Abstract
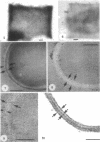
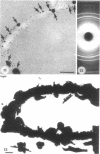
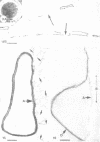
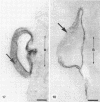
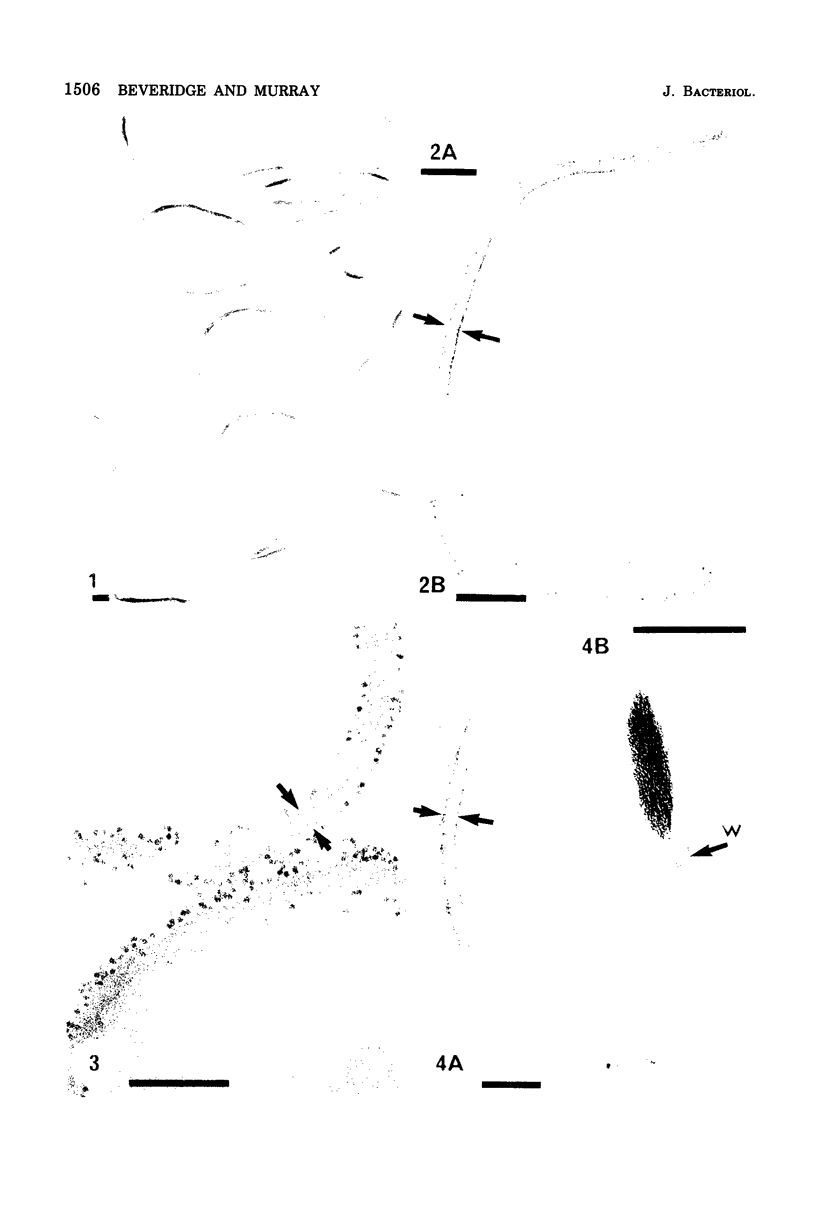
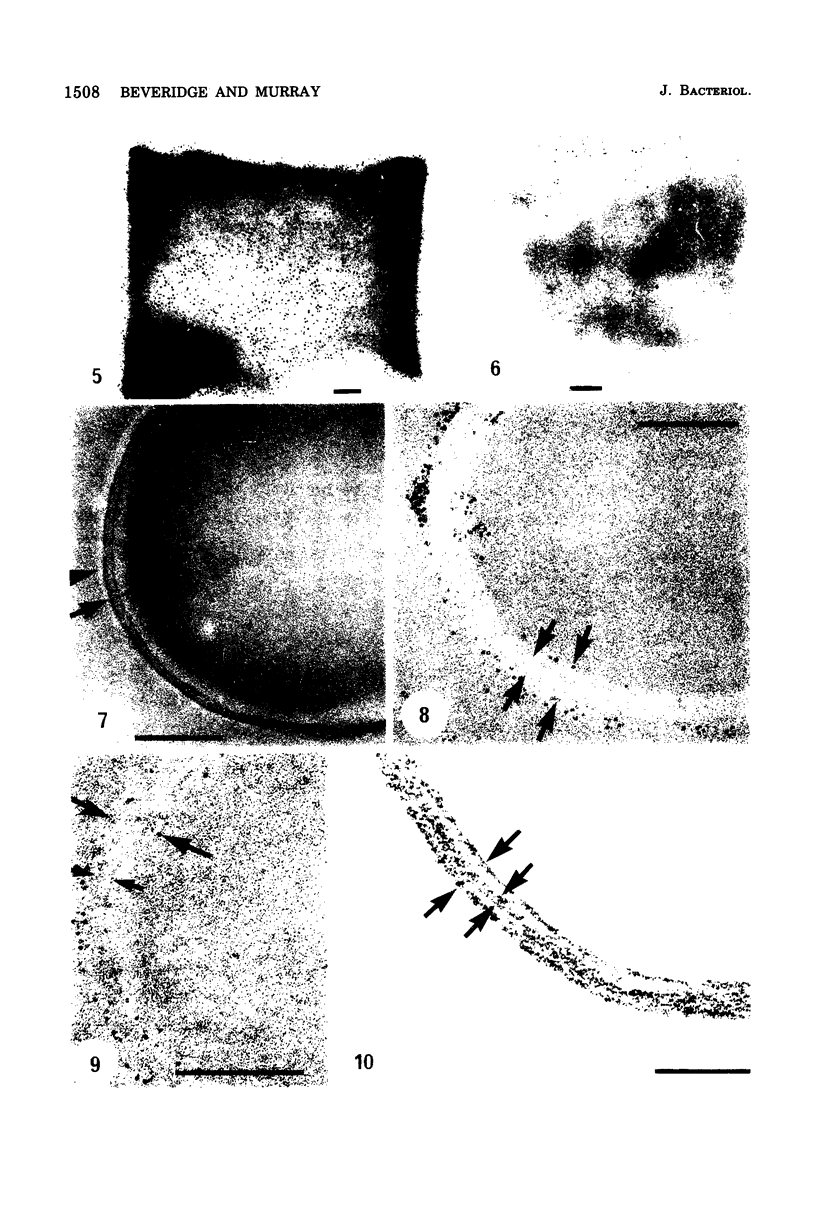
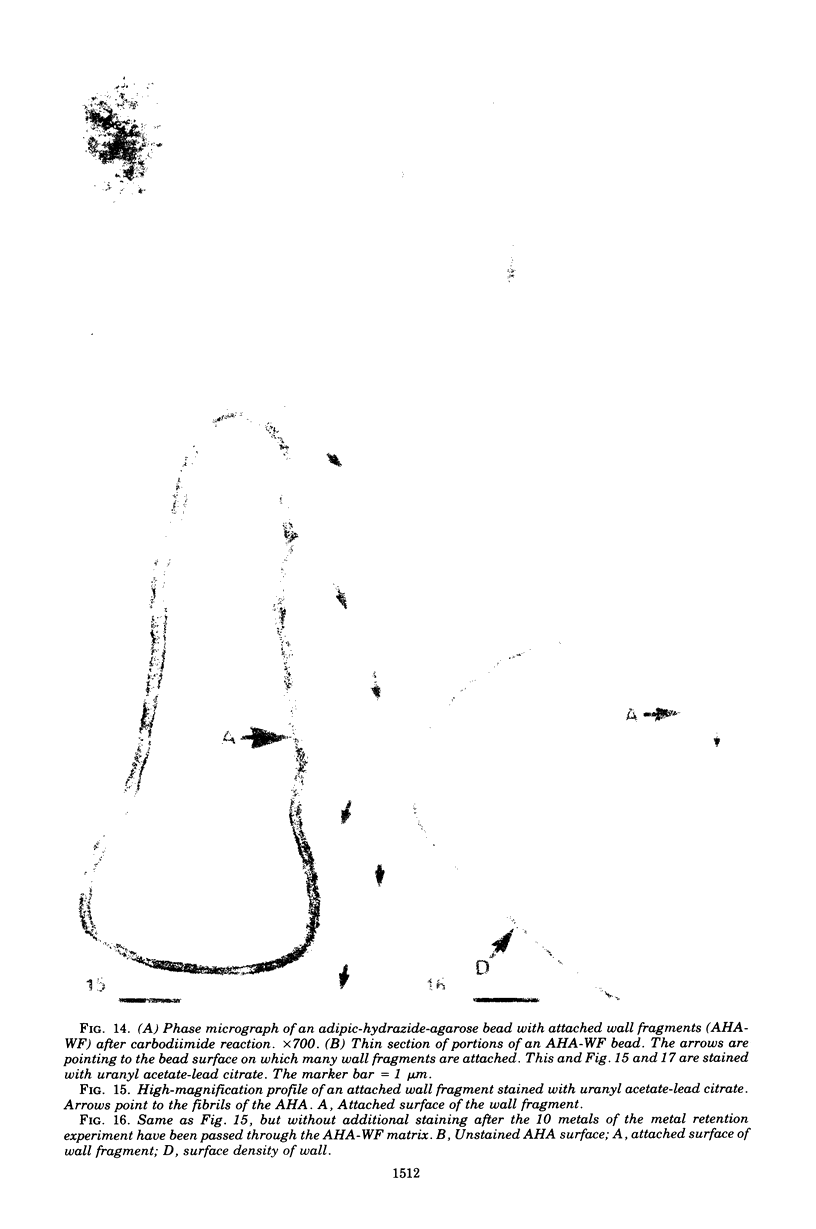
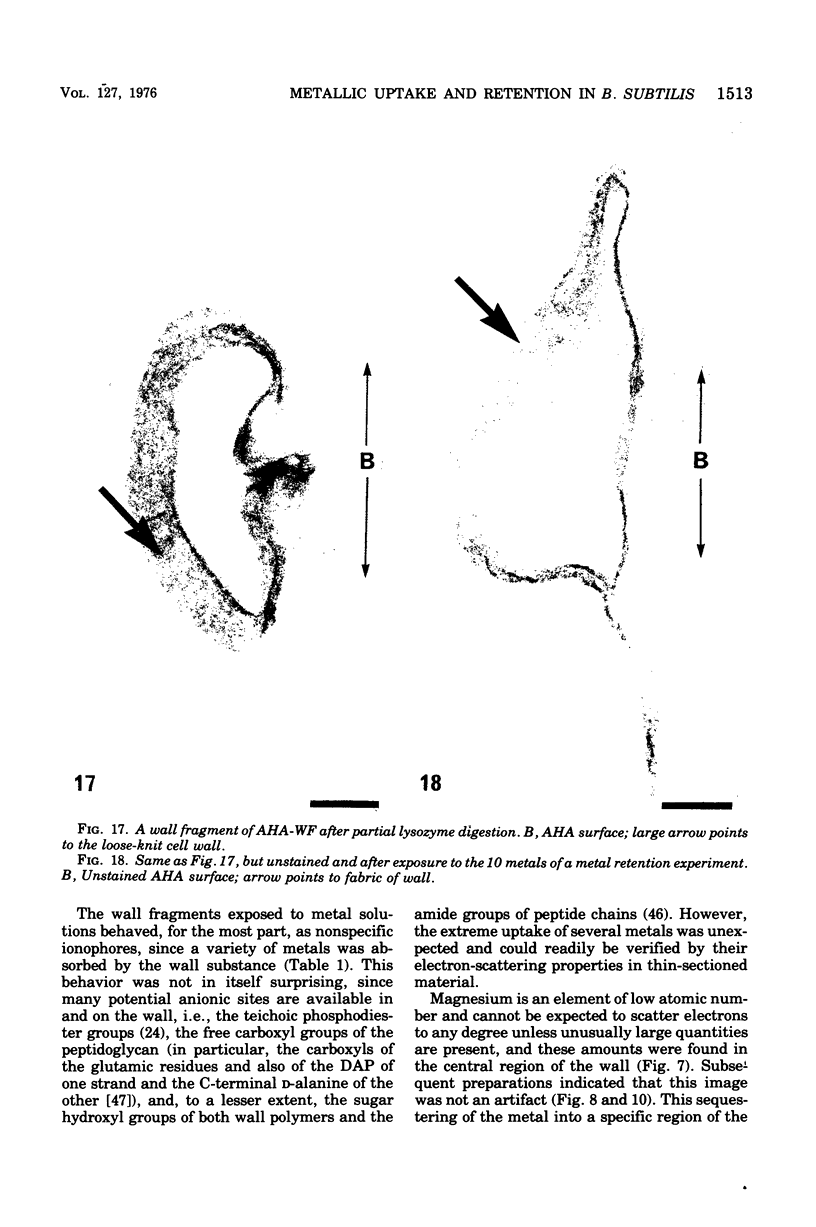
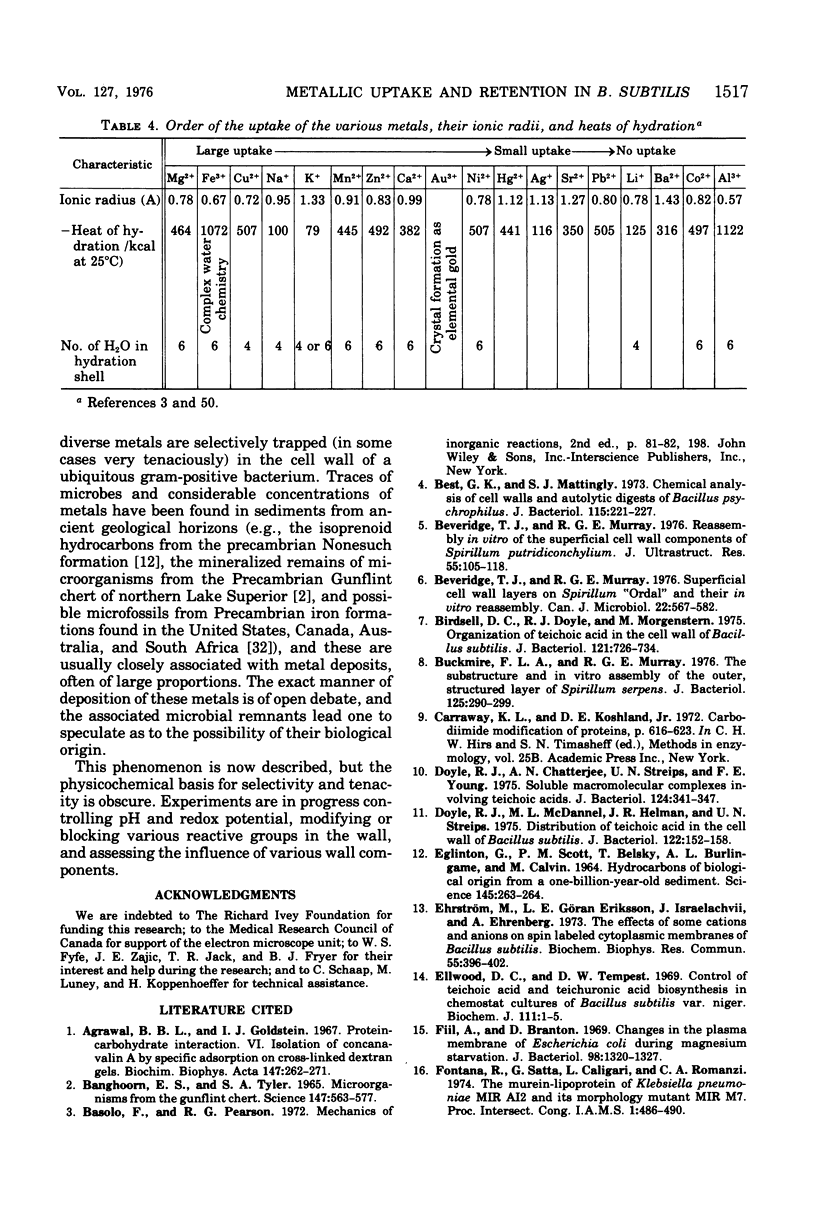
Isolated walls of Bacillus subtilis Marburg, prepared in a manner which avoided metal contamination other than by the growth medium, were incubated in dilute metal solutions, separated by membrane filtration (0.22 mum), and monitored by atomic absorption to give uptake data for 18 metals. Substantial amounts of Mg2+, Fe3+, Cu2+, Na+, and K+ (amounts which were often visible as Au3+, and Ni2+ (the higher atomic-numbered elements also visible as electron scattering), and small amounts of Hg2+, Sr2+, Pb2+, and Ag+ were taken into the wall. Some (Li+, Ba2+, Co2+, and Al3+) were not absorbed. Most metals which had atomic numbers greater than 11 and which could be detected by electron microscopy appeared to diffusely stain thin sections of the wall. Magnesium, on the other hand, partitioned into the central region, and these sections of walls resisted ruthenium red staining, which was not true for the other metals. Areas of the walls also acted as nucleation sites for the growth of microscopic elemental gold crystals when incubated in solutions of auric chloride. Retention or displacement of the metals was estimated by a "chromatographic" method using the walls cross-linked by the carbodiimide reaction to adipic hydrazide agarose beads (which did not take up metal but reduced the metal binding capacity of the walls by ca. 1%) packed in a column. When a series of 12 metal solutions was passed through the column, it became evident that Mg2+, Ca2+, Fe3+, and Ni2+ were strongly bound to the walls and could be detected by both atomic absorption and by their electron-scattering power in thin sections, qhereas the other metals were fisplaced or replaced. Partial lysozyme digestion of the walls (causing a 28% loss of a [3H]diaminopimelic acid label) greatly diminished the Mg2+ retention but not that of Ca2+, Fe3+, or Ni2+, indicating that there are select sites for various cations.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal B. B., Goldstein I. J. Protein-carbohydrate interaction. VI. Isolation of concanavalin A by specific adsorption on cross-linked dextran gels. Biochim Biophys Acta. 1967 Oct 23;147(2):262–271. [PubMed] [Google Scholar]
- Barghoorn E. S., Tyler S. A. Microorganisms from the Gunflint Chert: These structurally preserved Precambrian fossils from Ontario are the most ancient organisms known. Science. 1965 Feb 5;147(3658):563–575. doi: 10.1126/science.147.3658.563. [DOI] [PubMed] [Google Scholar]
- Best G. K., Mattingly S. J. Chemical analysis of cell walls and autolytic digests of Bacillus psychrophilus. J Bacteriol. 1973 Jul;115(1):221–227. doi: 10.1128/jb.115.1.221-227.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J., Murray R. G. Reassembly in vitro of the superficial cell wall components of Spirillum putridiconcyhylium. J Ultrastruct Res. 1976 Apr;55(1):105–118. doi: 10.1016/s0022-5320(76)80086-x. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J., Murray R. G. Superficial cell-wall layers on Spirillum "Ordal" and their in vitro reassembly. Can J Microbiol. 1976 Apr;22(4):567–582. doi: 10.1139/m76-085. [DOI] [PubMed] [Google Scholar]
- Birdsell D. C., Doyle R. J., Morgenstern M. Organization of teichoic acid in the cell wall of Bacillus subtilis. J Bacteriol. 1975 Feb;121(2):726–734. doi: 10.1128/jb.121.2.726-734.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Substructure and in vitro assembly of the outer, structured layer of Spirillum serpens. J Bacteriol. 1976 Jan;125(1):290–299. doi: 10.1128/jb.125.1.290-299.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle R. J., Chatterjee A. N., Streips U. N., Young F. E. Soluble macromolecular complexes involving bacterial teichoic acids. J Bacteriol. 1975 Oct;124(1):341–347. doi: 10.1128/jb.124.1.341-347.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle R. J., McDannel M. L., Helman J. R., Streips U. N. Distribution of teichoic acid in the cell wall of Bacillus subtilis. J Bacteriol. 1975 Apr;122(1):152–158. doi: 10.1128/jb.122.1.152-158.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglinton G., Scott P. M., Belsky T., Burlingame A. L., Calvin M. Hydrocarbons of Biological Origin from a One-Billion-Year-Old Sediment. Science. 1964 Jul 17;145(3629):263–264. doi: 10.1126/science.145.3629.263. [DOI] [PubMed] [Google Scholar]
- Ehrström M., Eriksson L. E., Israelachvili J., Ehrenberg A. The effects of some cations and anions on spin labeled cytoplasmic membranes of Bacillus subtilis. Biochem Biophys Res Commun. 1973 Nov 16;55(2):396–402. doi: 10.1016/0006-291x(73)91100-5. [DOI] [PubMed] [Google Scholar]
- Ellwood D. C., Tempest D. W. Control of teichoic acid and teichuronic acid biosyntheses in chemostat cultures of Bacillus subtilis var. niger. Biochem J. 1969 Jan;111(1):1–5. doi: 10.1042/bj1110001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiil A., Branton D. Changes in the plasma membrane of Escherichia coli during magnesium starvation. J Bacteriol. 1969 Jun;98(3):1320–1327. doi: 10.1128/jb.98.3.1320-1327.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formanek H., Formanek S., Wawra H. A three-dimensional atomic model of the murein layer of bacteria. Eur J Biochem. 1974 Jul 15;46(2):279–294. doi: 10.1111/j.1432-1033.1974.tb03620.x. [DOI] [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968 Apr;95(4):1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland J. M., Archibald A. R., Baddiley J. An electron microscopic study of the location of teichoic acid and its contribution to staining reactions in walls of Streptococcus faecalis 8191. J Gen Microbiol. 1975 Jul;89(1):73–86. doi: 10.1099/00221287-89-1-73. [DOI] [PubMed] [Google Scholar]
- Gerrard T. L., Telford J. N., Williams H. H. Detection of selenium deposits in Escherichia coli by electron microscopy. J Bacteriol. 1974 Sep;119(3):1057–1060. doi: 10.1128/jb.119.3.1057-1060.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Stinnett J. D., Eagon R. G. Ultrastructural and chemical alteration of the cell envelope of Pseudomonas aeruginosa, associated with resistance to ethylenediaminetetraacetate resulting from growth in a Mg2+-deficient medium. J Bacteriol. 1974 Jan;117(1):302–311. doi: 10.1128/jb.117.1.302-311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heptinstall S., Archibald A. R., Baddiley J. Teichoic acids and membrane function in bacteria. Nature. 1970 Feb 7;225(5232):519–521. doi: 10.1038/225519a0. [DOI] [PubMed] [Google Scholar]
- Hughes R. C. Autolysis of isolated cell walls of Bacillus licheniformis N.C.T.C. 6346 and Bacillus subtilis Marburg Strain 168. Separation of the products and characterization of the mucopeptide fragments. Biochem J. 1970 Oct;119(5):849–860. doi: 10.1042/bj1190849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin R. T., Chatterjee A. K., Sanderson K. E., Costerton J. W. Comparison of the cell envelope structure of a lipopolysaccharide-defective (heptose-deficient) strain and a smooth strain of Salmonella typhimurium. J Bacteriol. 1975 Nov;124(2):930–941. doi: 10.1128/jb.124.2.930-941.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernelöv A., Martin A. L. Ecological implications of metal metabolism by microorganisms. Annu Rev Microbiol. 1975;29:61–77. doi: 10.1146/annurev.mi.29.100175.000425. [DOI] [PubMed] [Google Scholar]
- Jost R., Miron T., Wilchek M. The mode of adsorption of proteins to aliphatic and aromatic amines coupled to cyanogen bromide-activated agarose. Biochim Biophys Acta. 1974 Aug 7;362(1):75–82. doi: 10.1016/0304-4165(74)90028-2. [DOI] [PubMed] [Google Scholar]
- Ketchum P. A., Owens M. S. Production of molybdenum-coordinating compound by Bacillus thuringiensis. J Bacteriol. 1975 May;122(2):412–417. doi: 10.1128/jb.122.2.412-417.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings W. N., Freese E. L-serine transport in membrane vesicles of Bacillus subtilis energized by NADH or reduced phenazine methosulfate. FEBS Lett. 1971 Apr 12;14(1):65–68. doi: 10.1016/0014-5793(71)80276-4. [DOI] [PubMed] [Google Scholar]
- Lambert P. A., Hancock I. C., Baddiley J. The interaction of magnesium ions with teichoic acid. Biochem J. 1975 Sep;149(3):519–524. doi: 10.1042/bj1490519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamed R., Levin Y., Wilchek M. Covalent coupling of nucleotides to agarose for affinity chromatography. Biochim Biophys Acta. 1973 Apr 28;304(2):231–235. doi: 10.1016/0304-4165(73)90239-0. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Macham L. P., Ratledge C. A new group of water-soluble iron-binding compounds from Mycobacteria: the exochelins. J Gen Microbiol. 1975 Aug;89(2):379–382. doi: 10.1099/00221287-89-2-379. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Singer S. J. Ferritin-conjugated plant agglutinins as specific saccharide stains for electron microscopy: application to saccharides bound to cell membranes. Proc Natl Acad Sci U S A. 1971 May;68(5):942–945. doi: 10.1073/pnas.68.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada Y., Une T., Ikeuchi T., Ogawa H. Divalent cation stimulation of the cell infectivity of Shigella flexneri 2a. Jpn J Microbiol. 1975 Apr;19(2):163–166. doi: 10.1111/j.1348-0421.1975.tb00863.x. [DOI] [PubMed] [Google Scholar]
- Osada Y., Une T., Ikeuchi T., Ogawa H. Effect of calcium on the cell infectivity of virulent Shigella flexneri 2a. Jpn J Microbiol. 1974 Jul;18(4):321–326. doi: 10.1111/j.1348-0421.1974.tb00816.x. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Site of synthesis of lipopolysaccharide. J Biol Chem. 1972 Jun 25;247(12):3973–3986. [PubMed] [Google Scholar]
- Ou L. T., Marquis R. E. Coccal cell-wall compactness and the swelling action of denaturants. Can J Microbiol. 1972 May;18(5):623–629. doi: 10.1139/m72-099. [DOI] [PubMed] [Google Scholar]
- Ou L. T., Marquis R. E. Electromechanical interactions in cell walls of gram-positive cocci. J Bacteriol. 1970 Jan;101(1):92–101. doi: 10.1128/jb.101.1.92-101.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao C. N., Rao K. G., Balasubramanian D. Binding of alkali and alkaline earth cations and of protons to the peptide group. FEBS Lett. 1974 Sep 15;46(1):192–194. doi: 10.1016/0014-5793(74)80366-2. [DOI] [PubMed] [Google Scholar]
- Rayman M. K., MacLeod R. A. Interaction of Mg-2+ with peptidoglycan and its relation to the prevention of lysis of a marine pseudomonad. J Bacteriol. 1975 May;122(2):650–659. doi: 10.1128/jb.122.2.650-659.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS R. J. Nature and properties of metal ions of biological interest and their coordination compounds. Fed Proc. 1961 Sep;2:5–14. [PubMed] [Google Scholar]
- Warth A. D., Strominger J. L. Structure of the peptidoglycan from vegetative cell walls of Bacillus subtilis. Biochemistry. 1971 Nov 23;10(24):4349–4358. doi: 10.1021/bi00800a001. [DOI] [PubMed] [Google Scholar]