Abstract
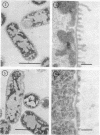
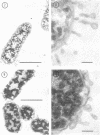
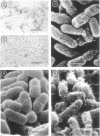
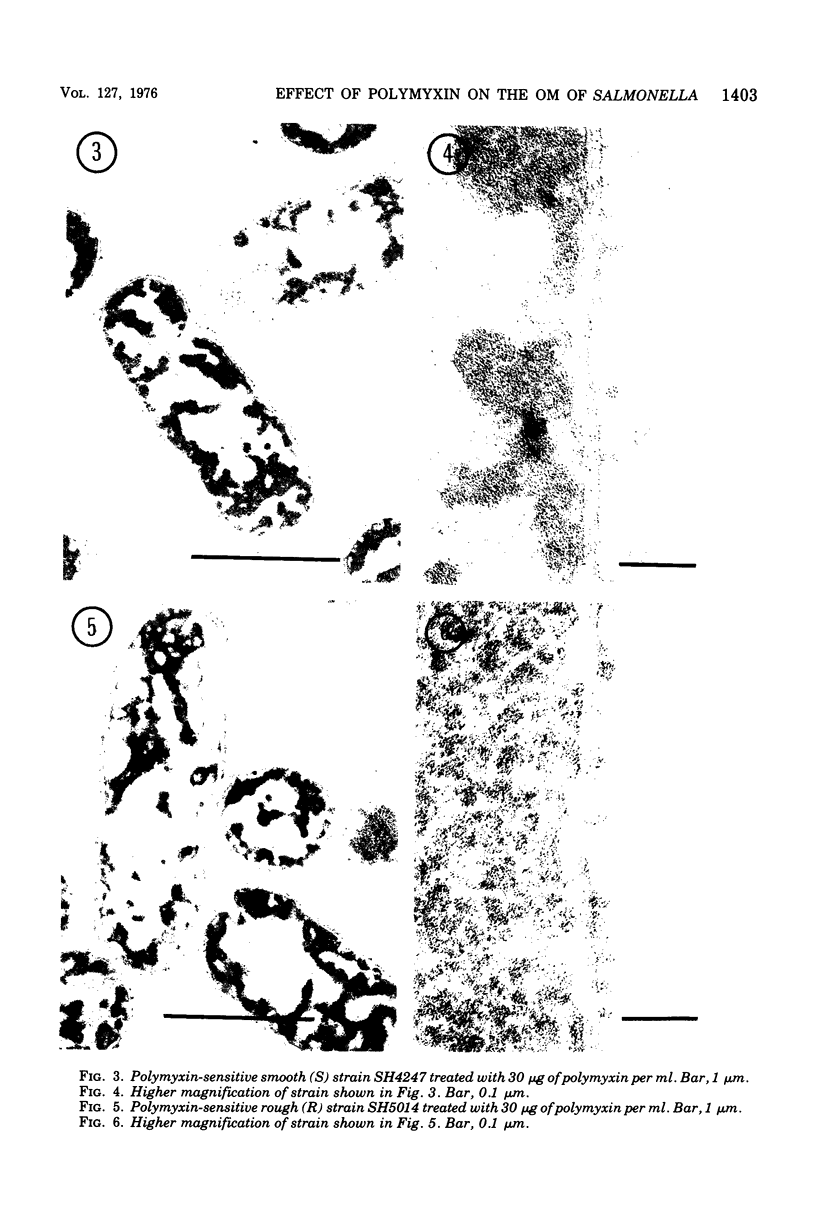
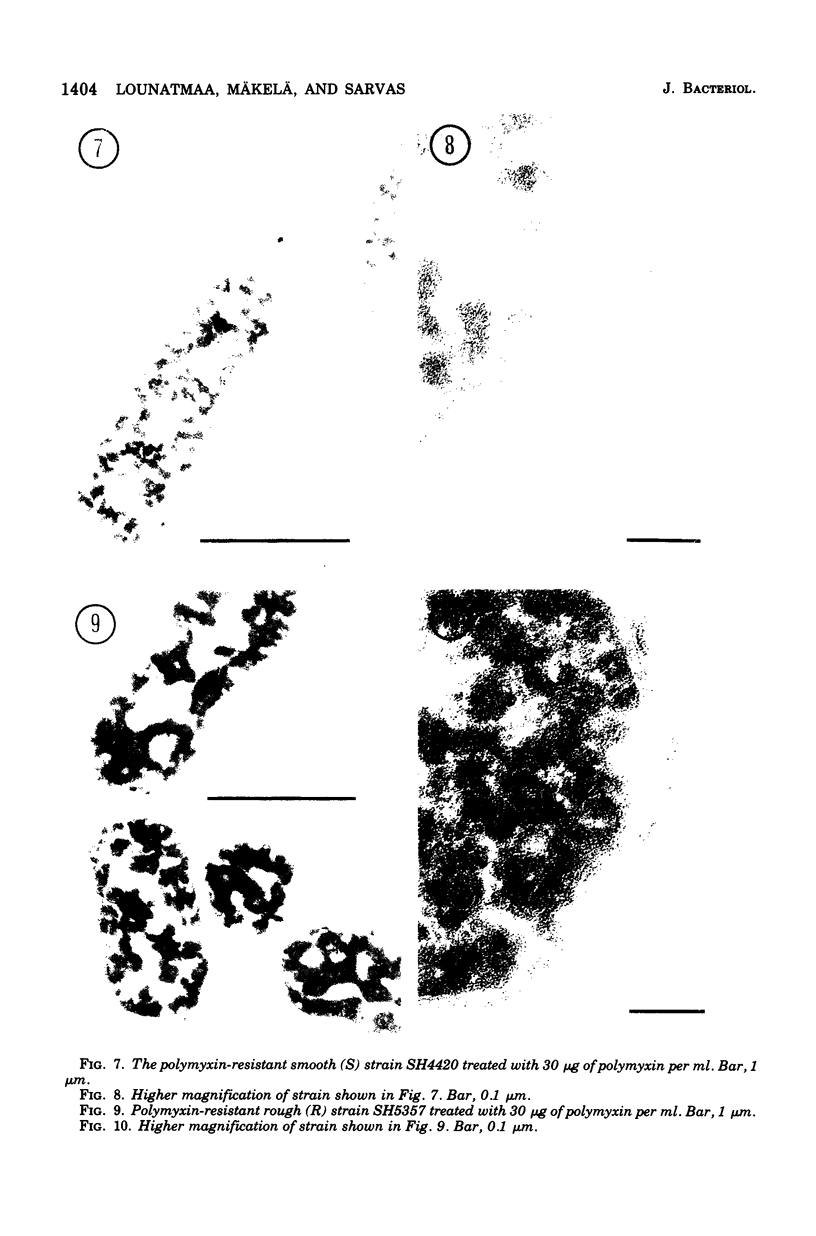
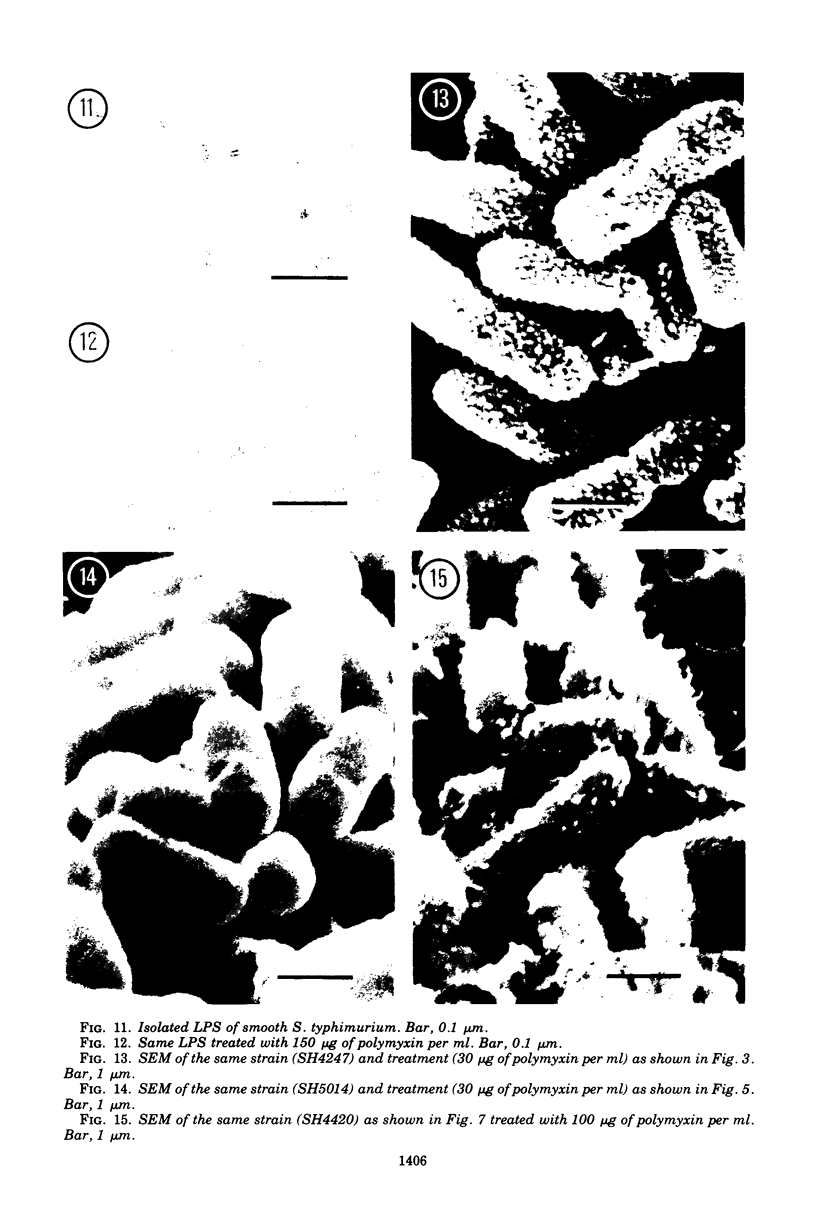
The effect of polymyxin on two sets of Salmonella mutants was studied by thin-section and scanning electron microscopy. Polymyxin (in increasing concentrations, starting just below bactericidal effect) caused the appearance of the previously described rodlike projections on the cell surface of wild-type (smooth, polymyxin-sensitive) bacteria. These projections seemed to involve the outer membrane of the cell wall. In rough mutants, which are deficient in lipopolysaccharide, the projections were much smaller and flat. Higher concentrations of polymyxin were required to produce morphological effects in polyxmin-resistant mutants of both smooth and rough forms. Furthermore, in these mutants polymyxin caused vesicle-like bulging of the total outer membrane quite different in appearance from the rodlike projections of the wild type.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader J., Teuber M. Action of polymyxin B on bacterial membranes. 1. Binding to the O-antigenic lipopolysaccharide of Salmonella typhimurium. Z Naturforsch C. 1973 Jul-Aug;28(7):422–430. [PubMed] [Google Scholar]
- Braun V., Bosch V. Repetitive sequences in the murein-lipoprotein of the cell wall of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Apr;69(4):970–974. doi: 10.1073/pnas.69.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN G. B. Cytological aspects of antimicrobial antibiosis. I. Cytological changes associated with the exposure of Escherichia coli to colistin sulfate. J Bacteriol. 1962 Jul;84:169–179. doi: 10.1002/path.1700840118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerny G., Teuber M. Differential release of periplasmic versus cytoplasmic enzymes from Escherichia coli B by polymixin B. Arch Mikrobiol. 1971;78(2):166–179. doi: 10.1007/BF00424873. [DOI] [PubMed] [Google Scholar]
- Glauert A. M. Electron microscopy of lipids and membranes. J R Microsc Soc. 1968;88(1):49–70. doi: 10.1111/j.1365-2818.1968.tb00597.x. [DOI] [PubMed] [Google Scholar]
- HsuChen C. C., Feingold D. S. The mechanism of polymyxin B action and selectivity toward biologic membranes. Biochemistry. 1973 May 22;12(11):2105–2111. doi: 10.1021/bi00735a014. [DOI] [PubMed] [Google Scholar]
- Koike M., Iida K. Effect of polymyxin on the bacteriophage receptors of the cell walls of gram-negative bacteria. J Bacteriol. 1971 Dec;108(3):1402–1411. doi: 10.1128/jb.108.3.1402-1411.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike M., Iida K., Matsuo T. Electron microscopic studies on mode of action of polymyxin. J Bacteriol. 1969 Jan;97(1):448–452. doi: 10.1128/jb.97.1.448-452.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes J., Gottfried S., Rothfield L. Leakage of periplasmic enzymes by mutants of Escherichia coli and Salmonella typhimurium: isolation of "periplasmic leaky" mutants. J Bacteriol. 1972 Feb;109(2):520–525. doi: 10.1128/jb.109.2.520-525.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes J., Inniss W. E. Electron microscopy of effect of polymyxin on Escherichia coli lipopolysaccharide. J Bacteriol. 1969 Nov;100(2):1128–1129. doi: 10.1128/jb.100.2.1128-1130.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlradt P. F., Golecki J. R. Asymmetrical distribution and artifactual reorientation of lipopolysaccharide in the outer membrane bilayer of Salmonella typhimurium. Eur J Biochem. 1975 Feb 21;51(2):343–352. doi: 10.1111/j.1432-1033.1975.tb03934.x. [DOI] [PubMed] [Google Scholar]
- NEWTON B. A. The release of soluble constituents from washed cells of Pseudomonas aeruginosa by the action of polymyxin. J Gen Microbiol. 1953 Aug;9(1):54–64. doi: 10.1099/00221287-9-1-54. [DOI] [PubMed] [Google Scholar]
- Shands J. W., Jr, Graham J. A., Nath K. The morphologic structure of isolated bacterial lipopolysaccharide. J Mol Biol. 1967 Apr 14;25(1):15–21. doi: 10.1016/0022-2836(67)90275-6. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Singer S. J. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4457–4461. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganuma A., Hara K., Kishida T., Nakajima K., Kawamata J. Cytological changes of Escherichia coli caused by polymyxin E. Biken J. 1968 Jun;11(2):149–155. [PubMed] [Google Scholar]
- Wahn K., Lutsch G., Rockstroh T., Zapf K. Morphological and physiological investigations on the action of polymyxin B on Escherichia coli. Arch Mikrobiol. 1968;63(2):103–116. doi: 10.1007/BF00412165. [DOI] [PubMed] [Google Scholar]
- Woldringh C. L., van Iterson W. Effects of treatment with sodium dodecyl sulfate on the ultrastructure of Escherichia coli. J Bacteriol. 1972 Sep;111(3):801–813. doi: 10.1128/jb.111.3.801-813.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa R., Levinthal M., Nikaido H. Biosynthesis of cell wall lipopolysaccharide in mutants of Salmonella. V. A mutant of Salmonella typhimurium defective in the synthesis of cytidine diphosphoabequose. J Bacteriol. 1969 Oct;100(1):433–444. doi: 10.1128/jb.100.1.433-444.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]