Abstract
Allogeneic hematopoietic cell transplantation (HCT) is an increasingly widely used treatment modality in hematological malignancies. Alloreactivity mediated by donor T cells (and, in some settings, by donor natural killer cells) can produce durable immunologic control or eradication of residual malignancy after allogeneic HCT. However, graft-vs.-tumor (GVT) effects are variably effective and are often accompanied by deleterious alloreactivity against normal host tissue, manifesting as graft-vs.-host disease (GVHD). A major focus of current research in HCT is the separation of beneficial GVT effects from GVHD. Here we review a number of approaches currently under investigation to specifically augment GVT effects, including the identification of minor histocompatibility antigens (mHA), adoptive immunotherapy with tumor-specific or mHA-specific cytotoxic T cells, vaccination of the donor or recipient to stimulate tumor-specific immunity, and adoptive transfer of natural killer cells. In addition, we review strategies being investigated to specifically suppress GVHD while sparing GVT, including the manipulation and infusion of regulatory T cells, the use of novel pharmacologic and biologic agents, and the use of mesenchymal stem cells. Ultimately, advances in separation of GVT from GVHD will further enhance the potential of allogeneic HCT as a curative treatment for hematological malignancies.
Keywords: Adoptive immunotherapy, allogeneic hematopoietic cell transplantation, minor histocompatibility antigens
INTRODUCTION
Allogeneic hematopoietic cell transplantation (HCT) presents unique immunological challenges and opportunities. In contrast to solid-organ transplantation, where the transplanted organ contains few immunologically active cells and where the major concern is rejection of the donor organ, HCT reconstitutes an active donor-derived immune system within the recipient. While rejection of the allograft by residual recipient immune cells remains a concern, donor-derived immunity can also be directed against the recipient. When directed against healthy recipient tissues, this alloreactivity produces the clinical syndrome of graft-vs.-host disease (GVHD). While the prevention and control of GVHD remain among the foremost challenges in hematopoietic cell transplantation, donor-vs.-host alloreactivity is also responsible for much of the benefit of allogeneic HCT. When directed against residual malignant cells in the host, this alloreactivity can provide immunologic control and eradication of otherwise incurable hematological malignancies. Disentangling these two facets of alloreactivity appears technically feasible and holds great promise; however, thus far few approaches have been translated to the clinical realm, and alloreactivity after allogeneic HCT remains a double-edged sword.
The existence of beneficial graft-vs.-tumor alloreactivity was posited in some of the earliest murine studies of allogeneic HCT by Barnes et al. These authors reported in 1957 that allogeneic HCT, in contrast to syngeneic HCT, could eradicate residual host leukemia in a murine model, but produced a fatal syndrome of diarrhea and wasting which would today be recognized as GVHD [1]. The antileukemic efficacy and the wasting syndrome associated with allogeneic HCT demonstrated both the positive and negative effects of donor-vs.-host immunologic alloreactivity. Further evidence of the role of immunologic GVT effects in human transplantation accumulated: it was noted that cessation of immunosuppressive therapy after allogeneic HCT could result in disease remissions, that the disease relapse rate was lower in humans after allogeneic HCT as compared with syngeneic HCT, and that T-cell depletion of the allograft substantially increased the disease relapse rate. Furthermore, the disease relapse rate was found to be lower in patients with GVHD, suggesting a link between GVHD and GVT effects. The most compelling evidence of a role for immunological alloreactivity in disease control came from the efficacy of donor lymphocyte infusion (DLI); the infusion of donor lymphocytes in patients with relapsed chronic myelogenous leukemia after allogeneic HCT leads to a high rate of complete remission [2]. Finally, allogeneic HCT can induce remissions even after non-myeloablative conditioning lacking significant anti-tumor effectiveness; disease responses in this setting are largely or completely due to immunologic GVT reactions [3].
While GVHD and GVT effects are linked by a number of shared biological pathways, there is substantial evidence that these effects can occur independently [4], and that separation may be feasible. While a number of approaches have successfully induced GVT without GVHD in murine models, translation of these methods to human patients has been hampered by significant interspecies differences and shortcomings of the animal model. Recent reviews have summarized the overall immunobiology of allogeneic hematopoietic cell transplantation [5], the shared biology of GVHD and GVT [6,7], new developments in the understanding of acute and chronic GVHD [8], and autoimmunity after HCT [9]. We will therefore focus specifically on strategies to augment the GVT effect as well as complementary strategies to promote specific immunoregulatory responses which suppress GVHD without impairing GVT.
AUGMENTATION OF GRAFT-VS.-TUMOR RESPONSE
The graft-vs.-tumor response is mediated largely by cytotoxic T-cells (CTLs); a role for natural killer (NK) cells as a separate effector arm is emerging, particularly in T-cell-depleted HLA-haploidentical HCT. In rare situations, tumors may express unique antigens capable of generating a CTL response (e.g. the BCR-ABL fusion protein in chronic myelogenous leukemia). More commonly, malignant cells express so-called tumor-associated antigens, which are found on normal cells but overexpressed in malignant tissue. For example, Wilms tumor protein (WT-1) is overexpressed by leukemic blasts; CTLs specific for WT-1 lyse leukemic blasts in vitro without affecting normal hematopoietic cells [10]. Although these tumor-associated antigens are expressed at low levels on normal cells, T cells appear to recognize them only on tumor cells, perhaps due to a threshold density necessary for recognition, making such antigens attractive targets for adoptive immunotherapy.
Minor histocompatibility antigens
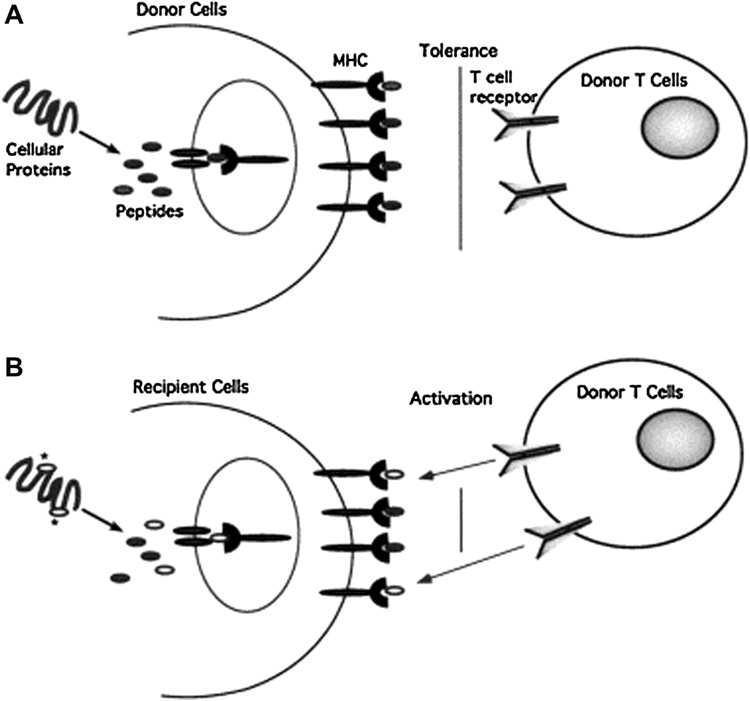
In addition to such tumor-associated antigens, more broadly expressed minor histocompatibility antigens (mHA) play a significant role in GVHD and GVT effects. These antigens are peptides presented in conjunction with HLA class I and II, and are derived from self proteins encoded by polymorphic genes and inherited separately from HLA. Disparities in mHA can induce significant T-cell responses, accounting for the development of GVHD (and GVT) even with HLA-identical sibling allografts (see Fig. 1). The first mHA to be described were those encoded by the Y chromosome; male recipients of female allografts experience both a higher severity of GVHD and more effective GVT responses, presumably due to recognition of Y-chromosome-encoded mHA by the female donor T cells [11]. The potential usefulness of mHA in adoptive immunotherapy was demonstrated by Bonnet et al., who showed that mHA-specific CTL targeted and lysed leukemic stem cells in a NOD/SCID mouse model [12]. Currently, nearly 30 human mHA have been identified; they are listed in a recent review by Hambach et al [13].
Figure 1.
Minor histocompatibility antigens represent distinct MHC-bound peptides displayed by MHC identical recipient cells. (A) Peptides derived from cellular proteins are displayed on the surface of cells complexed to MHC molecules and autologous T cells are tolerant to these self-peptides. (B) Due to polymorphisms in the genome, cellular proteins expressed by recipient cells may contain amino acid substitutions (depicted by the asterisks) compared with the homologous proteins in donor cells. After processing, these sequences may provide unique peptides that bind to MHC molecules and are displayed at the cell surface. T cells of the donor will recognize the unique peptides on recipient cells as foreign. Reproduced with permission from Riddell SR, Berger C, Murata M, et al. The graft versus leukemia response after allogeneic hematopoietic stem cell transplantation. Blood Rev. 2003 Sep;17(3):153-62.
The tissue distribution of mHA provides a potential window to specifically enhance GVT effects without GVHD. Many mHA are widely expressed and play a role in the development of GVHD in the HLA-identical setting. However, some mHA are unique to hematopoietic cells [14]. CTL clones specific for these hematopoietic mHA can target host hematological malignancies; “bystander” damage in this setting affects only the host hematopoietic system, which is replaced by donor hematopoiesis after allogeneic HCT in any case. Thus, by targeting hematopoietic mHA, GVT effects could be generated against hematological malignancies without the concurrent development of GVHD. Experimental support for this approach comes from a study by Marijt et al., who demonstrated the development of CTL clones specific for hematopoietic mHA after donor lymphocyte infusion in 3 patients with relapsed hematological malignancies; the development of these CTL clones was temporally associated with complete remission of their malignancies [15].
Thus, research has focused on the generation of mHA-specific CTL for infusion. A number of methods have been described. Donor T cells can be stimulated with donor dendritic cells pulsed with the relevant mHA [16]; this approach, while technically feasible, has been utilized only at specialized centers on small numbers of patients due to the cost and expertise required. Alternately, genes for mHA-specific T-cell receptors can be transferred to donor (or autologous) T cells using a retroviral vector. However, hybridization of the transferred gene product with endogenous T-cell receptor subchains may result in diminished specificity and undesirable alloreactivity [17], and safety concerns regarding the use of retroviral vectors remain an issue. Finally, allograft donors can be vaccinated prior to stem cell collection with mHA peptides to generate mHA-specific CTLs in vivo; alternately, allograft recipients can be vaccinated with mHA peptides after HCT, possibly in combination with unmanipulated donor lymphocyte infusion [13]. The potential of a vaccination-based approach was demonstrated by Neelapu et al., who vaccinated allograft donors with myeloma idiotype proteins and then vaccinated recipients with a “booster” after allogeneic HCT. A specific T-cell response was generated and transferred via HCT, and 3 of the 5 patients treated entered durable complete remissions while the remaining 2 died of early transplant-related complications [18]. Vaccination is theoretically appealing in that it avoids the expense, technical requirements, and individualized patient-specific aspects of ex vivo expansion and adoptive transfer of CTL. However, vaccine-based approaches to cancer immunotherapy have proven difficult to translate to the clinic, and issues of antigen and adjuvant selection remain unsettled.
There are several additional barriers to the clinical use of mHA-specific CTLs. Selection of donors on the basis of mHA typing holds great theoretical promise, but given the diversity of mHA and the small number of hematopoietic mHA that have thus far been characterized, it is currently difficult or impossible to prospectively identify target mHA in a given donor/host pairing. Additionally, even after successful adoptive transfer, mHA-specific CTL have shown a disappointing lack of persistence in vivo. Riddell et al. described successful initial treatment of relapsed leukemia with mHA-specific CTL; however, the CTL clone did not persist and the leukemia later relapsed [19]. IL-2 has been administered systemically to enhance the persistence of CTL clones, but its drawbacks include systemic toxicity and the expansion of unwanted cell types. An intriguing approach to circumvent these limitations was recently described by Quintarelli et al., who found that transgenic expression of IL-2 and IL-15 by CTL clones themselves improved the persistence of these clones; the authors further incorporated a suicide gene into the CTL clone to enable elimination of these cells in the event of undesired or autonomous growth [20]. Other potential barriers to the use of mHA-specific CTL include tumor cell evasion by mHA or MHC down-regulation and the possibility that the pro-inflammatory cytokine milieu resulting from generalized alloreactivity and GVHD is necessary to enhance GVT through non-specific stimulation.
Natural killer cells
In addition to T cells, natural killer (NK) cells play a significant role in GVT effects in some transplant settings and provide a possible mechanism to augment GVT effects while avoiding GVHD. The role of NK cells was first recognized in the setting of HLA-haploidentical transplantation. Allografts from HLA-haploidentical donors are typically extensively T-cell-depleted to prevent the serious or fatal GVHD which would result from transplantation across such an HLA barrier. In the absence of T-cell-mediated effects, GVT is nevertheless apparent and appears to be mediated by NK cells. NK-cell cytotoxicity is regulated by a variety of stimulatory and inhibitory signals; autoreactivity is prevented primarily through the expression by NK cells of an inhibitory killer cell immunoglobulin-like receptor (KIR), which interacts with MHC class I epitopes. KIR recognition of self-MHC class I inhibits NK cytotoxicity. Certain donor-recipient pairings in HLA-haploidentical HCT involve a KIR-ligand mismatch, in which KIR on donor NK cells do not recognize host MHC class I; in these settings, NK cells are not inhibited and lyse recipient cells. KIR-ligand mismatching appears to be beneficial in HLA-haploidentical HCT; Ruggeri et al. reported that KIR-ligand-mismatched recipients had a lower risk of disease relapse and better overall survival, presumably due to NK-cell-mediated GVT effects [21]. Low rates of acute and chronic GVHD have also been reported after HLA-haploidentical HCT, possibly due to extensive allograft T-cell depletion or tissue specificity of NK-cell cytotoxicity, which preferentially affects lymphohematopoietic tissue. Additionally, KIR-ligand mismatching is associated with a lower risk of GVHD in this setting, suggesting that NK-cell killing of host antigen-presenting cells (necessary for the development of GVHD) plays a role. In contrast to the HLA-haploidentical related-donor setting, a beneficial effect of KIR-ligand mismatching has not been consistently observed after T-cell-replete HCT from unrelated donors. Several explanations have been proposed for these findings. T cells in the allograft have been reported to reduce NK-cell KIR expression [22]. An intriguing recent study by Wang et al. suggested that cyclosporine A, given as GVHD prophylaxis after T-cell-replete HCT, may also downregulate KIR expression by NK cells [23]. Thus, while NK-cell-mediated GVT is evident in T-cell-depleted HLA-haploidentical HCT, it appears that several factors conspire to reduce or obscure the role of NK cells in other settings.
Nonetheless, given the ability of NK cells to induce GVT without GVHD, they remain an attractive candidate for adoptive immunotherapy. The University of Minnesota group demonstrated this potential in the non-transplant setting; 19 poor-prognosis patients with acute myeloid leukemia were treated with high-dose cyclophosphamide and fludarabine, followed by infusion of allogeneic NK cells from an HLA-haploidentical related donor. NK cell infusion was followed by a rise in IL-15 levels, in vivo expansion of the donor NK cells, and complete hematologic remission in 5 of the 19 patients [24]. Notably, there was no evidence of GVHD, and 4 of the 5 responding patients were KIR-ligand-mismatched with their donors. Recent animal studies in the setting of allogeneic HCT have shown similarly suggestive results; infusion of alloreactive NK cells after allogeneic HCT in a murine model resulted in both a reduction in GVHD and enhancement of GVT effects [25]. Given these promising preliminary results, adoptive therapy via NK cell infusion is an area of active investigation in human HCT.
Donor lymphocyte infusion
The infusion of unmanipulated donor lymphocytes to treat relapsed disease after allogeneic HCT was first described by Kolb et al. in 1990, and remains the only form of adoptive immunotherapy in widespread clinical use. Donor lymphocyte infusion (DLI) is highly effective against relapsed chronic myelogenous leukemia; its activity against acute leukemias is significantly poorer (see Table 1). Disease regression after DLI is often accompanied by the development of GVHD, and significant pre-existing active GVHD is generally a contraindication to DLI. Efforts to improve DLI by infusing only specific CTL clones have been described above. A number of other approaches are being explored to improve the anti-tumor efficacy of DLI and lessen the associated risk of GVHD. Selective depletion of CD8+ cells from the DLI product has shown promise in separating a GVT effect from the associated GVHD in a small pilot study [26]. T-cell anergy due to lack of costimulatory ligands on tumor cells has been hypothesized as a mechanism of tumor escape from GVT; a recent phase I trial explored ex vivo expansion and activation of donor lymphocytes as a means of circumventing T-cell anergy, and reported encouraging disease responses without an excess of GVHD [27]. The transfer of so-called “suicide genes” into donor lymphocytes has also been studied as a means of curtailing donor T-cell expansion in the event of severe GVHD. Herpes simplex virus thymidine kinase (HSV-TK) is the most widely studied suicide gene; however, its usefulness has been limited by the immunogenicity of the HSV-TK transgene, which results in rapid clearance of the infused lymphocytes [28]. Given this limitation, several non-immunogenic suicide genes such as caspase 9 have been described which may be more useful in this setting [29]. Ultimately, a combination of approaches may be necessary to augment the GVT effectiveness of DLI in diseases other than chronic myelogenous leukemia, while reducing the serious limitation of accompanying GVHD. Nonetheless, the proven clinical utility of DLI is a starting point for the development of more specific forms of adoptive immunotherapy.
Table 1. Complete response rates after donor lymphocyte infusion.
High response rates are seen in relapsed chronic myelogenous leukemia, but results are significantly poorer in other disease states, particularly acute leukemia. Abbreviations: CR, complete response; EBMT, European Group for Blood and Marrow Transplantation. EBMT data taken from Kolb HJ, Schmid C, Barrett AJ, Schendel DJ. Graft-versus-leukemia reactions in allogeneic chimeras. Blood 2004 Feb 1;103(3):767-76. North American data taken from Collins RH Jr, Shpilberg O, Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997 Feb;15(2):433-44.
| Disease |
Complete responses/evaluable patients (% CR) |
|
|---|---|---|
| EBMT | North America | |
| Chronic myelogenous leukemia | ||
| Cytogenetic/molecular relapse | 40/50 (80%) | 3/3 (100%) |
| Hematological relapse | 88/114 (77%) | 25/34 (74%) |
| Accelerated phase/blast crisis | 13/36 (36%) | 5/18 (28%) |
| Acute myeloid leukemia/myelodysplastic syndrome | 15/58 (26%) | 8/44 (18%) |
| Acute lymphoblastic leukemia | 3/20 (15%) | 2/11 (18%) |
| Multiple myeloma | 5/17 (29%) | 2/4 (50%) |
| Non-Hodgkin lymphoma | None evaluated | 0/6 (0%) |
AUGMENTATION OF REGULATORY RESPONSE
While the above approaches seek largely to augment the power or specificity of the GVT effect, a complementary area of research involves more effective and specific suppression of the graft-vs.-host response. Building on current proven strategies to prevent GVHD, such as post-transplant immunosuppression with calcineurin inhibitors, methotrexate, or mycophenolate mofetil [30,31], investigation has focused on harnessing regulatory T-cell populations and on novel pharmacologic and biologic agents which may more specifically inhibit GVHD without affecting GVT responses.
Regulatory T cells
Sakaguchi et al. initially described a population of regulatory T cells (Treg) which exert a suppressive effect on the T-cell immune response, controlling autoreactivity and maintaining self-tolerance. The role of these Treg in tolerization after allogeneic HCT is an area of active investigation, and has recently been reviewed by Zorn [32]. In the murine model of allogeneic HCT, infusion of Treg suppresses both acute GVHD and graft rejection while maintaining GVT effects, while Treg depletion augments GVHD. However, one of the major barriers to utilizing Treg therapeutically in humans is their identification. The transcription factor FOXP3 is used as a marker for Treg, but identification of FOXP3 requires cell solubilization, rendering the cells unusable for re-infusion. Using a CD4+CD25+ pattern alone, without FOXP3, does not reliably identify Treg. Furthermore, while FOXP3 is highly specific for Treg in murine models, recent reports have suggested that in humans FOXP3 is transiently expressed by activated T cells and thus may not be specific enough to isolate human Treg [33]. Recently, the cell surface marker CD127 has been used in conjunction with CD4 and CD25 to identify a highly suppressive population of T cells; this marker may be a useful substitute for FOXP3, particularly where viable cells are required [34]. Given the utility of Treg in animal models of HCT and the development of reliable methods to expand human Treg ex vivo, human trials of Treg infusion have been initiated. A number of issues remain to be explored in these trials, including the effect of Treg on GVT in humans, the optimal timing and cell dose of Treg, and the effect of post-transplant immunosuppressive medication on infused Treg.
Pharmacologic agents
The current mainstays of GVHD prophylaxis include calcineurin inhibitors, methotrexate, mycophenolate mofetil, and anti-thymocyte globulin, as described above. While combinations of these medications are highly effective in the prevention of GVHD and permit successful transplantation from unrelated and HLA-mismatched donors, GVHD continues to be a significant source of morbidity and mortality, and these agents may impair the GVT response to some extent along with GVHD. Therefore, novel immunoregulatory agents are being sought which will preserve GVT while effectively preventing GVHD.
The proteosome inhibitor bortezomib has shown promise in animal models of HCT; its administration immediately after HCT reduced GVHD while preserving GVT. In fact, in this model bortezomib sensitized tumor cells to lysis by donor T cells, suggesting a synergistic GVT effect in addition to the suppression of GVHD [35]. Bortezomib’s efficacy in this setting is likely mediated by down-regulation of NF-kappa B; thus, more specific investigational inhibitors of NF-kappa B may also be useful in the setting of allogeneic HCT [36]. However, the efficacy of bortezomib in the human setting remains to be demonstrated. Rapamycin is an immunosuppressant which inhibits the mammalian target of rapamycin (mTOR); in addition to direct anti-tumor effects which are under investigation, rapamycin may augment the pool of regulatory T cells (in contrast to cyclosporine, which inhibits Treg) [37]. Given these findings, rapamycin-containing regimens for GVHD prophylaxis after allogeneic HCT are currently being evaluated in randomized controlled human trials. Finally, the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) is under investigation; in the murine model, SAHA prevented GVHD by downregulating inflammatory cytokines, while preserving tumor-specific CTL activity [38].
Mesenchymal stem cells
Recent reports have excited interest in mesenchymal stem cell (MSC) infusion as a means of suppressing the graft-vs.-host reaction. MSC are undifferentiated, pluripotent cells which generate mesodermal tissue, including the bone marrow stroma. MSC have low immunogenicity and can be transplanted without donor-recipient matching; furthermore, they exert an immunoregulatory effect, although the exact mechanisms of this effect (soluble factors vs. direct cell-to-cell interaction) remain uncertain. A case report authored by Le Blanc et al. in 2004 described the use of third-party HLA-haploidentical MSC in a patient with severe and refractory acute GVHD after HCT; the patient had a striking clinical response [39]. The same group recently published a pilot study reporting that MSC infusion successfully treated steroid-refractory acute GVHD in 6 of 8 treated patients [40]. The efficacy of MSC in this setting, as well as their downstream effects on GVT and the risk of ectopic tissue formation, are currently being evaluated further in larger multi-center studies. The advantages of MSC include their universality, in that third-party donor MSC can be infused without the need for histocompatibility testing or matching. In addition, one group has reported the use of MSC transduced to express IL-10 to suppress inflammatory cytokines and ameliorate GVHD in the murine model [41], demonstrating the potential to tailor these cells as a treatment for GVHD.
CONCLUSION
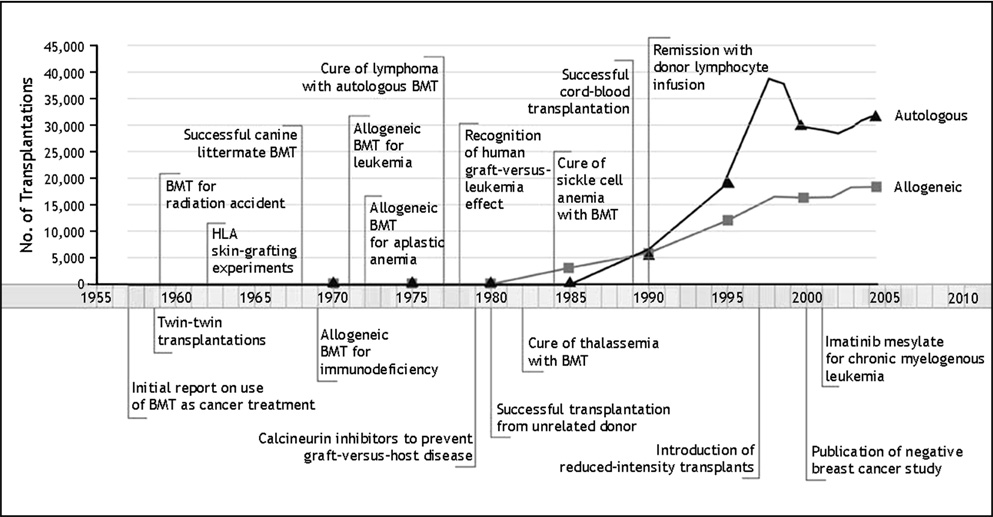
Allogeneic HCT is an increasingly widely used therapy which has advanced dramatically in the half-century since its clinical introduction and contributed greatly to the understanding of immunology and transplantation biology (see Fig. 2). The donor pool has been expanded with substantial unrelated-donor registries, HLA-haploidentical transplantation, and umbilical cord blood banking; the range of eligible patients has been significantly broadened by the development of reduced-intensity and non-myeloablative conditioning regimens; and more effective antimicrobials and supportive care have rendered once formidable toxicities and complications more manageable. Allogeneic HCT from an HLA-matched sibling is currently the treatment of choice for severe aplastic anemia, and has been shown to produce survival advantages over conventional chemotherapy in adults with acute leukemia in first complete remission. In addition, allogeneic HCT remains the only potentially curative therapy for a number of chronic hematological diseases, such as the myelodysplastic syndrome. However, significant challenges and limitations remain. The GVT effect is dramatically evident in some donor-recipient pairings but absent from others, and GVT continues to be closely associated with GVHD, particularly chronic GVHD. Meanwhile, acute and particularly chronic GVHD continue to be major causes of morbidity and mortality after allogeneic HCT. In order to advance further, methods to augment GVT while suppressing GVHD are required. A broad range of strategies have been effective in dissecting GVT from GVHD in murine models; however, many of these have proven disappointing when translated to the human setting. Unmanipulated donor lymphocyte infusion is the only method of GVT augmentation currently in widespread clinical use; while effective in chronic myelogenous leukemia, DLI is inconsistent against other hematological malignancies, and is often accompanied by severe GVHD. Investigational approaches to augment GVT which are currently being studied in humans include the use of mHA-specific CTL infusion, vaccination of the donor or recipient with mHA peptides, adoptive transfer of NK cells, and modified donor lymphocyte infusion. Complementary methods under investigation to reduce the burden of GVHD while sparing GVT focus on immunoregulatory strategies, such as ex vivo expansion and infusion of regulatory T cells, the use of novel pharmacologic agents such as SAHA, bortezomib, or rapamycin, and the use of biological modifiers of alloreactivity such as mesenchymal stem cells. The curative potential of allogeneic HCT in hematological malignancy has been well-demonstrated. With ongoing advances in our understanding of transplant immunology and alloreactivity, the separation of beneficial GVT from GVHD appears to be a feasible goal, and holds the promise of realizing the full potential of this modality to treat and cure otherwise fatal disease. For additional readings on the use of bone marrow transplantation for autoimmunity, we refer the reader to the companion papers published in this special issue [42–52]
Figure 2.
Allogeneic hematopoietic cell transplantation is an increasingly widely used therapy. Important milestones and developments in the field of hematopoietic cell transplantation are shown on the timeline. Reproduced with permission from Appelbaum FR. Hematopoietic-cell transplantation at 50. N Engl J Med. 2007 Oct 11;357(15):1472-5. Copyright © 2007 Massachusetts Medical Society. All rights reserved.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrew R. Rezvani, Email: arezvani@fhcrc.org.
Rainer F. Storb, Email: rstorb@fhcrc.org.
References
- 1.Barnes DWH, Loutit JF. Treatment of murine leukaemia with x-rays and homologous bone marrow: II. Br. J. Haematol. 1957;3:241–252. doi: 10.1111/j.1365-2141.1957.tb05793.x. Ref ID: 2339. [DOI] [PubMed] [Google Scholar]
- 2.Fefer A. Graft-vs.tumor responses. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas' Hematopoieitic Cell Transplantation. Third ed. Oxford, UK: Blackwell Publishing Ltd.; 2004. pp. 369–379. Ref ID: 24181. [Google Scholar]
- 3.Baron F, Storb R. Allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning as treatment for hematologic malignancies and inherited blood disorders (Review) Molecular Therapy. 2006;13:26–41. doi: 10.1016/j.ymthe.2005.09.011. Ref ID: 28836. [DOI] [PubMed] [Google Scholar]
- 4.Ringden O, Labopin M, Gorin NC, Schmitz N, Schaefer UW, Prentice HG, Bergmann L, Jouet JP, Mandelli F, Blaise D, Fouillar L, Frassoni F. Is there a graft-versus-leukaemia effect in the absence of graft-versus-host disease in patients undergoing bone marrow transplantation for acute leukaemia? Br. J. Haematol. 2000;111:1130–1137. doi: 10.1046/j.1365-2141.2000.02493.x. Ref ID: 33473. [DOI] [PubMed] [Google Scholar]
- 5.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation (Review) Annu. Rev. Immunol. 2007;25:139–170. doi: 10.1146/annurev.immunol.25.022106.141606. Ref ID: 32604. [DOI] [PubMed] [Google Scholar]
- 6.Fowler DH. Shared biology of GVHD and GVT effects: potential methods of separation (Review) Critical Reviews in Oncology-Hematology. 2006;57:225–244. doi: 10.1016/j.critrevonc.2005.07.001. Ref ID: 32699. [DOI] [PubMed] [Google Scholar]
- 7.Shlomchik WD. Graft-versus-host disease. Nature Reviews Immunology. 2007 doi: 10.1038/nri2000. Ref Type: In Press Ref ID: 32166. [DOI] [PubMed] [Google Scholar]
- 8.Riddell SR, Appelbaum FR. Graft versus host disease - a surge of developments provide a new way forward. PLoS Medicine. doi: 10.1371/journal.pmed.0040198. 9999. Ref Type: In Press Ref ID: 31805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daikeler T, Tyndall A. Autoimmunity following haematopoietic stem-cell transplantation. Bailliere's Best Practice in Clinical Haematology. 2007;20:349–360. doi: 10.1016/j.beha.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Bellantuono I, Gao L, Parry S, Marley S, Dazzi F, Apperley J, Goldman JM, Stauss HJ. Two distinct HLA-A0201-presented epitopes of the Wilms tumor antigen 1 can function as targets for leukemia-reactive CTL. Blood. 2002;100:3835–3837. doi: 10.1182/blood.V100.10.3835. Ref ID: 32631. [DOI] [PubMed] [Google Scholar]
- 11.Randolph SSB, Gooley TA, Warren EH, Appelbaum FR, Riddell SR. Female donors contribute to a selective graft-versus-leukemia effect in male recipients of HLA-matched, related hematopoietic cell transplants. Blood. 2004;103:347–352. doi: 10.1182/blood-2003-07-2603. Ref ID: 23469. [DOI] [PubMed] [Google Scholar]
- 12.Bonnet D, Warren EH, Greenberg PD, Dick JE, Riddell SR. CD8+ minor histocompatibility antigen-specific cytotoxic T lymphocyte clones eliminate human acute myeloid leukemia stem cells. Proc. Natl. Acad. Sci. USA. 1999;96:8639–8644. doi: 10.1073/pnas.96.15.8639. Ref ID: 16298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hambach L, Spierings E, Goulmy E. Risk assessment in haematopoietic stem cell transplantation: minor histocompatibility antigens (Review) Bailliere' s Best Practice in Clinical Haematology. 2007;20:171–187. doi: 10.1016/j.beha.2006.09.002. Ref ID: 32595. [DOI] [PubMed] [Google Scholar]
- 14.de Bueger M, Bakker A, van Rood JJ, Van der Woude F, Goulmy E. Tissue distribution of human minor histocompatibility antigens. Ubiquitous versus restricted tissue distribution indicates heterogeneity among human cytotoxic T lymphocyte-defined non-MHC antigens. J. Immunol. 1992;149:1788–1794. Ref ID: 15524. [PubMed] [Google Scholar]
- 15.Marijt WA, Heemskerk MH, Kloosterboer FM, Goulmy E, Kester MG, van der Hoorn MA, van Luxemburg-Heys SA, Hoogeboom M, Mutis T, Drijfhout JW, van Rood JJ, Willemze R, Falkenburg JH. Hematopoiesis-restricted minor histocompatibility antigens HA-1- or HA-2-specific T cells can induce complete remissions of relapsed leukemia. Proc. Natl. Acad. Sci. USA. 2003;100:2742–2747. doi: 10.1073/pnas.0530192100. Ref ID: 23537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mutis T, Verdijk R, Schrama E, Esendam B, Brand A, Goulmy E. Feasibility of immunotherapy of relapsed leukemia with ex vivo-generated cytotoxic T lymphocytes specific for hematopoietic system-restricted minor histocompatibility antigens. Blood. 1999;93:2336–2341. Ref ID: 16514. [PubMed] [Google Scholar]
- 17.Mommaas B, van Halteren AG, Pool J, Van d V, Wieles B, Heemskerk MH, Goulmy E. Adult and cord blood T cells can acquire HA-1 specificity through HA-1 T-cell receptor gene transfer. Haematologica. 2005;90:1415–1421. Ref ID: 32643. [PubMed] [Google Scholar]
- 18.Neelapu SS, Munshi NC, Jagannath S, Watson TM, Pennington R, Reynolds C, Barlogie B, Kwak LW. Tumor antigen immunization of sibling stem cell transplant donors in multiple myeloma. Bone Marrow Transplant. 2005;36:315–323. doi: 10.1038/sj.bmt.1705057. Ref ID: 33480. [DOI] [PubMed] [Google Scholar]
- 19.Riddell SR, Bleakley M, Nishida T, Berger C, Warren EH. Adoptive transfer of allogeneic antigen-specific T cells. Biol Blood Marrow Transplant. 2006;12:9–12. doi: 10.1016/j.bbmt.2005.10.025. Ref ID: 29206. [DOI] [PubMed] [Google Scholar]
- 20.Quintarelli C, Vera JF, Savoldo B, Giordano Attianese GM, Pule M, Foster AE, Heslop HE, Rooney CM, Brenner MK, Dotti G. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110:2793–2802. doi: 10.1182/blood-2007-02-072843. Ref ID: 33471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T, Stern M, Pende D, Perruccio K, Burchielli E, Topini F, Bianchi E, Aversa F, Martelli MF, Velardi A. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. doi: 10.1182/blood-2006-07-038687. 9999; prepublished online March 19, 2007; Ref ID: 32294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooley S, McCullar V, Wangen R, Bergemann TL, Spellman S, Weisdorf DJ, Miller JS. KIR reconstitution is altered by T cells in the graft and correlates with clinical outcomes after unrelated donor transplantation. Blood. 2005;106:4370–4376. doi: 10.1182/blood-2005-04-1644. Ref ID: 33472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Grzywacz B, Sukovich D, McCullar V, Cao Q, Lee AB, Blazar BR, Cornfield DN, Miller JS, Verneris MR. The unexpected effect of cyclosporin A on CD56+CD16- and CD56+CD16+ natural killer cell subpopulations. Blood. 2007;110:1530–1539. doi: 10.1182/blood-2006-10-048173. Ref ID: 33476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. Ref ID: 27318. [DOI] [PubMed] [Google Scholar]
- 25.Lundqvist A, McCoy JP, Samsel L, Childs R. Reduction of GVHD and enhanced antitumor effects after adoptive infusion of alloreactive Ly49-mismatched NK cells from MHC-matched donors. Blood. 2007;109:3603–3606. doi: 10.1182/blood-2006-05-024315. Ref ID: 33477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alyea EP, Canning C, Neuberg D, Daley H, Houde H, Giralt S, Champlin R, Atkinson K, Soiffer RJ. CD8+ cell depletion of donor lymphocyte infusions using cd8 monoclonal antibody-coated high-density microparticles (CD8-HDM) after allogeneic hematopoietic stem cell transplantation: a pilot study. Bone Marrow Transplant. 2004;34:123–128. doi: 10.1038/sj.bmt.1704536. Ref ID: 33482. [DOI] [PubMed] [Google Scholar]
- 27.Porter DL, Levine BL, Bunin N, Stadtmauer EA, Luger SM, Goldstein S, Loren A, Phillips J, Nasta S, Perl A, Schuster S, Tsai D, Sohal A, Veloso E, Emerson S, June CH. A phase 1 trial of donor lymphocyte infusions expanded and activated ex vivo via CD3/CD28 costimulation. Blood. 2006;107:1325–1331. doi: 10.1182/blood-2005-08-3373. Ref ID: 32701. [DOI] [PubMed] [Google Scholar]
- 28.Berger C, Flowers ME, Warren EH, Riddell SR. Analysis of transgene-specific immune responses that limit the in vivo persistence of adoptively transferred HSV-TK-modified donor T cells after allogeneic hematopoietic cell transplantation. Blood. 2006;107:2294–2302. doi: 10.1182/blood-2005-08-3503. Ref ID: 29189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Straathof KC, Pule MA, Yotnda P, Dotti G, Vanin EF, Brenner MK, Heslop HE, Spencer DM, Rooney CM. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–4254. doi: 10.1182/blood-2004-11-4564. Ref ID: 33481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W, Buckner CD, Clift R, Doney K, Farewell V, Hansen J, Hill R, Lum L, Martin P, McGuffin R, Sanders J, Stewart P, Sullivan K, Witherspoon R, Yee G, Thomas ED. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N. Engl. J. Med. 1986;314:729–735. doi: 10.1056/NEJM198603203141201. Ref ID: 756. [DOI] [PubMed] [Google Scholar]
- 31.Niederwieser D, Maris M, Shizuru JA, Petersdorf E, Hegenbart U, Sandmaier BM, Maloney DG, Storer B, Lange T, Chauncey T, Deininger M, Pönisch W, Anasetti C, Woolfrey A, Little M-T, Blume KG, McSweeney PA, Storb RF. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101:1620–1629. doi: 10.1182/blood-2002-05-1340. Ref ID: 21841. [DOI] [PubMed] [Google Scholar]
- 32.Zorn E. CD4+CD25+ regulatory T cells in human hematopoietic cell transplantation (Review) Semin. Cancer Biol. 2006;16:150–159. doi: 10.1016/j.semcancer.2005.11.008. Ref ID: 32642. [DOI] [PubMed] [Google Scholar]
- 33.Seidel MG, Ernst U, Printz D, Juergens B, Pichler J, Attarbaschi A, Fritsch G, Gadner H, Heitger A. Expression of the putatively regulatory T-cell marker FOXP3 by CD4(+)CD25+ T cells after pediatric hematopoietic stem cell transplantation. Haematologica. 2006;91:566–569. Ref ID: 33478. [PubMed] [Google Scholar]
- 34.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St. Groth B. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. Ref ID: 30186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun K, Wilkins DE, Anver MR, Sayers TJ, Panoskaltsis-Mortari A, Blazar BR, Welniak LA, Murphy WJ. Differential effects of proteasome inhibition by bortezomib on murine acute graft-versus-host disease (GVHD): delayed administration of bortezomib results in increased GVHD-dependent gastrointestinal toxicity. Blood. 2005;106:3293–3299. doi: 10.1182/blood-2004-11-4526. Ref ID: 32617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vodanovic-Jankovic S, Hari P, Jacobs P, Komorowski R, Drobyski WR. NF-kappaB as a target for the prevention of graft-versus-host disease: comparative efficacy of bortezomib and PS-1145. Blood. 2006;107:827–834. doi: 10.1182/blood-2005-05-1820. Ref ID: 32612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valmori D, Tosello V, Souleimanian NE, Godefroy E, Scotto L, Wang Y, Ayyoub M. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J. Immunol. 2006;177:944–949. doi: 10.4049/jimmunol.177.2.944. Ref ID: 32641. [DOI] [PubMed] [Google Scholar]
- 38.Reddy P, Maeda Y, Hotary K, Liu C, Reznikov LL, Dinarello CA, Ferrara JL. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proc. Natl. Acad. Sci. USA. 2004;101:3921–3926. doi: 10.1073/pnas.0400380101. Ref ID: 26758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. Ref ID: 27463. [DOI] [PubMed] [Google Scholar]
- 40.Ringdén O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, Marschall HU, Dlugosz A, Szakos A, Hassan Z, Omazic B, Aschan J, Barkholt L, Le Blanc K. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. Ref ID: 32656. [DOI] [PubMed] [Google Scholar]
- 41.Min CK, Kim BG, Park G, Cho B, Oh IH. IL-10-transduced bone marrow mesenchymal stem cells can attenuate the severity of acute graft-versus-host disease after experimental allogeneic stem cell transplantation. Bone Marrow Transplant. 2007;39:637–645. doi: 10.1038/sj.bmt.1705644. Ref ID: 32655. [DOI] [PubMed] [Google Scholar]
- 42.Abraham N, Li M, Vanella L, Peterson S, Ikehara S, Asprinio D. Bone marrow stem cell transplant into intra-bone cavity prevent Type 2 diabetes: Role of heme oxygenase and CO. J Autoimmunity. 2007 doi: 10.1016/j.jaut.2007.12.005. In press. [DOI] [PubMed] [Google Scholar]
- 43.Boren E, Cheema G, Naguwa S, Ansari A, Gershwin M. Progressive multifocal leukoencephalopathy (PML) and its emergence in rheumatic diseases. J Autoimmunity. 2007 doi: 10.1016/j.jaut.2007.11.013. In press. [DOI] [PubMed] [Google Scholar]
- 44.Burt R, Craig R, Cohen B, Suffit R, Barr W. Hematopoietic stem cell transplantation for autoimmune diseases: What have we learned? J Autoimmunity. 2007 doi: 10.1016/j.jaut.2007.12.010. In press. [DOI] [PubMed] [Google Scholar]
- 45.Deane S, Meyers F, Gershwin M. On reversing the persistence of memory: Hematopoietic stem cell transplant for autoimmune disease in the first ten years. J Autoimmunity. 2007 doi: 10.1016/j.jaut.2007.12.001. In press. [DOI] [PubMed] [Google Scholar]
- 46.Gershwin M. Bone marrow transplantation, refractory autoimmunity and the contributions of Susumu Ikehara. J Autoimmunity. 2007 doi: 10.1016/j.jaut.2007.12.006. In press. [DOI] [PubMed] [Google Scholar]
- 47.Hara M, Murakami T, Kobayashi E. In vivo bioimaging using photogenic rats: fate of injected bone marrow-derived mesenchymal stromal cells. J Autoimmunity. 2007 doi: 10.1016/j.jaut.2007.12.007. In press. [DOI] [PubMed] [Google Scholar]
- 48.Ikehara S. A novel BMT method for intractable diseases. J Autoimmunity. 2007 In press. [Google Scholar]
- 49.Marmont A. Will hematopoietic stem cell transplantation cure human autoimmune diseases? J Autoimmunity. 2007 doi: 10.1016/j.jaut.2007.12.009. In press. [DOI] [PubMed] [Google Scholar]
- 50.Ozawa K, Sato K, Oh I, Ozaki K, Uchibori R, Obara Y, Kikuchi Y, Ito T, Okada T, Urabe M, Mizukami H, Kume A. Cell and gene therapy using mesenchymal stem cells (MSCs) J Autoimmunity. 2007 doi: 10.1016/j.jaut.2007.12.008. In press. [DOI] [PubMed] [Google Scholar]
- 51.Ratajczak M, Zuba-Surma E, Wysoczynski M, Wan W, Ratajczak J, Kucia M. Hunt for pluripotent stem cell - regenerative medicine search for almighty cell. J Autoimmunity. 2007 doi: 10.1016/j.jaut.2007.12.003. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sonoda Y. Immunophenotype and functional characteristics of human primitive CD34-negative hematopoietic stem cells: The significance of the intra-bone marrow injection. J Autoimmunity. 2007 doi: 10.1016/j.jaut.2007.12.004. In press. [DOI] [PubMed] [Google Scholar]