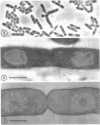
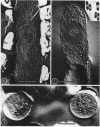
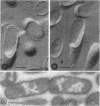
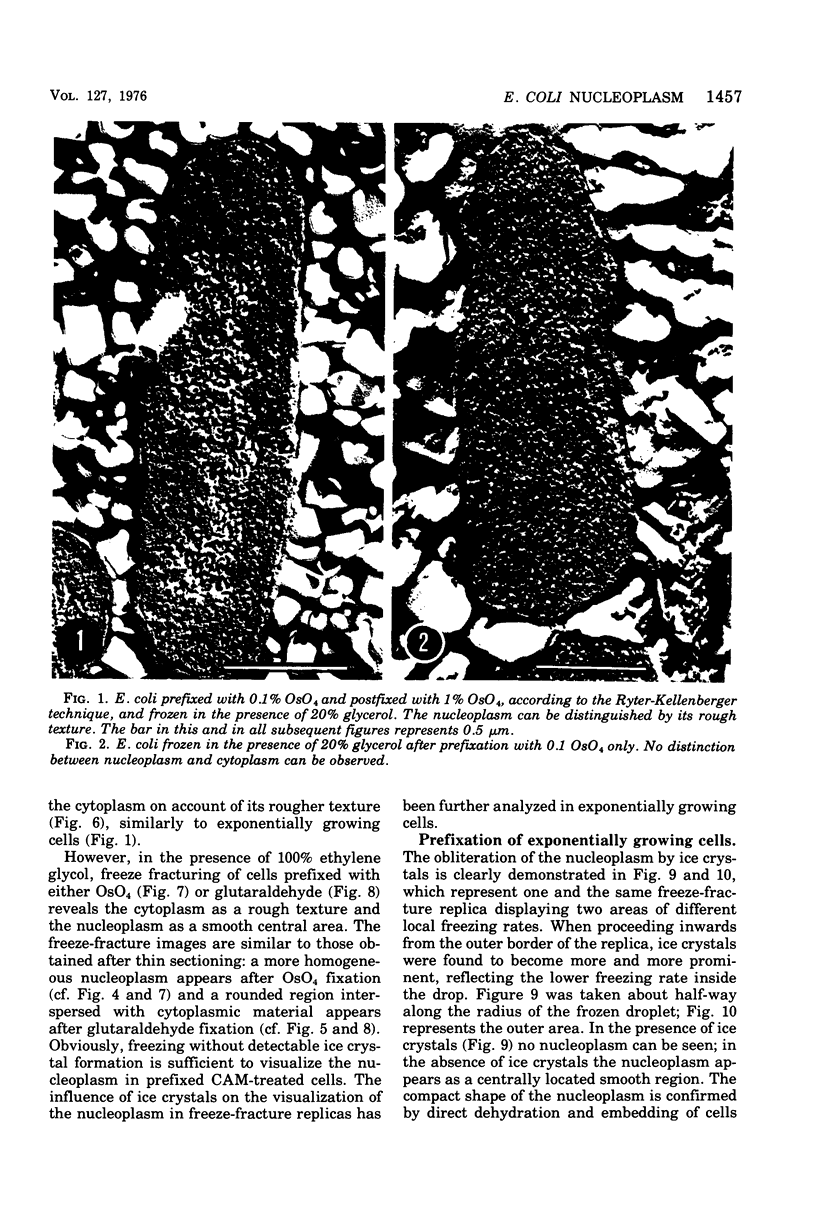
Abstract
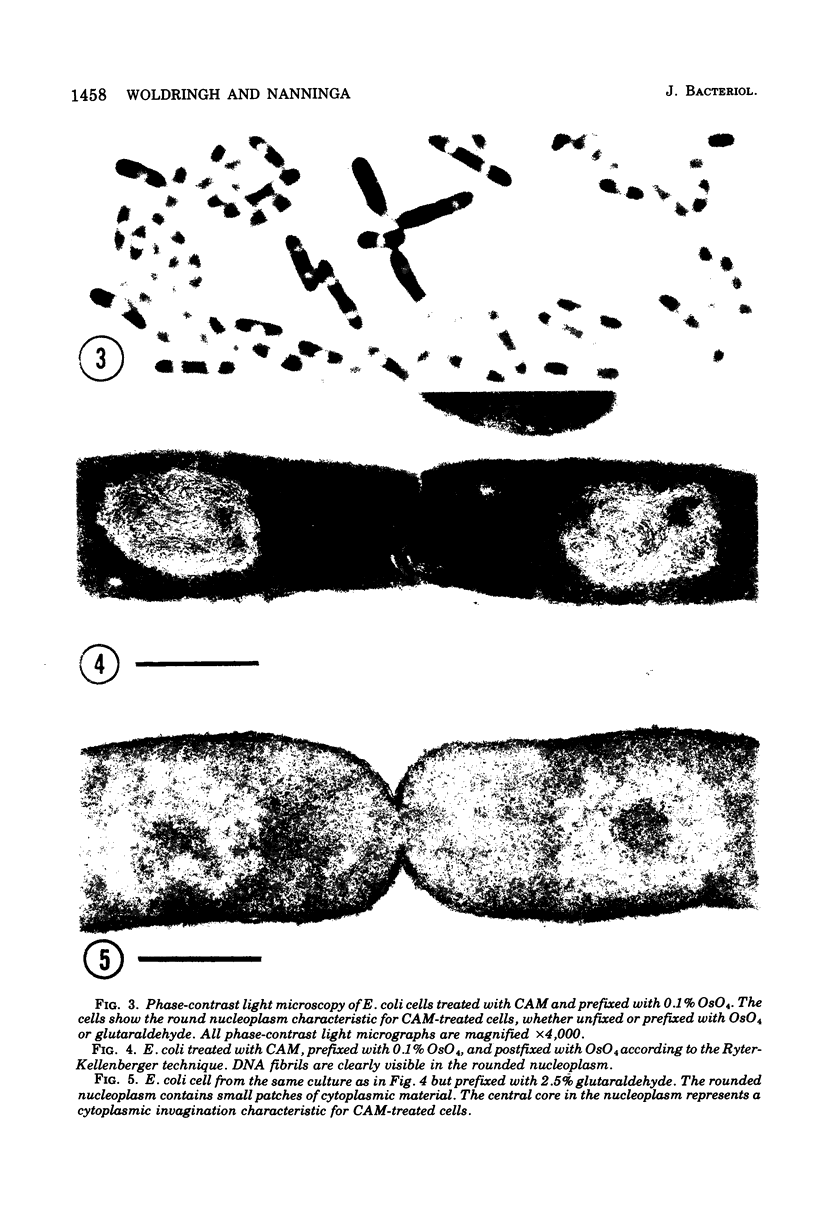
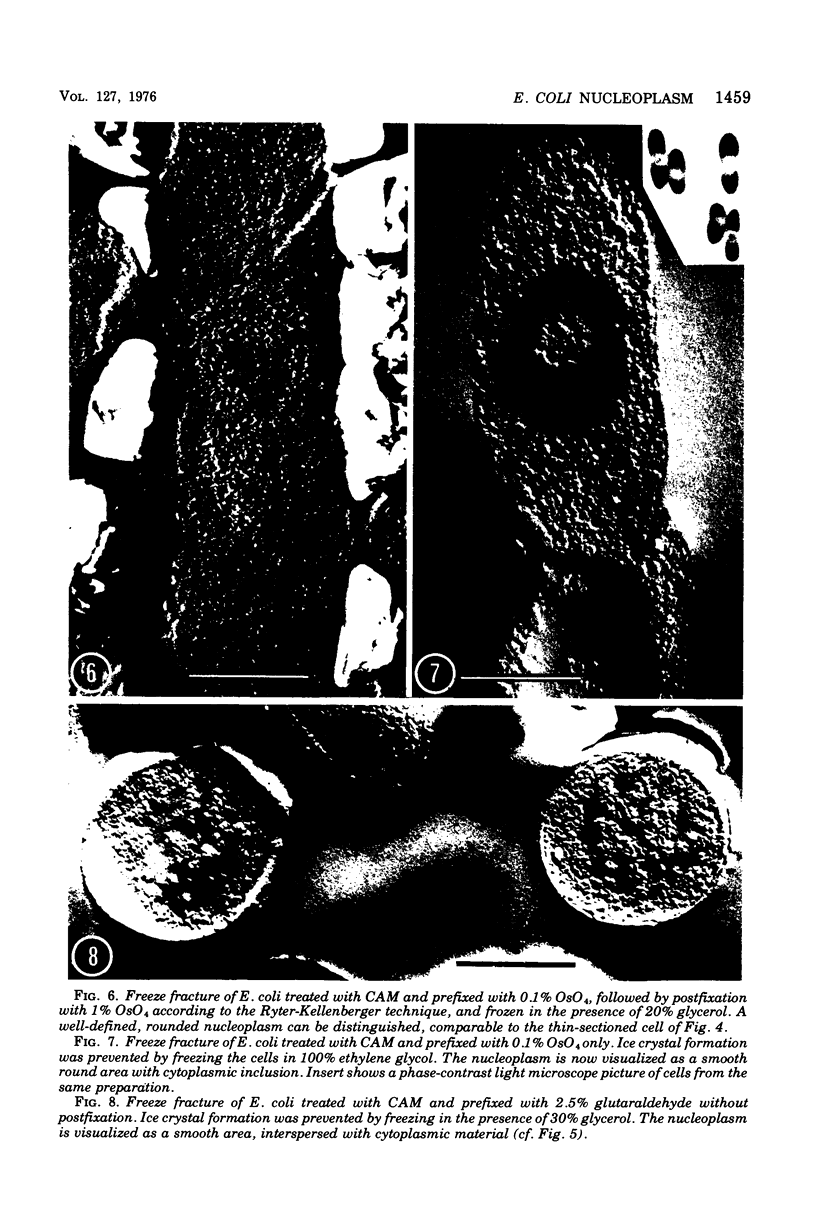
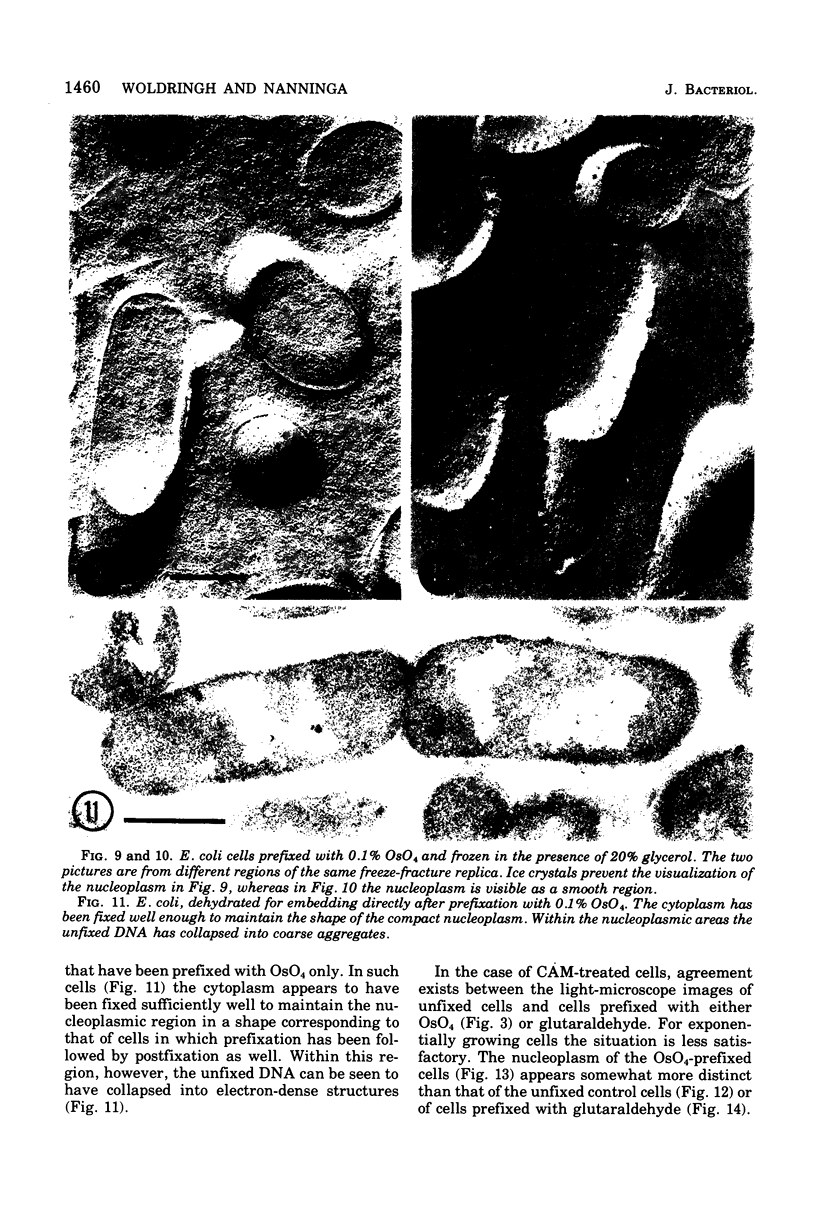
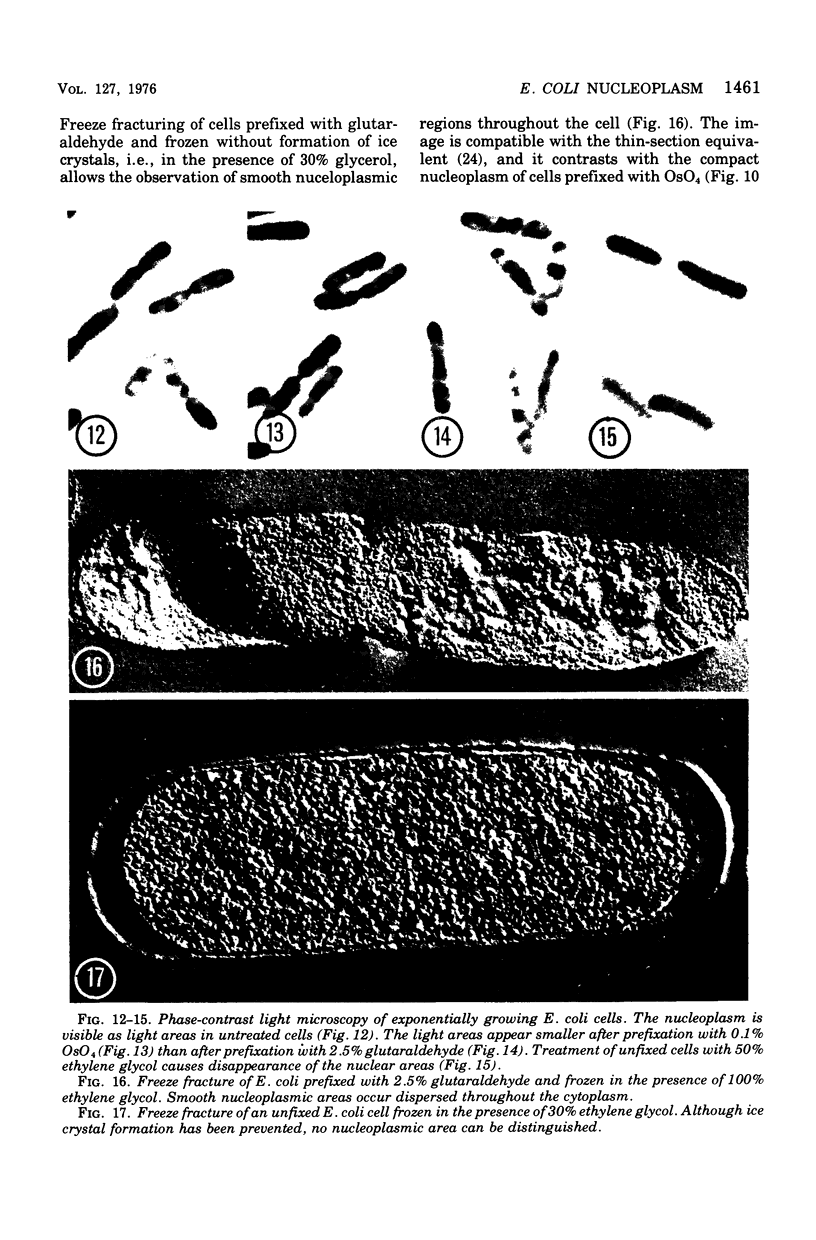
The organization of the nucleoplasm in Escherichia coli was studied by comparing the results obtained by freeze fracturing and thin sectioning. In addition to exponentially growing cells, we used chloramphenicol-treated cells which show a well-defined nucleoplasm, in the phase-contrast light microscope and can therefore function as a control for treatments necessary for electron microscopy. Two factors were found to determine the visibility of the nucleoplasm in freeze fractures: first, the state of lateral aggregation of deoxyribonucleic and fibrils, which is enhanced by postfixation with OsO4 according to the Ryter-Kellenberger technique; second, the presence of ice crystals. When their formation is prevented by the use of high concentration of freeze-protecting agents, the nucleoplasm appears as a smooth region in cells that have been prefixed. In unfixed cells, however, the freeze-protecting agent causes disappearance of the nucleoplasm by rearrangement of structures within the cell. This observation makes it hard to determine whether the deoxyribonucleic acid in vivo dispersed, as found after glutaraldehyde prefixation, or compact, as after OsO4 prefixation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daneo-Moore L., Higgins M. L. Morphokinetic reaction of Streptococcus faecalis (ATCC 9790) cells to the specific inhibition of macromolecular synthesis: nucleoid condensation on the inhibition of protein synthesis. J Bacteriol. 1972 Mar;109(3):1210–1220. doi: 10.1128/jb.109.3.1210-1220.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius H., Worcel A. Letter: Electron microscopic visualization of the folded chromosome of Escherichia coli. J Mol Biol. 1974 Jan 5;82(1):107–109. doi: 10.1016/0022-2836(74)90577-4. [DOI] [PubMed] [Google Scholar]
- Herskovits T. T., Harrington J. P. Solution studies of the nucleic acid bases and related model compounds. Solubility in aqueous alcohol and glycol solutions. Biochemistry. 1972 Dec 5;11(25):4800–4811. doi: 10.1021/bi00775a025. [DOI] [PubMed] [Google Scholar]
- Hopwood D. The reactions of glutaraldehyde with nucleic acids. Histochem J. 1975 May;7(3):267–276. doi: 10.1007/BF01003595. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D. Regular superstructures of purified DNA in ethanolic solutions. J Mol Biol. 1973 Aug 5;78(2):247–254. doi: 10.1016/0022-2836(73)90113-7. [DOI] [PubMed] [Google Scholar]
- Lickfeld K. G. Der frostgeätzte Bakterienkern. Ein Beitrag zur Klärung seiner Tertiärstruktur. Z Zellforsch Mikrosk Anat. 1968;88(4):560–564. [PubMed] [Google Scholar]
- MASON D. J., POWELSON D. M. Nuclear division as observed in live bacteria by a new technique. J Bacteriol. 1956 Apr;71(4):474–479. doi: 10.1128/jb.71.4.474-479.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margaretten W., Morgan C., Rosenkranz H. S., Rose H. M. Effect of hydroxyurea on virus development. I. Electron microscopic study of the effect on the development of bacteriophage T4. J Bacteriol. 1966 Feb;91(2):823–833. doi: 10.1128/jb.91.2.823-833.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller O. L., Jr, Hamkalo B. A., Thomas C. A., Jr Visualization of bacterial genes in action. Science. 1970 Jul 24;169(3943):392–395. doi: 10.1126/science.169.3943.392. [DOI] [PubMed] [Google Scholar]
- Morgan C., Rosenkranz H. S., Carr H. S., Rose H. M. Electron microscopy of chloramphenicol-treated Escherichia coli. J Bacteriol. 1967 Jun;93(6):1987–2002. doi: 10.1128/jb.93.6.1987-2002.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanninga N. Preservation of the ultrastructure of Bacillus subtilis by chemical fixation as verified by freeze-etching. J Cell Biol. 1969 Sep;42(3):733–744. doi: 10.1083/jcb.42.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanninga N. Structural features of mesosomes (chondrioids) of Bacillu subtilis after freeze-etching. J Cell Biol. 1968 Nov;39(2):251–263. doi: 10.1083/jcb.39.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Remsen C. C. Fine structure of the mesosome and nucleoid in frozen-etched Bacillus subtilis. Arch Mikrobiol. 1968;61(1):40–47. doi: 10.1007/BF00704290. [DOI] [PubMed] [Google Scholar]
- SCHREIL W. H. STUDIES ON THE FIXATION OF ARTIFICIAL AND BACTERIAL DNA PLASMS FOR THE ELECTRON MICROSCOPY OF THIN SECTIONS. J Cell Biol. 1964 Jul;22:1–20. doi: 10.1083/jcb.22.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stent G. S. Genetic transcription. Proc R Soc Lond B Biol Sci. 1966 Mar 22;164(995):181–197. doi: 10.1098/rspb.1966.0022. [DOI] [PubMed] [Google Scholar]
- Stonington O. G., Pettijohn D. E. The folded genome of Escherichia coli isolated in a protein-DNA-RNA complex. Proc Natl Acad Sci U S A. 1971 Jan;68(1):6–9. doi: 10.1073/pnas.68.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séchaud J., Kellenberger E. Electron microscopy of DNA-containing plasms. IV. Glutaraldehyde-uranyl acetate fixation of virus-infected bacteria for thin sectioning. J Ultrastruct Res. 1972 Jun;39(5):598–607. doi: 10.1016/s0022-5320(72)90124-4. [DOI] [PubMed] [Google Scholar]
- Worcel A., Burgi E. On the structure of the folded chromosome of Escherichia coli. J Mol Biol. 1972 Nov 14;71(2):127–147. doi: 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]
- Zusman D. R., Carbonell A., Haga J. Y. Nucleoid condensation and cell division in Escherichia coli MX74T2 ts52 after inhibition of protein synthesis. J Bacteriol. 1973 Sep;115(3):1167–1178. doi: 10.1128/jb.115.3.1167-1178.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]