Abstract
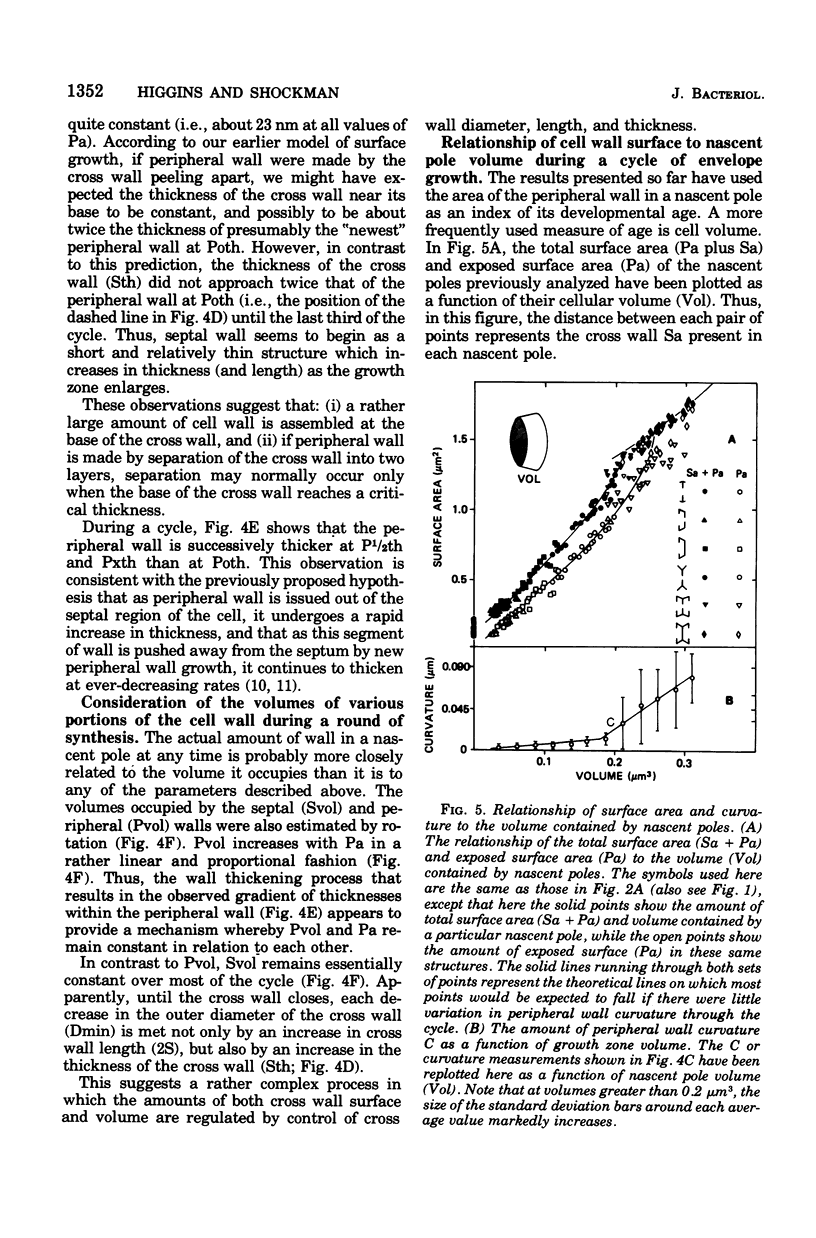
A new ultrastructural method was used to study rounds of envelope synthesis that occur in Streptococcus faecalis in "growth zones" found between pairs of naturally occurring surface markers. The technique consists of producing three-dimensional reconstructions of these growth zones from the mathematical rotation, about a central axis, of measurements taken from central, longitudinal thin sections of cells. A cycle of exponential-phase envelope growth was then simulated by arranging a series of these reconstructions in increasing order of the amount of peripheral wall surface area or the amount of cell volume that each was calculated to contain. Using this simulated cycle of growth, the geometry of a single growth zone during a round of synthesis was studied. Based on this analysis, a model was developed for the assembly of the cell wall of S. faecalis. The model states that new cell wall surface is synthesized by the regulated flow of essentially two channels of cell wall precursors into a single growth zone. One channel of precursors would be involved in the assembly of a bilayered cross wall that would proceed at a fairly constant rate until the cross wall closes. The second channel of precursors would be involved in the separation of the bilayered cross wall into two segments of peripheral wall. These precursors would intercalate into and thicken the separating layers of the cross wall. The flow of precursors through this channel would be progressively reduced through a cycle. These decreases, when coupled with internal hydrostatic pressure, apparently would result in the enlarging peripheral wall becoming increasingly more curved and would also promote cell division by reducing the total amount of cell wall that must be assembled in order for septation to occur.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briles E. B., Tomasz A. Radioautographic evidence for equatorial wall growth in a gram-positive bacterium. Segregation of choline-3H-labeled teichoic acid. J Cell Biol. 1970 Dec;47(3):786–790. doi: 10.1083/jcb.47.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLE R. M., HAHN J. J. Cell wall replication in Streptococcus pyogenes. Science. 1962 Mar 2;135(3505):722–724. doi: 10.1126/science.135.3505.722. [DOI] [PubMed] [Google Scholar]
- Daneo-Moore L., Coyette J., Sayare M., Boothby D., Shockman G. D. Turnover of the cell wall peptidoglycan of Lactobacillus acidophilus. The presence of a fraction immune to turnover. J Biol Chem. 1975 Feb 25;250(4):1348–1353. [PubMed] [Google Scholar]
- Dezélée P., Shockman G. D. Studies of the formation of peptide cross-links in the cell wall peptidoglycan of Streptococcus faecalis. J Biol Chem. 1975 Sep 10;250(17):6806–6816. [PubMed] [Google Scholar]
- Fordham W. D., Gilvarg C. Kinetics of cross-linking of peptidoglycan in Bacillus megaterium. J Biol Chem. 1974 Apr 25;249(8):2478–2482. [PubMed] [Google Scholar]
- Higgins M. L., Daneo-Moore L., Boothby D., Shockman G. D. Effect of inhibition of deoxyribonucleic acid and protein synthesis on the direction of cell wall growth in Streptococcus faecalis. J Bacteriol. 1974 May;118(2):681–692. doi: 10.1128/jb.118.2.681-692.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Pooley H. M., Shockman G. D. Reinitiation of cell wall growth after threonine starvation of Streptococcus faecalis. J Bacteriol. 1971 Mar;105(3):1175–1183. doi: 10.1128/jb.105.3.1175-1183.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Pooley H. M., Shockman G. D. Site of initiation of cellular autolysis in Streptococcus faecalis as seen by electron microscopy. J Bacteriol. 1970 Aug;103(2):504–512. doi: 10.1128/jb.103.2.504-512.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Model for cell wall growth of Streptococcus faecalis. J Bacteriol. 1970 Feb;101(2):643–648. doi: 10.1128/jb.101.2.643-648.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Procaryotic cell division with respect to wall and membranes. CRC Crit Rev Microbiol. 1971 May;1(1):29–72. doi: 10.3109/10408417109104477. [DOI] [PubMed] [Google Scholar]
- Higgins M. L. Three-dimensional reconstruction of whole cells of Streptococcus faecalis from thin sections of cells. J Bacteriol. 1976 Sep;127(3):1337–1345. doi: 10.1128/jb.127.3.1337-1345.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M., Shockman G. D., Higgins M. L., Porres-Juan J. Some properties of two autolytic-defective mutants of Streptococcus faecalis ATCC 9790. J Bacteriol. 1972 Jan;109(1):423–431. doi: 10.1128/jb.109.1.423-431.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M. Turnover and spreading of old wall during surface growth of Bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1127–1138. doi: 10.1128/jb.125.3.1127-1138.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Bacterial growth and the cell envelope. Bacteriol Rev. 1970 Jun;34(2):194–214. doi: 10.1128/br.34.2.194-214.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A., Hirota Y., Schwarz U. Process of cellular division in Escherichia coli growth pattern of E. coli murein. J Mol Biol. 1973 Jun 25;78(1):185–195. doi: 10.1016/0022-2836(73)90437-3. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Daneo-Moore L., Higgins M. L. Problems of cell wall and membrane growth, enlargement, and division. Ann N Y Acad Sci. 1974 May 10;235(0):161–197. doi: 10.1111/j.1749-6632.1974.tb43265.x. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Pooley H. M., Thompson J. S. Autolytic enzyme system of Streptococcus faecalis. 3. Localization of the autolysin at the sites of cell wall synthesis. J Bacteriol. 1967 Nov;94(5):1525–1530. doi: 10.1128/jb.94.5.1525-1530.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Hsu K. C., Gotschlich E. C. Electron microscopic studies on streptococci. I. M antigen. J Exp Med. 1969 Nov 1;130(5):1063–1091. doi: 10.1084/jem.130.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M. Studien mit fluoreszierenden Antikörpern an wachsenden Bakterien. I. Die Neubildung der Zellwand bei Diplococcus pneumoniae. Zentralbl Bakteriol Orig. 1964 Dec;195(1):87–93. [PubMed] [Google Scholar]



