Abstract
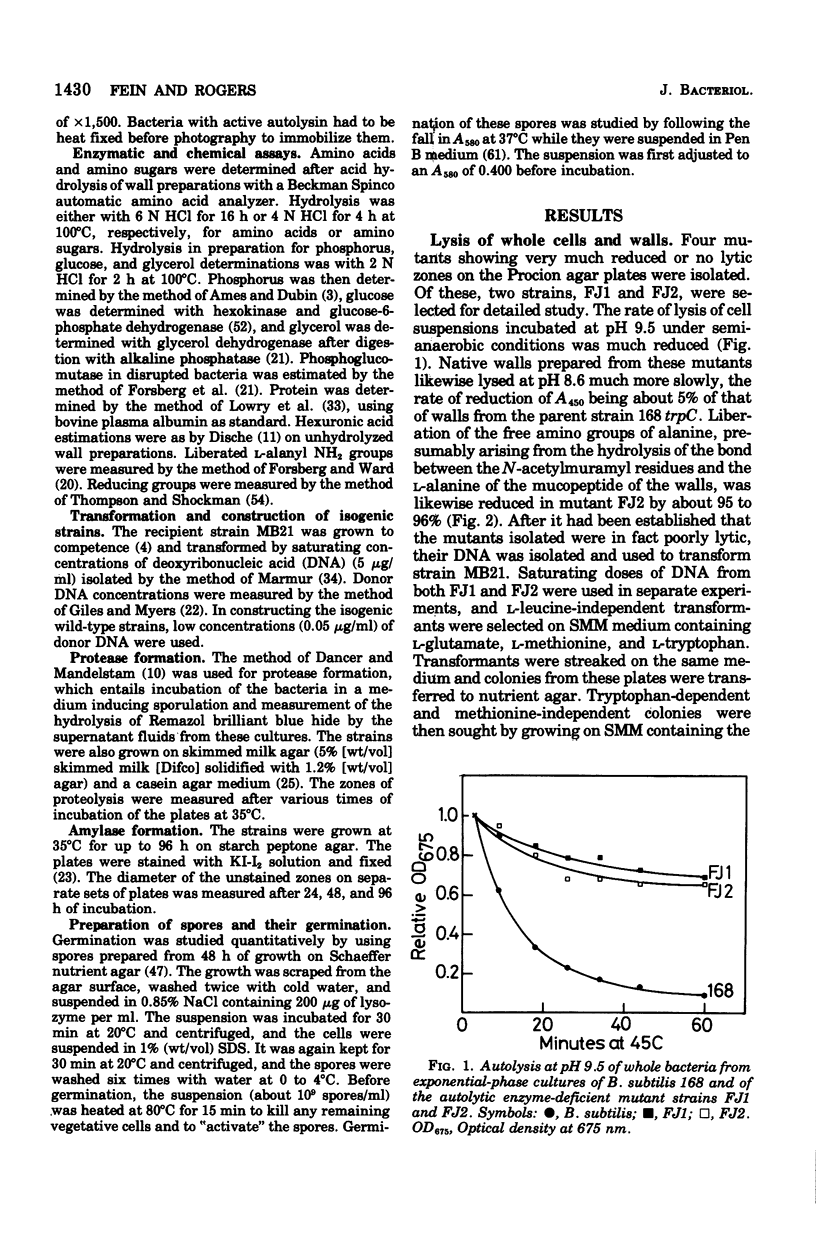
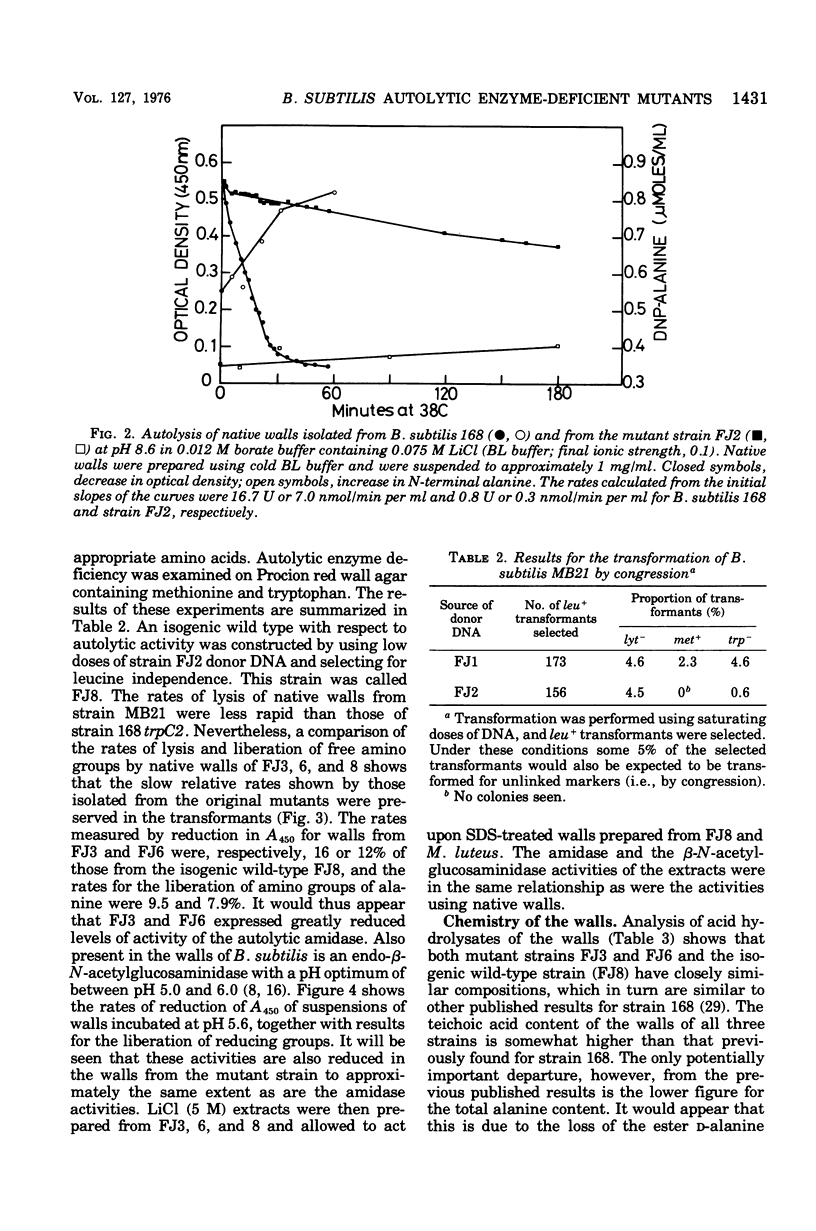
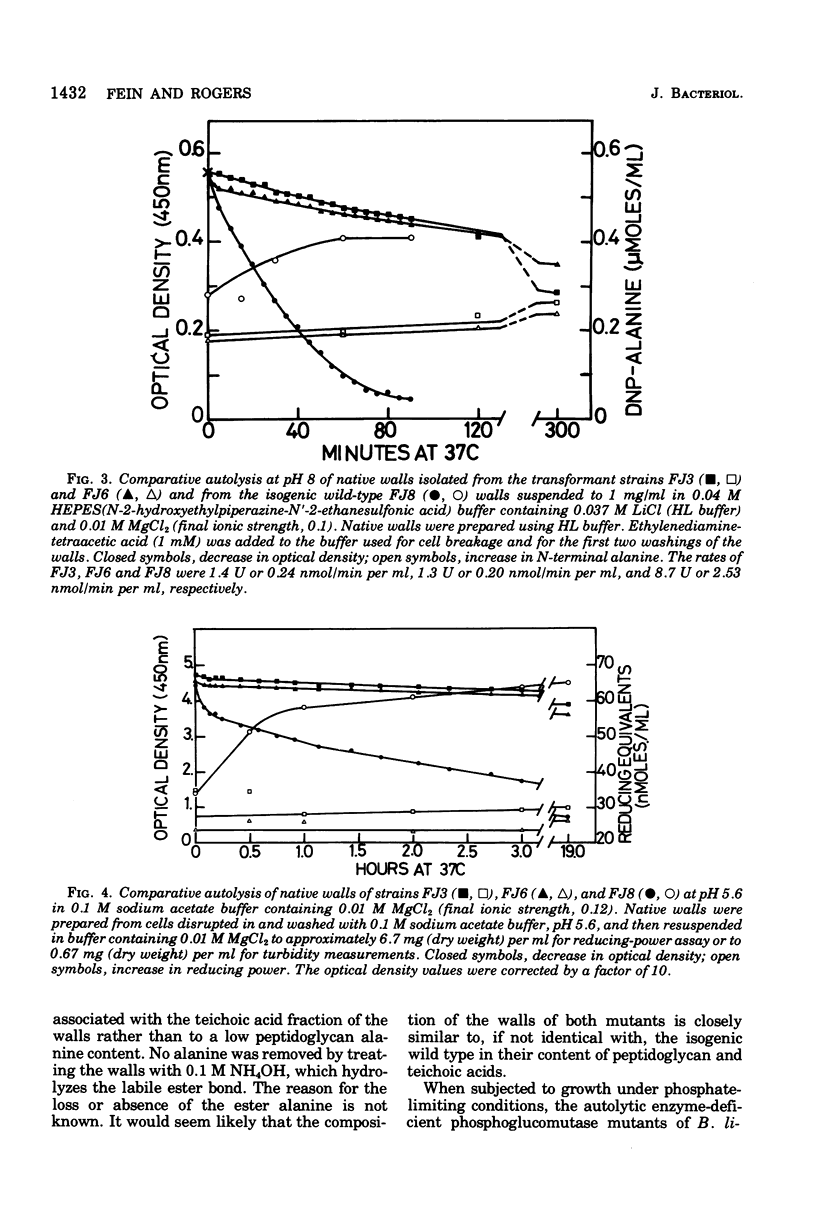
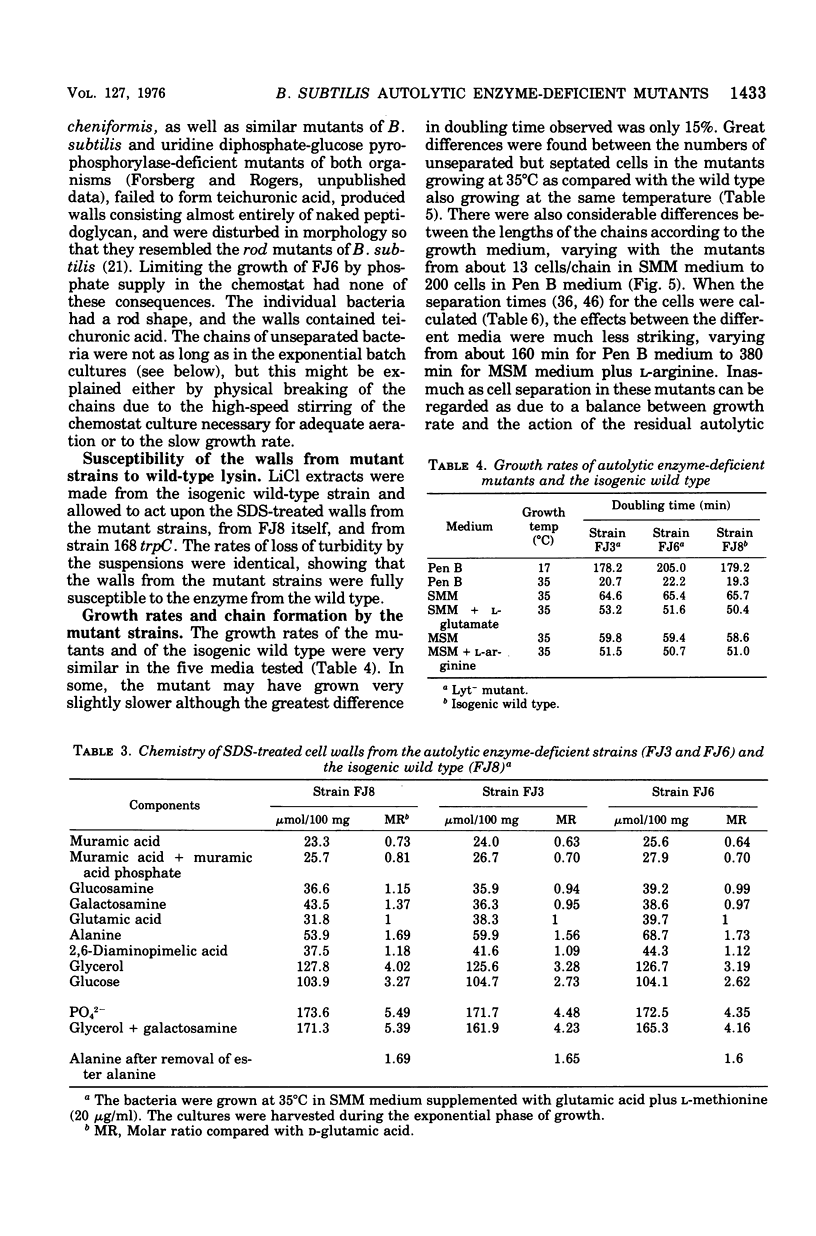
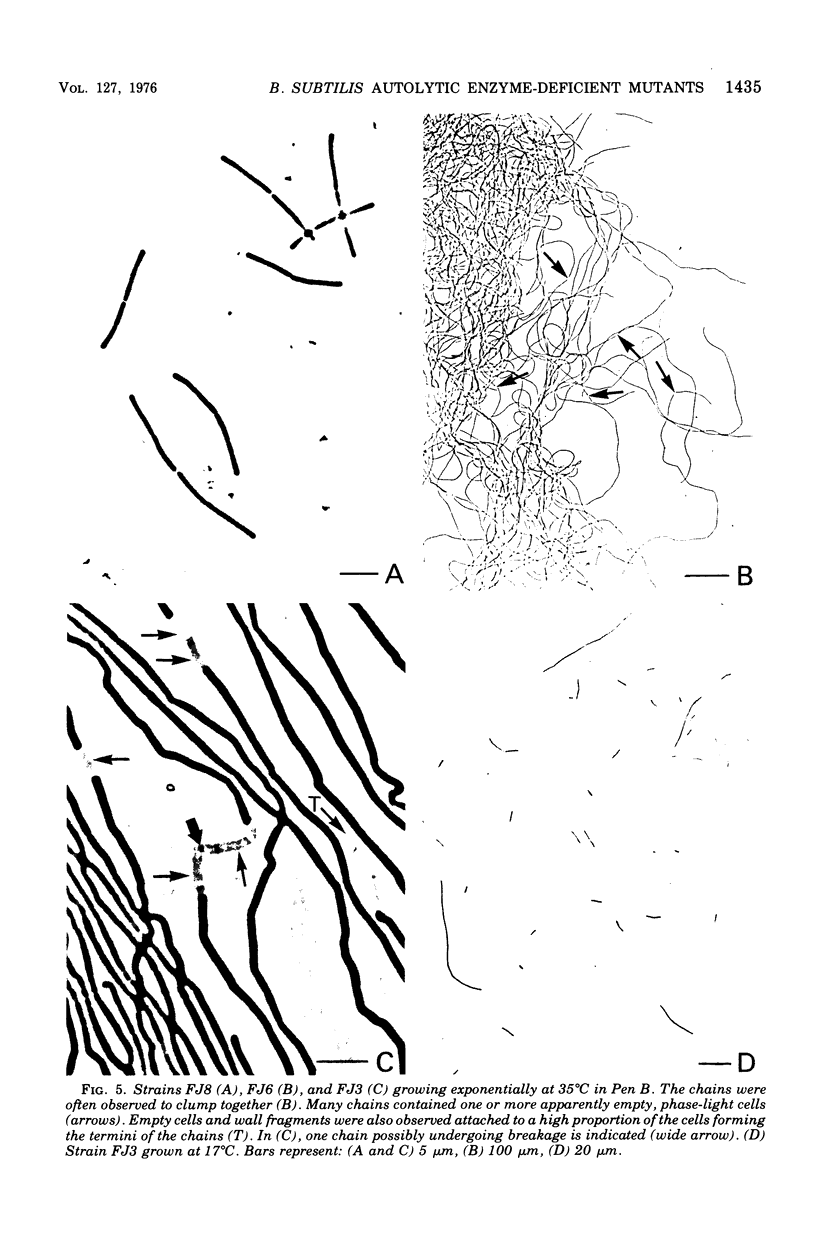
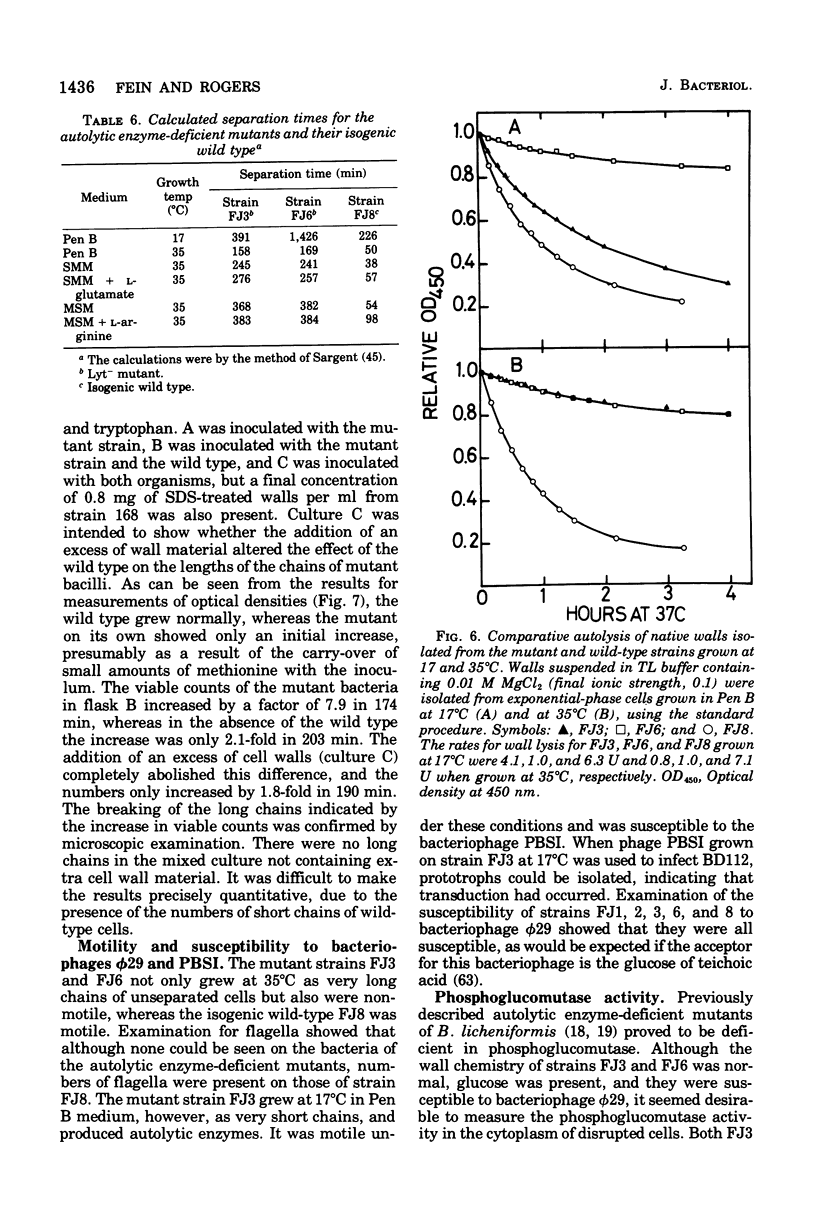
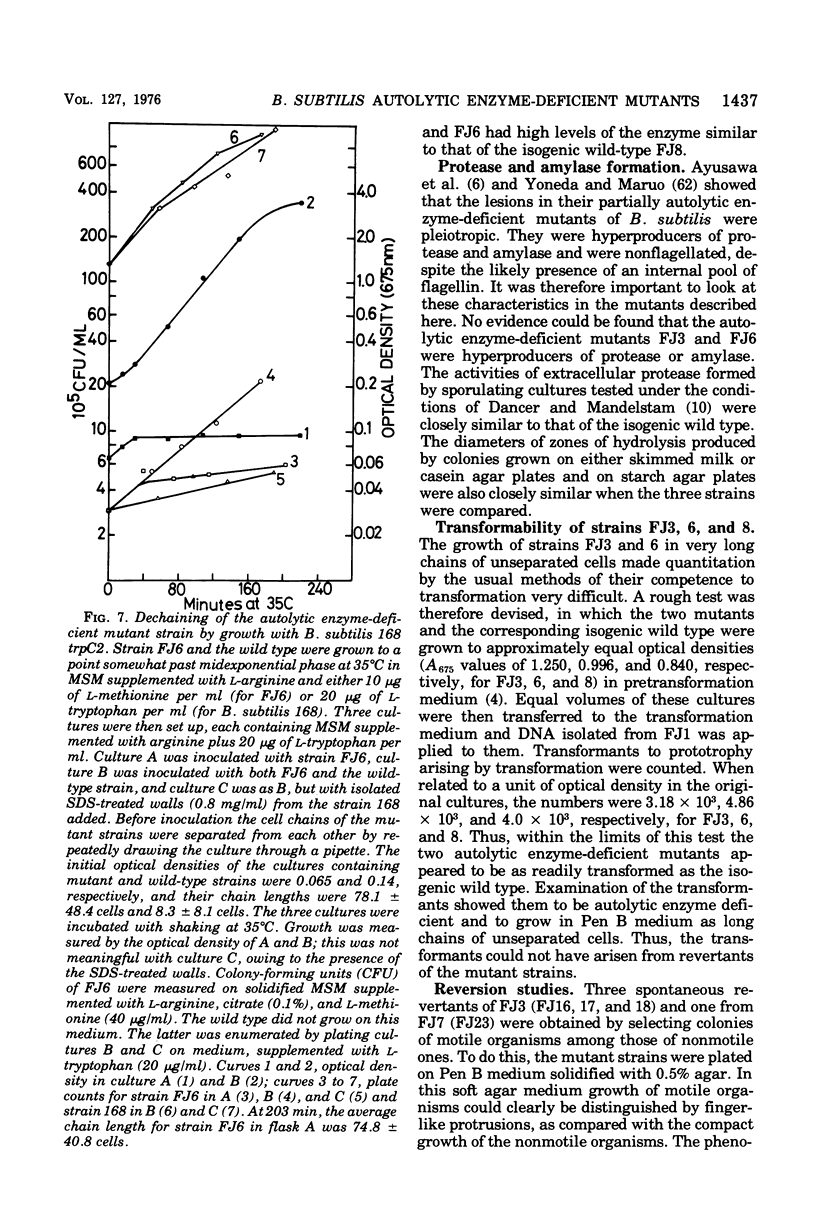
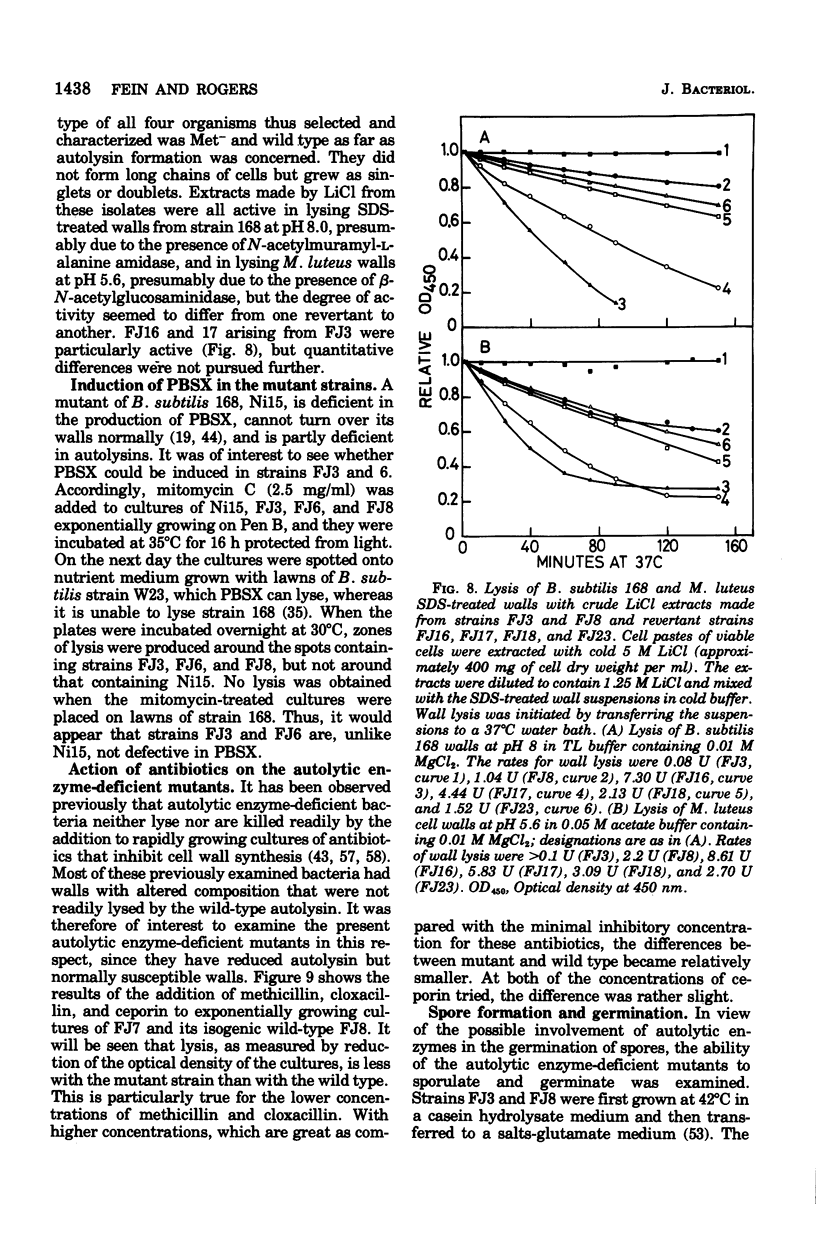
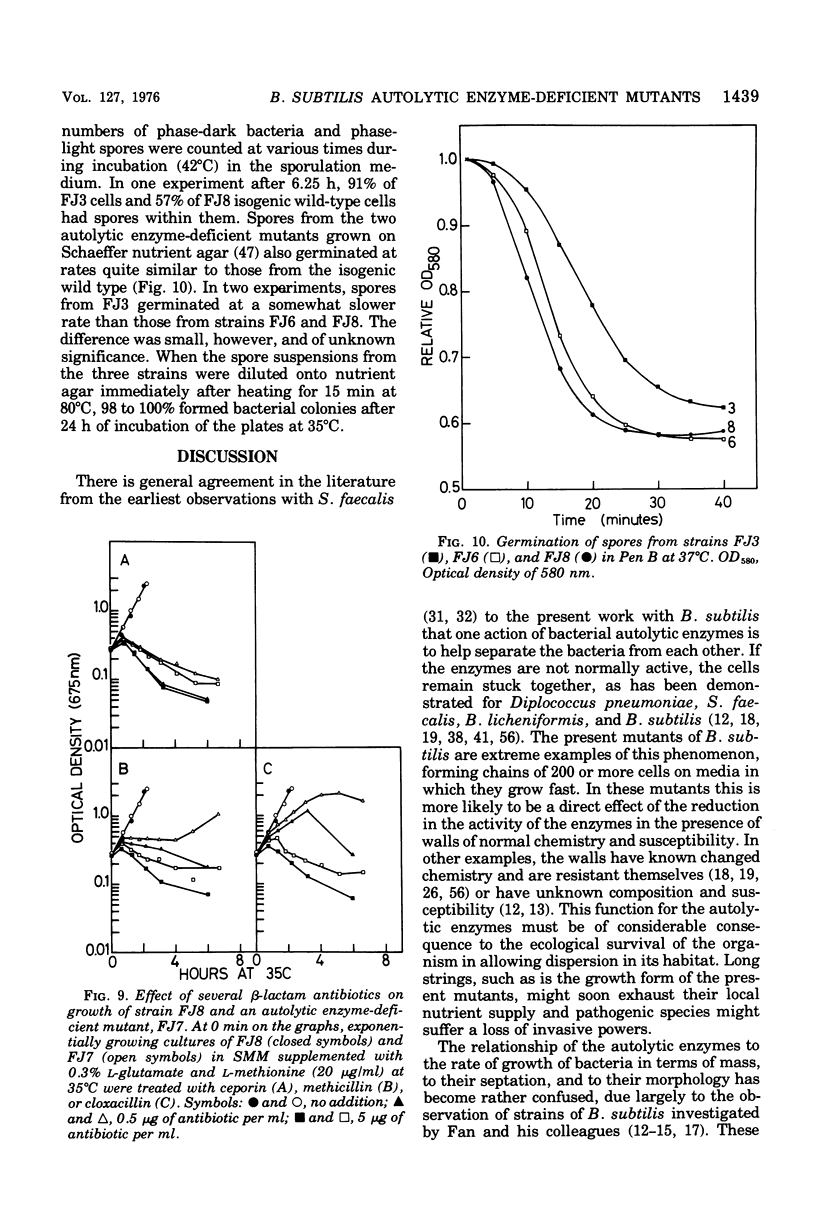
Mutants of Bacillus subtilis strain 168 have been isolated that are at least 90 to 95% deficient in the autolytic enzymes N-acetylmuramyl-L-alanine amidase and endo-beta-N-acetylglucosaminidase. These mutants grow at normal rates as very long chains of unseparated cells. The length of the chains is directly related to the growth rates. They are nonmotile and have no flagella, but otherwise appear to have normal cell morphology. Their walls are fully sysceptible to enzymes formed by the wild type and have the same chemical composition as the latter. Cell wall preparations from the mutants lyse at about 10% of the rate of those from the isogenic wild type, with the correspondingly small liberation of both the amino groups of alanine at pH 8.0 and of reducing groups at pH 5.6. Likewise, Microcococcus luteus walls at pH 5.6 and B. subtilis walls at pH 8 are lysed only very slowly by LiCl extracts made from the mutants as compared with rates obtained with wild-type extracts. Thus, the activity of both autolytic enzymes in the mutants is depressed. The frequencies of transformation, the isolation of revertants, and observations with a temperature-sensitive mutant all point to the likelihood that the pleiotropic, phenotypic properties of the strains are due to a single mutation. The mutants did not produce more protease or amylase than did the wild type. They sporulate and the spores germinate normally. The addition of antibiotics to exponentially growing cultures prevents wall synthesis but leads to less lysis than is obtained with the wild type. The bacteriophage PBSX can be induced in the mutants by treatment with mitomycin C.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Akrigg A., Ayad S. R., Barker G. R. The nature of a competence-inducing factor in Bacillus subtilis. Biochem Biophys Res Commun. 1967 Sep 27;28(6):1062–1067. doi: 10.1016/0006-291x(67)90090-3. [DOI] [PubMed] [Google Scholar]
- Akrigg A., Ayad S. R. Studies on the competence-inducing factor of Bacillus subtilis. Biochem J. 1970 Apr;117(2):397–403. doi: 10.1042/bj1170397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayad S. R., Barker G. R. The integration of donor and recipient deoxyribonucleic acid during transformation of Bacillus subtilis. Biochem J. 1969 Jun;113(1):167–174. doi: 10.1042/bj1130167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayad S. R., Shimmin E. R. Properties of the competence-inducing factor of Bacillus subtilis 168I-. Biochem Genet. 1974 Jun;11(6):455–474. doi: 10.1007/BF00486078. [DOI] [PubMed] [Google Scholar]
- Ayusawa D., Yoneda Y., Yamane K., Maruo B. Pleiotropic phenomena in autolytic enzyme(s) content, flagellation, and simultaneous hyperproduction of extracellular alpha-amylase and protease in a Bacillus subtilis mutant. J Bacteriol. 1975 Oct;124(1):459–469. doi: 10.1128/jb.124.1.459-469.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. C. Binding and release from cell walls: a unique approach to the purification of autolysins. Biochem Biophys Res Commun. 1972 Jun 9;47(5):993–996. doi: 10.1016/0006-291x(72)90930-8. [DOI] [PubMed] [Google Scholar]
- Brown W. C., Young F. E. Dynamic interactions between cell wall polymers, extracellular proteases and autolytic enzymes. Biochem Biophys Res Commun. 1970 Feb 20;38(4):564–568. doi: 10.1016/0006-291x(70)90618-2. [DOI] [PubMed] [Google Scholar]
- Cleveland R. F., Holtje J. V., Wicken A. J., Tomasz A., Daneo-Moore L., Shockman G. D. Inhibition of bacterial wall lysins by lipoteichoic acids and related compounds. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1128–1135. doi: 10.1016/0006-291x(75)90791-3. [DOI] [PubMed] [Google Scholar]
- Dancer B. N., Mandelstam J. Production and possible function of serine protease during sporulation of Bacillus subtilis. J Bacteriol. 1975 Feb;121(2):406–410. doi: 10.1128/jb.121.2.406-410.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P. Autolysin(s) of Bacillus subtilis as dechaining enzyme. J Bacteriol. 1970 Aug;103(2):494–499. doi: 10.1128/jb.103.2.494-499.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Beckman M. M., Cunningham W. P. Ultrastructural studies on a mutant of Bacillus subtilis whose growth is inhibited due to insufficient autolysin production. J Bacteriol. 1972 Mar;109(3):1247–1257. doi: 10.1128/jb.109.3.1247-1257.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Beckman M. M. Micrococcus lysodeikticus bacterial walls as a substrate specific for the autolytic glycosidase of Bacillus subtilis. J Bacteriol. 1973 May;114(2):804–813. doi: 10.1128/jb.114.2.804-813.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Beckman M. M. Mutant of Bacillus subtilis demonstrating the requirement of lysis for growth. J Bacteriol. 1971 Feb;105(2):629–636. doi: 10.1128/jb.105.2.629-636.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Beckmann M. M. Mutant of Bacillus subtilis with a temperature-sensitive autolytic amidase. J Bacteriol. 1973 May;114(2):798–803. doi: 10.1128/jb.114.2.798-803.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P. Cell wall binding properties of the Bacillus subtilis autolysin(s). J Bacteriol. 1970 Aug;103(2):488–493. doi: 10.1128/jb.103.2.488-493.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Rogers H. J. Characterization of Bacillus licheniformis 6346 mutants which have altered lytic enzyme activities. J Bacteriol. 1974 May;118(2):358–368. doi: 10.1128/jb.118.2.358-368.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Ward J. B. N-acetylmuramyl-L-alanine amidase of Bacillus licheniformis and its L-form. J Bacteriol. 1972 Jun;110(3):878–888. doi: 10.1128/jb.110.3.878-888.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Wyrick P. B., Ward J. B., Rogers H. J. Effect of phosphate limitation on the morphology and wall composition of Bacillus licheniformis and its phosphoglucomutase-deficient mutants. J Bacteriol. 1973 Feb;113(2):969–984. doi: 10.1128/jb.113.2.969-984.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C., Rogers H. J. Autolytic enzymes in growth of bacteria. Nature. 1971 Jan 22;229(5282):272–273. doi: 10.1038/229272a0. [DOI] [PubMed] [Google Scholar]
- GREEN D. M., COLARUSSO L. J. THE PHYSICAL AND GENETIC CHARACTERIZATION OF A TRANSFORMABLE ENZYME: BACILLUS SUBTILIS ALPHA-AMYLASE. Biochim Biophys Acta. 1964 Aug 26;89:277–290. doi: 10.1016/0926-6569(64)90216-0. [DOI] [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- Higerd T. B., Hoch J. A., Spizizen J. Hyperprotease-producing mutants of Bacillus subtilis. J Bacteriol. 1972 Nov;112(2):1026–1028. doi: 10.1128/jb.112.2.1026-1028.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hranueli D., Piggot P. J., Mandelstam J. Statistical estimate of the total number of operons specific for Bacillus subtilis sporulation. J Bacteriol. 1974 Sep;119(3):684–690. doi: 10.1128/jb.119.3.684-690.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. C. Autolysis of isolated cell walls of Bacillus licheniformis N.C.T.C. 6346 and Bacillus subtilis Marburg Strain 168. Separation of the products and characterization of the mucopeptide fragments. Biochem J. 1970 Oct;119(5):849–860. doi: 10.1042/bj1190849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. C., Pavlik J. G., Rogers H. J., Tanner P. J. Organization of polymers in the cell walls of some bacilli. Nature. 1968 Aug 10;219(5154):642–644. doi: 10.1038/219642a0. [DOI] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Lipoteichoic acid: a specific inhibitor of autolysin activity in Pneumococcus. Proc Natl Acad Sci U S A. 1975 May;72(5):1690–1694. doi: 10.1073/pnas.72.5.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOMINSKI I., CAMERON J., WYLLIE G. Chaining and unchaining Streptococcus faecalis; a hypothesis of the mechanism of bacterial cell separation. Nature. 1958 May 24;181(4621):1477–1477. doi: 10.1038/1811477a0. [DOI] [PubMed] [Google Scholar]
- LOMINSKI I., GRAY S. Inhibition of lysozyme by 'Suramin'. Nature. 1961 Nov 18;192:683–683. doi: 10.1038/192683a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacks S. Mutants of Diplococcus pneumoniae that lack deoxyribonucleases and other activities possibly pertinent to genetic transformation. J Bacteriol. 1970 Feb;101(2):373–383. doi: 10.1128/jb.101.2.373-383.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Mudd J. A., Mangan J., Huang W. M., Subbaiah T. V., Marmur J. Properties of the defective phage of Bacillus subtilis. J Mol Biol. 1968 Jun 28;34(3):413–428. doi: 10.1016/0022-2836(68)90169-1. [DOI] [PubMed] [Google Scholar]
- Paulton R. J. Analysis of the multiseptate potential of Bacillus subtilis. J Bacteriol. 1970 Nov;104(2):762–767. doi: 10.1128/jb.104.2.762-767.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M., Shockman G. D., Higgins M. L., Porres-Juan J. Some properties of two autolytic-defective mutants of Streptococcus faecalis ATCC 9790. J Bacteriol. 1972 Jan;109(1):423–431. doi: 10.1128/jb.109.1.423-431.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M. Turnover and spreading of old wall during surface growth of Bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1127–1138. doi: 10.1128/jb.125.3.1127-1138.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranhand J. M., Leonard C. G., Cole R. M. Autolytic activity associated with competent group H streptococci. J Bacteriol. 1971 Apr;106(1):257–268. doi: 10.1128/jb.106.1.257-268.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Bacterial growth and the cell envelope. Bacteriol Rev. 1970 Jun;34(2):194–214. doi: 10.1128/br.34.2.194-214.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Forsberg C. W. Role of autolysins in the killing of bacteria by some bactericidal antibiotics. J Bacteriol. 1971 Dec;108(3):1235–1243. doi: 10.1128/jb.108.3.1235-1243.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Pooley H. M., Thurman P. F., Taylor C. Wall and membrane growth in bacilli and their mutants. Ann Microbiol (Paris) 1974 Sep;125 B(2):135–147. [PubMed] [Google Scholar]
- Rogers H. J. The killing of bacteria by cell wall inhibitors. Contrib Microbiol Immunol. 1973;1:117–134. [PubMed] [Google Scholar]
- Sargent M. G. Control of cell length in Bacillus subtilis. J Bacteriol. 1975 Jul;123(1):7–19. doi: 10.1128/jb.123.1.7-19.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G. Synchronous cultures of Bacillus subtilis obtained by filtration with glass fiber filters. J Bacteriol. 1973 Nov;116(2):736–740. doi: 10.1128/jb.116.2.736-740.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto H., Tomasz A. Protoplast formation and leakage of intramembrane cell components: induction by the competence activator substance of pneumococci. J Bacteriol. 1975 Jan;121(1):344–353. doi: 10.1128/jb.121.1.344-353.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh M. R., Stewart-Tull D. E. Streptococcus faecalis chain disruption. J Gen Microbiol. 1975 Nov;91(1):195–197. doi: 10.1099/00221287-91-1-195. [DOI] [PubMed] [Google Scholar]
- Sharpe J. E. Disrupter for bacteriological and mammalian cells. Lab Pract. 1976 Jan;25(1):28–29. [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterlini J. M., Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969 Jun;113(1):29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. S., Shockman G. D. A modification of the Park and Johnson reducing sugar determination suitable for the assay of insoluble materials: its application to bacterial cell walls. Anal Biochem. 1968 Feb;22(2):260–268. doi: 10.1016/0003-2697(68)90315-1. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Albino A., Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970 Jul 11;227(5254):138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of Pneumococcus: chanin formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. The role of autolysins in cell death. Ann N Y Acad Sci. 1974 May 10;235(0):439–447. doi: 10.1111/j.1749-6632.1974.tb43282.x. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Westphal M. Abnormal autolytic enzyme in a pneumococus with altered teichoic acid composition. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2627–2630. doi: 10.1073/pnas.68.11.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowsdale J., Smith D. A. Isolation, characterization, and mapping of Bacillus subtilis 168 germination mutants. J Bacteriol. 1975 Jul;123(1):83–95. doi: 10.1128/jb.123.1.83-95.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda Y., Maruo B. Mutation of Bacillus subtilis causing hyperproduction of alpha-amylase and protease, and its synergistic effect. J Bacteriol. 1975 Oct;124(1):48–54. doi: 10.1128/jb.124.1.48-54.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E. Requirement of glucosylated teichoic acid for adsorption of phage in Bacillus subtilis 168. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2377–2384. doi: 10.1073/pnas.58.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]