil studies, LTP, and oxidative stress studies.
Hippocampal recording
Hippocampal slice preparation.
Hippocampal slices (400 μm thick) were prepared from 8- to 10-wk-old CD-1 mice maintained on regular chow using methods described previously (11). Recordings were performed in a submersion recording chamber constantly perfused with artificial cerebrospinal fluid (ACSF) containing (in millimoles): 124 NaCI, 5 KCI, 2.5 CaCI2, 1.5 MgCI2, 1.3 NaH2PO4, and 10 glucose, bubbled with 95% O2-5% CO2 at 30–31 C and a rate of approximately 5 cc/min. After obtaining stable baseline responses in ACSF, the solution was switched to ACSF with 20 cc/liter skim milk, ACSF with 20 cc/liter low-fat milk (2.1 g milk fat per cubic centimeter milk), or ACSF with 1 mg/ml triolein [9-octadecenoic acid-, 1,2,3-propanetriyl ester].
Electrophysiology.
Extracellular recordings were obtained from the stratum radiatum of the CA1 region of hippocampus using glass electrodes filled with 2M NaCI (∼5 mΩ DC resistance). Bipolar constant-current pulses (0.1–0.2 msec) were applied to the Schaffer collateral pathway to elicit excitatory postsynaptic potentials (EPSPs). The stimulus intensity used was one that would evoke 50% of maximal response. A θ burst stimulus consisted of a train of five pulses at 100 Hz applied at 200-msec intervals 10 times; LTP induction was achieved by applying 4 θ burst stimuli (total of 200 pulses).
Oxidative stress
Protein carbonyls.
Samples (5 μl) of whole brain were incubated for 20 min at room temperature with 5 μl of 12% sodium dodecyl sulfate and 10 μl of 2,4-dinitrophenylhydrazine that was diluted 10 times with chelexed water from a 200 mm stock. The samples were neutralized with 7.5 μl of neutralization solution (2 m Tris in 30% glycerol). The resulting sample (250 ng) was loaded per well in the slot blot apparatus. Samples were loaded onto a nitrocellulose membrane under vacuum pressure. The membrane was blocked with 3% BSA in PBS containing 0.01% (wt/vol) sodium azide and 0.2% (vol/vol) Tween 20 wash blot for 1 h and incubated with a 1:100 dilution of anti-dinitrophenylhydrazone polyclonal primary antibody (Chemicon-Millipore, Billerica, MA) in wash blot for 1 h. After completion of the primary antibody incubation, the membranes were washed three times in wash blot for 5 min each. An antirabbit IgG alkaline phosphatase secondary antibody (Sigma) was diluted 1:8000 in wash blot and added to the membrane for 1 h. The membrane was washed in wash blot three times for 5 min and developed using Sigmafast tablets (5-bromo-4-chloro-3-indoyl-phosphate, 4-toluidine salt/4-nitro blue tetrazolium chloride substrate). Blots were dried, scanned with Adobe Photoshop (San Jose, CA), and quantitated with Scion Image.
4-Hydroxynonenal (HNE).
Samples (10 μl) were incubated with 10 μl of modified Laemmli buffer containing 0.125 m Tris base (pH 6.8), 4% (vol/vol) sodium dodecyl sulfate, and 20% (vol/vol) glycerol. The resulting sample (250 ng) was loaded per well in the slot blot apparatus onto a nitrocellulose membrane under vacuum pressure. The membrane was blocked with 3% (wt/vol) BSA in wash blot for 1 h and incubated with a 1:5000 dilution of anti-HNE polyclonal antibody (Alpha Diagnostics, San Antonio, TX) in wash blot for 90 min. After completion of the primary antibody incubation, the membranes were washed three times in wash blot for 5 min each. An antirabbit IgG alkaline phosphatase secondary antibody was diluted 1:8000 in wash blot and added to the membrane for 90 min. The membrane was washed in wash blot three times for 5 min and developed using Sigmafast tablets (5-bromo-4-chloro-3-indoyl-phosphate, 4-toluidine salt/4-nitro blue tetrazolium chloride substrate). Blots were dried, scanned with Adobe Photoshop, and quantitated with Scion Image.
3-Nitrotyrosine (3NT).
The methodology followed for measuring the 3NT levels in the samples is the same as the above-mentioned HNE method except for the primary antibody used in this method is anti-3NT antibody (Millipore).
Results
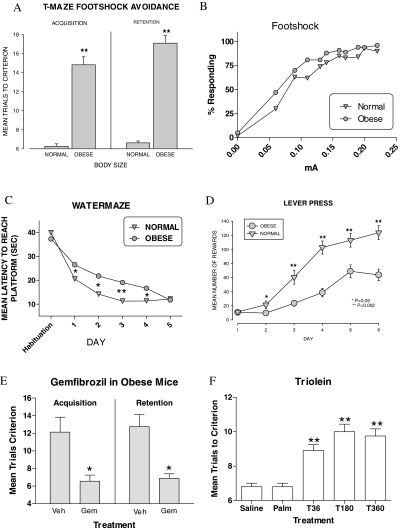
We examined the effects of obesity on memory in CD-1 mice receiving a 10% fat diet (Teklad Mouse Breeder Diet), compared with mice on a 5% fat diet (5001 Rodent Chow, PMI Nutrition International). The obese mice weighed 65.8 ± 9 g vs. regular chow mice of 43.02 ± 6 g (P < 0.01). The obese mice were impaired in the T-maze foot shock avoidance for both acquisition (t = 7.77, P < 0.01) and retention (t = 4.94, P < 0.01; n = 16 controls and 30 obese; Fig. 1A). Obese mice were more sensitive than normal mice to foot shock (Fig. 1B): two-way repeated measures ANOVA for group (control vs. obese): F(1, 484) = 185,004, P < 0.001; milliamp: F(10, 484) = 734,786, P < 0.001; interaction: F(10, 484) = 7,130, P < 0.001. Tukey’s posttest found differences at each milliamp setting.
Figure 1.
Effects of obesity and triglycerides on three tasks measuring memory. A, Comparison of obese and normal mice in the T-maze foot shock avoidance test. Obese mice performed less well in both measures of cognition. B, Response to foot shock. Obese mice were more sensitive to foot shock than normal mice. C, Comparison of time to reach the platform in the water maze. Obese mice performed more poorly than the normal mice. D, Comparison of acquisition of the lever press task in obese and normal mice. Obese mice performed more poorly than normal mice. E, Effect of triglyceride lowering with gemfibrozil on acquisition in obese mice. Mice with serum triglycerides lowered with gemfibrozil (Gem) performed better than vehicle (Veh)-treated mice. F, Effect of triolein or palmitate injected into the third ventricle on memory in the T-maze foot shock avoidance test. Triolein but not palmitate impaired memory. *, P < 0.05; **, P < 0.01.
In the water maze, the two-way repeated measures ANOVA for latency showed a significant effect for group (control vs. obese) F(1, 152) = 8.64, P < 0.006), day F(4, 152) = 15.30, P < 0.0001), and the interaction of group × day F(4, 152) = 2.57, P < 0.04. Tukey’s posttest analysis showed that obese mice took longer to find the hidden platform on d 1–4. By d 5 the obese and control mice were performing at the same level (see Fig. 1C). The two-way ANOVA for distance swam (data not shown) showed a significant effect for group F(1, 152) = 4.059, P < 0.05 and d F(4, 152) = 15.51, P < 0.0001, but not for interaction. Tukey’s posttest showed that on d 2–4 the obese mice traveled a significantly greater distance than the control mice. The two-way ANOVA for swim speed was not significantly different for group, day, or the interaction. There were no differences in probe trial test run 1 h after the final trial on d 5. There were also no significant differences in the cued version of the water maze between the obese and the control mice.
Acquisition for lever press operant conditioning was impaired in the obese mice, compared with the normal mice on d 2 (t = 1.92, P < 0.05), 3 (t = 3.41, P < 0.01), 4 (t = 5.34, P < 0.01), 5 (t = 2.88, P < 0.01), and 6 (t = 3.63, P < 0.01) of training (n = 27 controls and 24 obese; see Fig. 1D). There was no difference between the obese and normal mice in overnight milk consumption or amount of milk drunk during the first hour after overnight food and water deprivation, thus controlling for potential differences in appetitive behavior.
Previously we found that triglycerides are elevated in obese mice and that this disrupts leptin transport across the blood brain barrier, the mechanism underlying peripheral leptin resistance (8). To test whether elevated triglycerides could be responsible for the impaired learning and memory seen in the obese mice, we treated eight obese or eight normal mice with oral gemfibrozil, a drug that specifically lowers triglycerides, and eight obese or eight normal mice with the vegetable oil vehicle. After 2 wk on the twice-daily oral treatment with gemfibrozil (1 mg/kg), we tested acquisition in the T-maze. The t test indicated that the gemfibrozil-treated obese mice took significantly fewer trials to make one avoidance on the acquisition test than the mice that received vehicle (t = 3.394, P < 0.004) (Fig. 1E). One week later and after 21 d of gemfibrozil, retention was tested. The t test for the 1-wk retention test indicated that the mice that received gemfibrozil were also less impaired (t = 4.487, P < 0.0004) (Fig. 1E). Triglyceride levels were 125.5 ± 5.37 mg/dl in obese mice and 67.87 ± 9.60 mg/dl in the mice receiving gemfibrozil (t = 5.07, P < 0.0001) but showed no differences in body weight. Normal control mice treated with gemfibrozil were not significantly different from one another on acquisition of T-maze foot shock avoidance T (13) = 1.899; P = NS; (7.43 ± 1.52 oil; 11.75 ± 1.85 gemfibrozil). The triglyceride levels were 67.42 ± 9.39 for oil and 98.40 ± 7.36 for gemfibrozil (t = 2.59, P < 0.05).
To verify that triglycerides in the brain can impair cognition, we injected the triglyceride triolein into the third ventricle (ICV) immediately after training in the T-maze. As controls, other mice received the free fatty acid palmitate and another saline as the posttraining injections. Eight mice were used per group. The one-way ANOVA for trials to criterion avoidance showed a significant effect on memory [F(4, 40) = 24.06, P < 0.0001] (Fig. 1F). Tukey’s posttest indicated that the mice that received triolein (36–360 μg) took significantly more trials to reach criterion than the mice that received saline or palmitate. The mice that received palmitate were not significantly different from the mice that received saline.
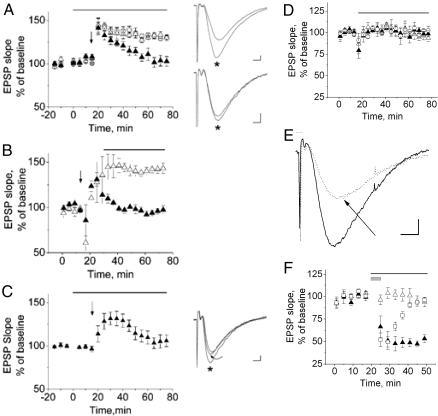
Hippocampal LTP is a useful assessment of synapse-specific plasticity. Spatial learning deficits in the water maze task are often accompanied by impaired hippocampal LTP (12), a neurophysiological correlate to learning and memory (13). Therefore, because the obese mice exhibited deficits in learning and memory, we evaluated the effect of triglycerides on hippocampal LTP at Shaffer collateral pathway synapses in the CA1 region of the hippocampus. First, we compared the slopes of EPSPs after LTP induction by θ burst stimulation (TBS) in the presence of ACSF, ACSF plus skim milk, and ACSF plus low-fat milk (Fig. 2A). Milk was used as a triglyceride source that readily dissolved in ACSF (97% of milk fat is triglycerides), and skim milk, which contains no fats, was used to control for nonfat milk contents. In ACSF or skim milk, there was potentiation of the EPSP slope after TBS lasting at more than 1 h, indicating successful induction and maintenance of LTP (Fig. 2, open triangles and circles). EPSP potentiation 60 min after TBS was 130 ± 5.5% of the baseline slope in ACSF (open circles, n = 8 slices, seven mice) and 130 ± 9.2% in ACSF plus skim milk (open triangles, n = 8 slices, six mice). In contrast, although the EPSP slope in ACSF containing low-fat milk showed initial potentiation after TBS by 60 min, the response reverted to 103 ± 15.9% of the baseline response (Fig. 2A, closed triangles), indicating loss of LTP maintenance (n = 8 slices, seven mice). If ACSF with skim or low-fat milk were applied to the slices 10 min after LTP induction, LTP was maintained in ACSF plus skim milk (Fig. 2B, open triangles, n = 5 slices, four mice) but not in ACSF with low-fat milk (Fig. 2B, closed triangles, n = 5 slices, four mice), indicating that triglycerides can inhibit LTP maintenance after TBS induction.
Figure 2.
Milk fat and triolein impair LTP maintenance but not post-TBS-induced synaptic potentiation (A–C). A, EPSPs normalized to baseline responses show potentiation after TBS (applied at arrow) in hippocampal slices continuously bathed in ACSF (open circles). Synaptic potentiation persists 60 min after TBS, demonstrating maintenance of LTP. Slices bathed in skim milk in ACSF (1% by volume) also exhibit LTP (open triangles). The bar over the graph indicates when ACSF plus milk was applied. In 1% low-fat milk in ACSF, post-TBS synaptic potentiation is observed but reverts to baseline over approximately 30 min, indicating impaired maintenance of LTP (closed triangles). Note that ACSF plus milk does not affect the baseline responses obtained in ACSF alone (t < 0 min). To the right of the graphs are representative traces before and 60 min after (*) TBS. To the right of graph in A, top panel, 60 min after TBS (*), the EPSP has a larger amplitude and steeper slope than the baseline response, indicating maintenance of LTP. The traces in the lower panel were obtained in 1% low-fat milk in ACSF at baseline and 60 min after TBS (*), demonstrating absent maintenance of LTP. B, If ACSF with skim or low-fat milk is applied 10 min after TBS induction of LTP (bar over graph indicates application of ACSF plus milk), LTP is maintained in ACSF plus skim milk but not in ACSF with low-fat milk. As in A, TBS is applied at the vertical arrow. C, EPSPs obtained in ACSF (t < 0 min) are unchanged after application of 1 mg/ml triolein in ACSF starting at t = 0. Triolein was applied from t = 0 to 75 min (bar over graph). TBS (arrow) results in potentiation, which reverts to baseline within 60 min, demonstrating absent LTP maintenance. Traces to the right of the graph (C) are representative traces at baseline, 30 min after TBS (*), and 60 min after TBS (curved arrow), demonstrating that despite demonstrating post-TBS potentiation, LTP is not maintained 60 min after TBS. Calibration for all traces: 2 msec, 0.2 mV. Skim milk, low-fat milk, and triolein do not affect the EPSP slope, but the NMDA component of the EPSP is significantly inhibited by low-fat milk (D–F). D, Slopes of EPSPs in ACSF alone were compared with EPSPs during 60 min of application of ACSF with 1% by volume skim milk (open triangles), 1% by volume low-fat milk (closed triangles), or 1 mg/ml triolein (open circles). The bar over the graph indicates when ACSF perfusion was switched to ACSF plus milk or triolein. The slopes of the EPSPs, dominated by AMPA receptor mediated depolarization are not significantly different. E, The NMDA component of the EPSP is isolated by removing magnesium from the ACSF and adding NBQX to block the AMPA-mediated component of the EPSP. Glycine is also present to facilitate activation of NMDA receptors. The slope of the isolated NMDA-mediated component of the EPSP in ACSF (solid trace) is reduced in ACSF containing low-fat milk (dotted trace, arrow). Calibration was 5 msec, 0.2 mV. F, Cumulative data indicate that the slope of the NMDA-mediated component of the EPSP is not affected by ACSF plus skim milk (open triangles), but ACSF plus low-fat milk significantly inhibits the NMDA component of the EPSP (closed triangles). The bar over the graph indicates when ACSF plus milk was applied. The inhibition approaches maximum after about 5 min of application, and is reversible within about 20 min of washout with ACSF (open squares). The gray box over the graph applies only to the open squares, indicating when the ACSF plus low-fat milk was transiently applied.
To more specifically examine whether a pure, homogeneous triglyceride would impair LTP maintenance, we applied 1 mg/ml of the triglyceride triolein after acquiring baseline EPSPs, applied TBS, and recorded EPSPs in the presence of triolein for another 60 min. Only one of 11 slices from four mice exhibited LTP 60 min after LTP induction. In seven of 11 slices, EPSP potentiation was observed after TBS, but the EPSP slopes returned to baseline within 60 min. In three of 11 slices, TBS failed to induce any EPSP potentiation. Analysis of the cumulative data from all 11 slices resulted in EPSP slope potentiation of 106 ± 13% 60 min after TBS (Fig. 2C, n = 11 slices, four mice).
ACSF containing skim milk, low-fat milk, or triolein did not affect the baseline EPSP slope when compared with the baseline EPSP slope in ACSF alone (Fig. 2D). Sixty minutes after application of ACSF plus milk or triolein, the EPSP slopes were 93 ± 9.4% in ACSF with skim milk (open triangles, n = 6 slices, four mice), 98 ± 8.5% in ACSF with low-fat milk (closed triangles, n = 6 slices, four mice), and 102.3 ± 10.1% in ACSF with triolein (open circles, n = 4 slices, two mice) when compared with the baseline EPSP slope in ACSF alone. Because LTP at Shaffer collateral synapses require NMDA receptor activation, we examined the effect of skim milk and low-fat milk on the NMDA component of the EPSP. To selectively evaluate the NMDA component of the EPSP, ACSF was modified by removal of magnesium, addition of 10 μm glycine and 10 μm 1,2,3,4-tetrahydro-6-nitro-2,3-dioxobenzo[f]quinoxaline-7-sulfonamide (NBQX) [a selective α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor antagonist]. After obtaining baseline NMDA-mediated EPSPs, the modified ACSF containing skim milk or low-fat milk was applied to the slices while continuing to record EPSPs. ACSF with skim milk had no effect on the NMDA component of the EPSP (Fig. 2E, solid trace, 2F open triangles, n = 5 slices, four mice), but after 30 min of perfusion with ACSF containing low-fat milk, the NMDA component of the EPSP was inhibited by 46 ± 4.5% (Fig. 2E, dotted trace and arrow, 2F closed triangles, n = 5 slices, four mice). The inhibition approached a stable maximal level after 5–10 min. When the ACSF containing low-fat milk was washed out by ACSF after 5 min, the NMDA component of the EPSP recovered to control baseline levels in approximately 20 min of washout (Fig. 2F, open squares, n = 5 slices one mouse).
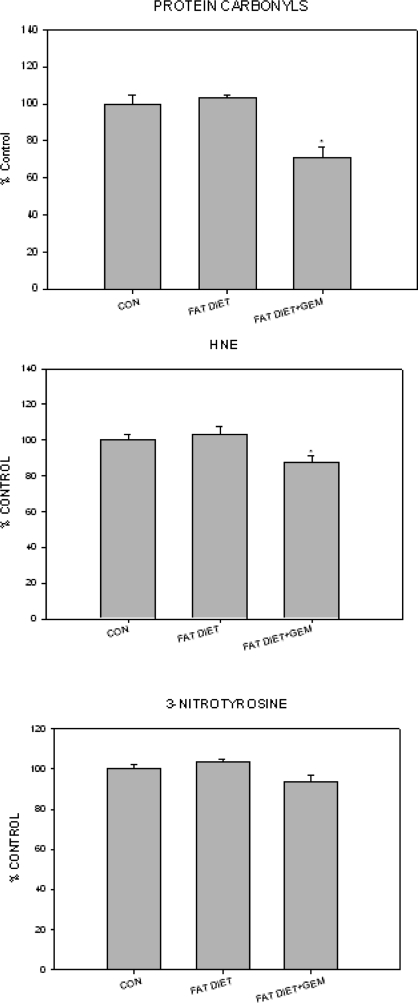
Parameters of oxidative stress are elevated in animal models of memory impairment and Alzheimer’s disease (14,15,16,17). To determine whether oxidative stress was altered in our model, we measured protein carbonyls and 3-nitrotyrosine (indices of protein oxidation) and HNE (an index of lipid peroxidation) levels in the brains of control, obese, and gemfibrozil-treated obese mice (Fig. 3, A–C). Whereas there were no statistically significant differences in the levels of these indices of oxidative stress between the mice on the 5 and 10% fat diet, there was a significant decline in protein carbonyl and HNE levels in the mice treated with gemfibrozil.
Figure 3.
Comparison of the effect of a high-fat diet, compared with normal diet (CON) and high-fat diet together with gemfibrozil (GEM) on parameters of oxidative stress in the brain. *, P < 0.05.
Discussion
The studies reported here show that triglycerides are likely a major cause of the cognitive disturbances in diet-induced obesity. Our results show that both hippocampal-dependent memory and nonhippocampal-dependent memory are impaired in diet-induced obesity. In addition, they show that triglycerides impair maintenance of NMDA-dependent hippocampal long-term synaptic potentiation. Hippocampal long-term potentiation is considered an assessment of synapse specific plasticity and a neurophysiological correlate of learning and memory (13). Lowering triglycerides pharmacologically with gemfibrozil reduced levels of protein carbonyls, an index of protein oxidation, and HNE levels, an index of membrane lipid oxidation, in the diet-induced obese mice. Together, these findings suggest that if oxidative stress plays a role in the learning and memory problems associated with obesity, lowering triglycerides may help reverse the cognitive impairments.
We found that obese mice performed more poorly than normal mice in three tests that inventory a variety of cognitive paradigms: hippocampal reference learning and memory tasks (water maze and T-maze), nonhippocampal-dependent response memory (lever press), episodic (T-maze and water maze) procedural (lever press), reward (lever press) aversion avoidance (T-maze). Together these results suggest that obesity affects several aspects of cognition.
Previously case reports in humans have suggested that hypertriglyceridemia may lead to delirium (18,19). In persons with type 2 diabetes mellitus, elevated triglycerides have been associated with poor cognitive performance (20). In a small study of humans, reducing hypertriglyceridemia with gemfibrozil improved cerebral blood flow and function on the cognitive capacity screening examination (6). Although gemfibrozil might act through pathways independent of its effects on triglycerides, these findings further support our hypothesis that triglycerides play a role in modulating cognitive performance.
Failure to maintain LTP in the presence of milk fat triglycerides is consistent with impaired learning and memory observed in CD-1 mice on high-fat diets or treated with triglyceride-containing emulsions. The results suggest that higher-than-normal levels of circulating triglycerides could impair hippocampal synaptic plasticity, resulting in impaired hippocampal-dependent learning and memory. Our studies suggest that milk fat could inhibit LTP induction by blocking NMDA receptor activation (Fig. 2, E and F). This effect occurs without effect on the AMPA-mediated component of the EPSP (Fig. 2, A and D) and is not detectable unless ACSF is modified to isolate the NMDA-mediated component of the EPSP (Fig. 2, E and F). During TBS, reduced NMDA receptor activation would reduce calcium entry and would impair LTP induction. However, it is interesting that there is post-TBS potentiation in the presence of low-fat milk, indicating that there is sufficient presynaptic calcium entry and no postsynaptic AMPA receptor inhibition, resulting in post-TBS synaptic potentiation. Moreover, we assume that in ACSF TBS induces normal NMDA receptor activation, postsynaptic calcium entry, and probably early events triggered by post-TBS calcium entry (Fig. 2B). Therefore, the fact that application of ACSF with low-fat milk 10 min after TBS prevents LTP maintenance suggests that milk fat may also exert a second, independent effect on LTP maintenance (Fig. 2B).
Triolein had similar effects, compared with those produced by milk fat (Fig. 2C). The baseline EPSP was not affected by triolein, and EPSP potentiation was elicited but not maintained in the presence of triolein. The triolein experiment also supports that LTP can be induced but not maintained in the presence of triglycerides. This is consistent with other findings that free fatty acids or their derivatives can affect early-phase LTP and glutamate release (21,22). Future studies will be necessary to definitively establish whether triglycerides inhibit LTP by NMDA receptor inhibition alone or in addition to a downstream effect upon LTP maintenance.
The mechanism(s) by which high triglyceride levels impair memory remain unclear, but our results suggest interesting possibilities. Acyl-CoA/free fatty acids (22) and linoleic derivatives (21) are capable of modifying glutamate release. Our work would suggest that triglycerides also affect the NMDA calcium channel. Oxidative stress may play a role as obesity itself is a proinflammatory, oxidatively stressed state. The work of Knapp and Klann (23,24,25) has suggested that increased reactive oxygen species (ROS) species can alter NMDA-dependent calcium influx, thus altering LTP. Superoxide acts as a signal in LTP at CA1 synapses in the hippocampus. The work of Knapp and Klann (24,25) in particular argues that low, long-term oxidative stress is the cause of decreased LTP in aging and that antioxidants can reverse the impaired LTP response. Activation of the NMDA channel can lead to increased ROS species (26) and release nitric oxide (27). Finally, nitric oxide and ROS can act synergistically to increase oxidative stress and damage (28). Therefore, obesity-induced oxidative stress could disrupt superoxide signaling in hippocampal neurons, with the resultant synaptic dysfunction accounting for impaired cognitive function. However, parameters of oxidative stress were decreased only after gemfibrozil treatment but not in the hypertriglyceridemic animals at baseline. Alternatively, we have shown that hypertriglyceridemia impairs leptin’s ability to cross the blood-brain barrier (8). Leptin enhances cognition (29,30), and leptin receptor-deficient animals have impaired hippocampal LTP and poor spatial memory (31,32). Thus, triglycerides could impair cognition by preventing leptin from reaching the brain regions important for learning and memory. Triglycerides may also affect cognition through their ability to modify release of feeding peptides (33), many of which affect cognition through nitric oxide-dependent pathways (34,35,36,37).
To conclude, we showed here that obesity in animal models is associated with cognitive impairments. Such impairments are mediated by triglycerides, which impair NMDA-mediated LTP. In view of the epidemic of obesity that is occurring in the developed world, we believe that these findings represent another reason for public health measures to focus on reducing obesity.
Footnotes
This work was supported by National Institute of Neurological Disorders and Stroke Grants 42774 and 051334 and Juvenile Diabetes Research Foundation Grant 1-2004-594.
Disclosure Statement: The authors have nothing to disclose. J.E.M. and D.A.B. conceived the studies and were responsible for their oversight and for writing the manuscript. S.A.F. undertook the memory studies and played a role in writing the manuscript. K.A.Y. and L.X. were responsible for the long-term potentiation studies and writing the manuscript. D.A.B. and H.M.A. were responsible for the studies on oxidative stress metabolism and contributed to writing the manuscript. N.E.M. conducted the memory studies. W.A.B. aided in the design and writing of the manuscript.
First Published Online February 14, 2008
Abbreviations: ACSF, Artificial cerebrospinal fluid; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid; EPSP, excitatory postsynaptic potential; HNE, 4-hydroxynonenal; ICV, intracerebroventricular; LTP, long-term potentiation; NMDA, N-methyl-d-aspartate; 3NT, 3-nitrotyrosine; ROS, reactive oxygen species; TBS, θ burst stimulation.
References
- Jeong SK, Nam HS, Son MH, Son EJ, Cho KH 2005 Interactive effect of obesity indexes on cognition. Dement Geriatr Cogn Disord 19:91–96 [DOI] [PubMed] [Google Scholar]
- Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB 2003 Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obes Relat Metab Disord 27:260–268 [DOI] [PubMed] [Google Scholar]
- Sorensen TI, Sonne-Holm S, Christensen U 1983 Cognitive deficiency in obesity independent of social origin. Lancet 14:1105–1106 [DOI] [PubMed] [Google Scholar]
- Morley JE 2004 The metabolic syndrome and aging. J Gerontol Med Sci 59A:139–142 [DOI] [PubMed] [Google Scholar]
- Reitz C, Luchsinger J, Tang MX, Manly J, Mayeux R 2005 Impact of plasma lipids and time on memory performance in healthy elderly without dementia. Neurology 64:1378–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RL, Meyer JS, McClintic K, Mortel KF 1989 Reducing hypertriglyceridemia in elderly patients with cerebrovascular disease stabilizes or improves cognition and cerebral perfusion. Angiology 40:260–269 [PubMed] [Google Scholar]
- Atmon g, Gabriely I, Greiner W, Davidson D, Schechter C, Barzilai N 2002 Plasma HDL levels highly correlate with cognitive function in exceptional longevity. J Gerontol Med Sci 57:M712–M715 [DOI] [PubMed] [Google Scholar]
- Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, Morley JE 2004 Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes 53:1253–1260 [DOI] [PubMed] [Google Scholar]
- Farr SA, Flood JF, Morley JE 2000 The effect of cholinergic, GABAergic, serotonergic and glutamatergic receptor modulation on post-trial memory processing in the hippocampus. Neurobiol Learn Mem 73:150–167 [DOI] [PubMed] [Google Scholar]
- Farr SA, Banks WA, La Scola ME, Flood JF, Morley JE 2000 Permanent and temporary inactivation of the hippocampus impairs T-maze footshock avoidance acquisition and retention. Brain Res 872:242–249 [DOI] [PubMed] [Google Scholar]
- Thio LL, Wong M, Yamada DA 2000 Ketone bodies do not directly alter excitatory or inhibitory hippocampal synaptic transmission. Neurology 54:325–331 [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA 1999 Long-term potentiation-a decade of progress? Science 285:1870–1874 [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MG 2004 LTP and LTD: an embarrassment of riches. Neuron 44:5–21 [DOI] [PubMed] [Google Scholar]
- Poon HF, Castegna A, Farr SA, Thongboonkerd V, Lynn BC, Banks WA, Morley JE, Klein JB, Butterfield DA 2004 Quantitative proteomics analysis of specific protein expression and oxidative modification in aged senescence-accelerated-prone 8 mice brain. Neuroscience 126:915–926 [DOI] [PubMed] [Google Scholar]
- Poon HF, Farr SA, Banks WA, Pierce WM, Klein JB, Morley JE, Butterfield DA 2005 Proteomic identification of less oxidized brain proteins in aged senescence-accelerated mice following administration of antisense oligonucleotide directed at the Aβ region of amyloid precursor protein. Mol Brain Res 138:8–13 [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Poon HF, St. Clair D, Kell JN, Pierce WM, Klein JB, Markesbery WR 2006 Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: insights into the development of Alzheimer’s disease. Neurobiol Dis 22:223–232 [DOI] [PubMed] [Google Scholar]
- Aslan M, Oxben T 2004 Reactive oxygen and nitrogen species in Alzheimer’s disease. Curr Alzheimer Res 1:111–119 [DOI] [PubMed] [Google Scholar]
- Heilman KM, Fisher WR 1974 Hyperlipidemic dementia. Arch Neurol 31:67–68 [DOI] [PubMed] [Google Scholar]
- Mathew NT, Meyer JS, Achari AN, Dodson RF 1976 Hyperlipidemia neuropathy and dementia. Eur Neurol 14:370–382 [DOI] [PubMed] [Google Scholar]
- Perlmuter LC, Nathan DM, Goldfinger SH, Russo PA, Yates J, Larkin M 1998 Triglyceride levels affect cognitive function in non-insulin-dependent diabetes. J Diabetes Complications 2:210–213 [DOI] [PubMed] [Google Scholar]
- Tanaka A, Nishizaki T 2003 The newly synthesized linoleic acid derivative FR236924 induces a long-lasting facilitation of hippocampal neurotransmission by targeting nicotinic acetylcholine receptors. Bioorg Med Chem Lett 13:1037–1040 [DOI] [PubMed] [Google Scholar]
- Shang Q, Yoshida S, Sakai K, Liu J, Fukunaga K 200 Changes of free fatty acids and acyl-CoAs in rat brain hippocampal slice with tetraethylammonium-induced long-term potentiation. Biochem Biophys Res Commun 267:208–212 [DOI] [PubMed] [Google Scholar]
- Klann E 1998 Cell-permeable scavengers of superoxide prevent long-term potentiation in hippocampal area CA1. J Neurophysiol 80:452–457 [DOI] [PubMed] [Google Scholar]
- Knapp LT, Klann E 2002 Potentiation of hippocampal synaptic transmission by superoxide requires the oxidative activation of protein kinase C. J Neurosci 22:674–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp LT, Klann E 2002 Role of reactive oxygen species in hippocampal long-term potentiation: contributory or inhibitory? J Neurosci Res 70:1–7 [DOI] [PubMed] [Google Scholar]
- Sanganahalli BG, Joshi PG, Joshi NB 2006 NMDA and non-NMDA receptors stimulation causes differential oxidative stress in rat cortical slices. Neurochem Int 49:475–480 [DOI] [PubMed] [Google Scholar]
- Arundine M, Aarts M, Lau A, Tymianski M 2004 Vulnerability of central neurons to secondary insults after in vitro mechanical stretch. J Neurosci 24:8106–8123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldyrec AA 2005 Homocysteinic acid causes oxidative stress in lymphocytes by potentiating toxic effect of NMDA. Bull Exp Biol Med 140:33–37 [DOI] [PubMed] [Google Scholar]
- Farr SA, Banks WA, Morley JE 2006 Effects of leptin on memory processing. Peptides 27:1420–1425 [DOI] [PubMed] [Google Scholar]
- Figlewicz DP 2004 Intraventricular insulin and leptin reverse place preference conditioned with high-fat diet in rats. Behav Neurosci 118:479–487 [DOI] [PubMed] [Google Scholar]
- Li XL 2002 Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience 113:607–615 [DOI] [PubMed] [Google Scholar]
- Wayner MJ, Armstrong DI, Phelix CF, Oomura Y 2004 Orexin-A (hypocretin-1) and leptin enhance LTP in the dentate gyrus of rats in vivo. Peptides 25:991–996 [DOI] [PubMed] [Google Scholar]
- Chang G-Q, Karatayev O, Daydova Z, Leibowitz SF 2006 Circulating triglycerides impact on orexigenic peptides and neuronal activity in hypothalamus. Endocrinology 145:3904–3912 [DOI] [PubMed] [Google Scholar]
- Diano S, Farr SA, Benoit SE, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschop MH, Horvath TL 2006 Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci 9:381–388 [DOI] [PubMed] [Google Scholar]
- Gaskin FS, Farr SA, Banks WA, Kumar VB, Morley JE 2003 Ghrelin-induced feeding is dependent on nitric oxide. Peptides 24:913–918 [DOI] [PubMed] [Google Scholar]
- Jaeger LB, Farr SA, Banks WA, Morley JE 2002 Effects of orexin-A on memory processing. Peptides 23:1683–1688 [DOI] [PubMed] [Google Scholar]
- Farr SA, Banks WA, Kumar VB, Morley JE 2005 Orexin-1-induced feeding is dependent on nitric oxide. Peptides 26:759–765 [DOI] [PubMed] [Google Scholar]