Abstract
Background and Aims
Epithelial stem cells in the stomach are responsible for constant renewal of the epithelium through generation of multiple gastric cell lineages that populate the gastric glands. However, gastric stem or progenitor cells have not been well-characterized due to the lack of specific markers that permit their prospective recognition. We identified an intestinal promoter that is active in a rare subpopulation of gastric epithelial cells and investigated whether these cells possess multi-lineage potential.
Methods
A marked allele of the endogenous mouse villin locus was used to visualize single β-galactosidase positive cells located in the lower third of antral glands. A 12.4Kb villin promoter/enhancer fragment drives several transgenes (EGFP, β-galactosidase and Cre recombinase) in these cells in a pattern similar to that of the marked villin allele. Reporter gene activity was used to track these cells during development and to examine cell number in the context of inflammatory challenge while Cre activity allowed lineage tracing in vivo.
Results
We demonstrate that these rare epithelial cells are normally quiescent, but multiply in response to interferon gamma. Lineage tracing studies confirm that these cells give rise to all gastric lineages of the antral glands. In the embryo, these cells are basally located in the stomach epithelium before completion of gastric gland morphogenesis.
Conclusions
We have identified a rare subpopulation of gastric progenitors with multilineage potential. The ability to prospectively identify and manipulate such progenitors in situ represents a major step forward in gastric stem cell biology and has potential implications for gastric cancer.
Introduction
The epithelium of the stomach is a continuously renewing tissue that is organized into repeating flask-like tubular extensions called glands. The multiple cell lineages that populate each gland are generated by controlled division of gastric epithelial stem cells located within the gland. Apart from the general importance of the stem cell population in the maintenance and regeneration of the gastric epithelium, it is widely held that the stem or progenitor cell also represents the target for the genetic changes that lead to tumorigenesis (for a review, see 1). This hypothesis has not been directly tested, however, since it has not been previously possible to follow single gastric stem or progenitor cells prospectively.
Despite the lack of specific markers to definitively identify gastric stem cells in situ, some of the characteristics of gastric stem cells have been inferred from elegant morphological and lineage tracing analyses. Labeling studies with thymidine analogs have identified a highly proliferative zone near the isthmus of the gland and electron microscopic studies have revealed immature looking cells in this region (i.e. small cells with prominent nucleoli and lacking secretory granules) 2; these are the presumed stem cells. The dynamics of stem cell division have been inferred retrospectively by following the patterns of X-chromosome inactivation, by tracking mutant clones after chemical mutagenesis, or by studying the pattern of strain-specific marker expression in chimeric or transgenic animals 3–8. Together, these studies revealed that in the antrum of adult mice and humans, the majority of glands are functionally monoclonal; that is, all cellular progeny of the glandular epithelium arise from a single stem cell 6, 7. Mathematical models predict that when murine antral glands are initially established, they contain approximately three stem cells 6. These initially polyclonal glands resolve to a primarily monoclonal state over the first six weeks of life 4, 6, 7, 9, though a few residual polyclonal glands (5–10%) persist in the adult 9.
One force that is believed to promote the resolution of a mixed gland to a monoclonal state is gland fission; the gland bifurcates, producing two daughter glands, each with half of the stem cell census of the original 10. Morphological evidence of stomach gland fission has been reported in the adult 7, 8 and in the neonate 6. The signal that initiates gland fission is unknown, but it has been proposed that intestinal crypts divide in response to a doubling of stem cell number 11, 12. In fact, recent studies reveal that in intestines of mice carrying a conditional PTEN deletion, stem cell numbers are increased and this appears to directly promote crypt budding as well as crypt fission13. It is possible that a similar stem cell-dependent counting mechanism might operate in gastric glands.
In this report, we identify a novel gastric cell population in situ that has properties similar to those of presumptive gastric stem cell populations described above. We call these cells gastric progenitor cells (GPC). We first identified murine GPC because they robustly express a marked allele of the Villin gene (Villinβ-gal/+) generated earlier in our laboratory 14. Villin is an intestinal gene and is not generally expressed in the stomach; GPC thus appear as rare β-gal positive cells in an otherwise β-gal negative adult gastric epithelium. GPC are predominantly found in the antrum, where they are located at or below the isthmus, the region thought to harbor stem cells. Indeed, we use lineage tracing reagents to show that GPC can populate entire antral glands with progeny of multiple gastric cell lineages. BrdU labeling studies suggest that GPC are normally quiescent, and that they have label retaining properties; these are thought to be characteristics of stem or progenitor cells. Administration of IFNγ, a potent pro-inflammtory cytokine, causes GPC amplification. This cytokine-stimulated division of GPC appears to be symmetric (i.e., creating more GPC). Interestingly, the pattern of resultant labeled glands suggests that the increase in GPC number might promote gland fission.
Materials and Methods
Mice
12.4KVil-EGFP transgenic mice were generated in the University of Michigan Transgenic Animal Core. These mice, as well as Villinβ-gal/+ 14, 12.4KVil-LacZ 15, 12.4KVil-Cre 15, Ctox7 transgenic mice 16 Cdx2 transgenic mice17 and ROSA26R mice 18 were maintained in our UCUCA-approved facility. All procedures were done in accord with previously approved guidelines. All lines were continuously maintained on a C57BL/6J background.
Administration of IFNγ
Surgically implanted Alzet microosmotic pumps (Durect Corporation, Cupertio, CA) were loaded with 250U/kg INFγ (R&D systems, MN) in PBS with 1% BSA as previously described 19. Control mice received pumps containing PBS with 1% BSA. Animals were sacrificed at two, eight or twelve weeks after pump implantation. In some experiments, mice were injected intraperitoneally with IFNγ (17 IU/Kg in PBS with 1% BSA) on a daily basis for 7–14 days prior to sacrifice.
Gland isolation and β-gal staining
Stomachs were removed, opened along the greater curvature, washed with PBS and incubated in 30mM EDTA in Hank’s Balanced Salt Solution (HBSS) for 15 minutes at 37°C. The stomach was then pinned to a wax surface and rinsed with HBSS. To loosen glands, excess HBSS was applied and a Pasteur pipette was used to gently aspirate and expel the liquid several times until glands were freed from the underlying stroma. Large mats of connected glands were further separated by trituration. Glands were fixed in 4% paraformaldehyde for 5 minutes before staining with X-gal.
β-gal staining and immunostaining of sections
Methods for tissue preparation, whole mount or section X-gal staining were as previously described 20, 21. Frozen or paraffin sections were used for antibody and/or lectin staining. Lectins used were: Griffonia simplicifolia II and Ulex europaeus agglutinin I (both 1:200, Vector Laboratories). Antibodies used for cell identification were: rabbit anti-H+, K+ ATPase α subunit (1:500, Medical and Biological Laboratories), rabbit anti-β-galactosidase (kind gift of J. Douglas Engel, department of Cell and Developmental Biology, University of Michigan; used at 1:500); mouse anti-BrdU (1:400, DSHB, Iowa); goat anti-serotonin (1:250, diluted in PBS, 0.1% triton, ImmunoStar, Wisconsin); rabbit anti-GFP (1:500, Invitrogen, CA); and chicken anti-GFP (1:500, ABCam, MA). Images were obtained on a Nikon ECLIPSE E800 microscope using a Spot CD camera. Confocal microscopy was done using an Olympus FV-500 Confocal microscope.
BrdU labeling studies
For pulse-labeling studies, 5-bromo-2’-deoxyuridine (BrdU, Sigma) was administered intraperitoneally (100 μg/g body weight) in 0.1 M phosphate buffered saline (PBS) with 1% bovine serum album (BSA, Sigma) one hour prior to sacrifice. For long term labeling, mice were given one injection of BrdU as above, and were then kept on oral administration of BrdU in the drinking water (2.2mg/ml as previously described22) for seven days prior to sacrifice. Some animals were provided with regular tap water for another seven days (chase period). Stomachs were removed, fixed in 4% PFA on ice for 10 min, washed in PBS (pH 7.4), snap frozen in O.C.T. (Electron Microscopy Sciences, FL) and stored at −80°C before use. Air dried sections were washed in PBS, acid denatured in 2N HCL for 15min., neutralized and further washed in PBS before immunostaining.
Results
A marked “intestine-specific” allele is active in rare gastric epithelial cells
Our laboratory previously generated mice that carry a marked villin allele, (Villinβ-gal/+)14. In adult mice, villin (and β-gal in the case of the marked allele) is highly expressed in intestinal cells, while the gastric epithelium is largely devoid of villin. However, careful analysis of Villinβ-gal/+ mice revealed rare β-gal positive cells in the antrum of the stomach (compare Figure 1A and 1B). The cells were located in the bottom one third of the antral glands and were oval or triangular in shape with a large pale nucleus (Figure 1B, inset, Figure 1C). Most glands did not appear to contain β-gal positive cells; when present, they commonly appeared as a single cell per sectioned gland, or rarely as two β-gal cells in a single gland (Figure 1D). To document the number and distribution of these cells, stomachs of three-month old Villinβ-gal/+ mice were opened along the greater curvature and each entire stomach was serially sectioned across the length of the stomach. The number of β-gal positive cells in every section was counted. For three animals, 192, 414 and 292 β-gal positive cells were found, the majority of which were located on the lesser curvature of the stomach, just lateral to the ventral midline. Within individual glands (Figure 1E and 1F), the majority of β-gal positive cells were located in the antrum, at or below the isthmus of the glands. Because the paucity of these cells in the corpus made them difficult to study in sufficient numbers, the remainder of the analysis here is concentrated on the β-gal positive cells found in the antrum.
Figure 1. The villin promoter is active in a rare sub-population cells in the adult gastric antrum.
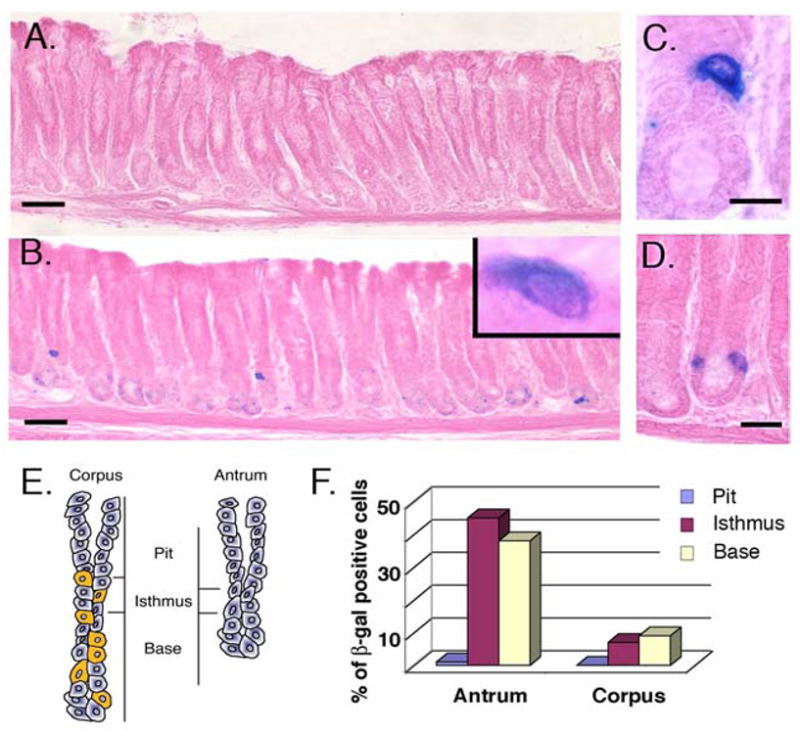
A) In the antrum of a control wild type mouse stained with X-gal, no β-gal positive cells can be detected. B) Widely spaced β-gal positive cells are detected in a section from the antrum of an untreated Villinβ-gal/+ mouse. The inset reveals an oval shaped cell with large nucleus to cytoplasm ratio. C) Cells are located in the lower one third of the gland. D) Rarely, two β-gal positive cells are observed in a single sectioned gland. E) Schematic views of gastric glands from corpus and antrum are shown. Yellow cells indicate parietal cells, diagnostic for the corpus. The regions designated as pit, isthmus and base are shown for each gland. F) The relative distribution of β-gal cells within one entire mouse stomach is plotted as the percent of cells at various positions along the gland axis in corpus and antrum. This distribution was similar in the three control animals examined. In A and B, Bar = 50μm; in B, Bar = 20μm; in C, bar = 10μm.
Behavior of β-gal positive cells in mouse models of metaplasia
The lesser curvature of the antrum is a common site for gastric tumor formation in humans23 as well as in several mouse models of gastric cancer 24–26. Commonly, the pathological cascade for gastric cancer27 begins with chronic inflammation and progresses through parietal cell atrophy, metaplasia (intestinal metaplasia or mucous metaplasia called SPEM, spasmolytic polypeptide-expressing metaplasia10, 28), to dysplasia and finally, tumors. To determine whether the number or location of β-gal positive cells is altered by the metaplasic progression, we examined these cells in the context of three mouse models characterized by metaplasia: a) Cdx2 transgenic mice17; b) Ctox-7 transgenic mice16; and c) IFNγ treated mice19.
Cdx2, a caudal-related homeobox transcription factor, is a master regulator of intestinal differentiation; transgenic mice ectopically expressing Cdx2 in gastric epithelium exhibit widespread intestinal metaplasia of the stomach17. Intestinal metaplasia was documented in the antrum of 3-month old Cdx2 transgenic mice by alkaline phosphatase staining in cells of the upper one third of antral glands (Figure 2A). X-gal staining of adjacent sections showed that the villin β-gal marker was expressed in some surface cells (Figure 2B), likely reflecting activation of the villin promoter in the metaplastic cells. However, the number, location and distribution of β-gal positive cells located deep in the antral glands was similar in Cdx2 transgenic mice and their wild type littermates; deep gland β-gal positive cells were not found in every gland with metaplastic changes. Thus, it seems unlikely that the deep gland β-gal cells are the source of the intestinal metaplasia in this model.
Figure 2. Behavior of β-gal positive cells in mouse models of metaplasia.
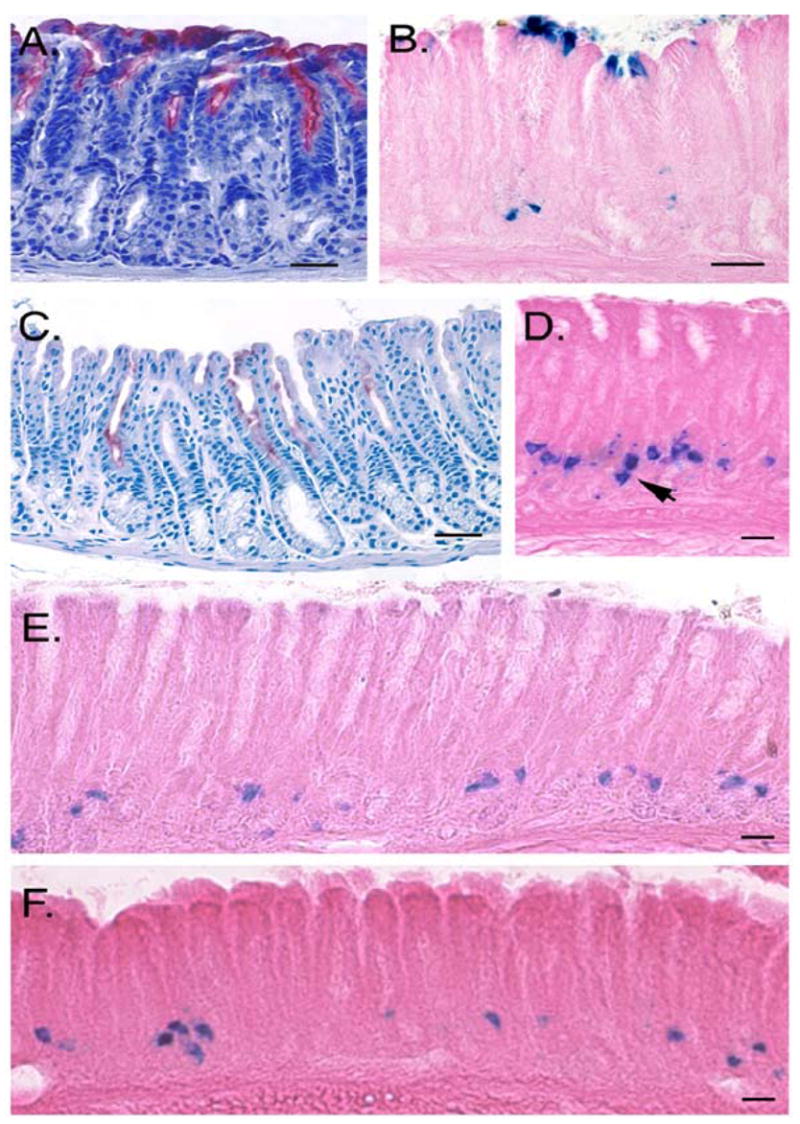
A, B) One year old Villinβ-gal/+/Cdx2 mice. A) Widespread intestinal metaplasia in the antrum is detected by alkaline phosphatase staining (red). B) X-gal staining in the same mouse reveals β-gal positive cells at the surface, within the metaplastic region. The number and location of deep gland β-gal positive cells is similar to wild type littermates. C–E) Six month old Villinβ-gal/+/Ctox-7 mice. C) Alkaline phosphatase staining (red) shows focally positive antral glands. D) The number of β-gal positive cells is greatly expanded. One gland contains two β-gal positive cells distributed along the long axis of the gland (arrow). E) Numerous β-gal positive cells are detectable in the lower region of the antral glands. F) IFNγ treated mice. The number of β-gal positive cells is increased in this model after only two weeks of treatment (compare to Figure 1B). In A–C, Bar = 50 μm; in D–F, Bar = 20μm.
Ctox-7 mice express the cholera toxin A1 subunit under control of the parietal cell- specific H+,K+-ATPase β-subunit promoter. These mice suffer parietal and chief cell loss and develop inflammatory infiltrates with high interferon gamma (IFNγ) expression and extensive mucous cell metaplasia, located primarily in the corpus 16. We recently identified antral tumors in some of these mice (L.C.S., X.T.Q., unpublished data). Ctox-7 mice also exhibit intestinal metaplasia of the corpus (data not shown) as well as focal regions of the antrum (Figure 2C). In contrast to the finding with Cdx2 mice, the population of antral β-gal positive cells was greatly expanded in the Ctox-7 model (compare Figures 2D and 2E to Figure 1B). Quantitation of cells in the entire stomach of these mice revealed 6,797 ± 1,523 cells (mean ± S.D., n = 3), a dramatic increase over the number seen in the wild type stomach (mean = 336, see above). The pattern of labeled cells within the glands was also altered: in wild type mice, glands containing labeled cells were widely spaced, while in Ctox-7 mice, adjacent glands often contained labeled cells (Figure 2D). However, amplified β-gal positive cells in Ctox-7/Villinβ-gal/+ mice remained concentrated on the lesser curvature of the antrum and did not appear to follow the focal distribution of intestinal metaplastia in the antrum.
Finally, surgical implantation of osmotic minipumps that deliver IFNγ into the peritoneum for two weeks produces gastritis, along with an expansion of SPEM-like cells in the corpus of the stomach 19. As in the Ctox-7 model (which also exhibits high IFNγ levels), β-gal positive cells were markedly expanded along the lesser curvature of the antrum in IFNγ-treated animals (Figure 2F). In support of this, more examples of glands with two β-gal positive cells were seen in both the IFNγ and Ctox-7 models (for example, Figure 2D, arrow) as compared to wild type mice. Quantitation of β-gal positive cells by serial sectioning in three IFNγ-infused mice and three PBS-infused littermates revealed 1,431 ± 477 cells and 175 ± 33 cells, respectively (mean ± SD).
While we have not ruled out the possibility that metaplastic cells can arise from β-gal positive cells, this seems unlikely given the different distributions of these two cell types in the three models. However, it appears that IFNγ itself, or some aspect of the pro-inflammatory cascade downstream of this cytokine, is a potent signal for amplification of the β-gal-marked cells. Since inflammation is closely connected to the evolution of gastric cancer, this finding could potentially signify a link between the concentration of β-gal positive cells on the lesser curvature and the frequency of tumors in this location.
Amplification in the number of β-gal positive cells is by cell division
Expansion of β-gal positive cells could result either from an IFNγ-induced mitotic stimulus, or could reflect stress-induced activation of the Villinβ-gal/+ allele in postmitotic cells. To clarify this, we carried out BrdU labeling studies in animals treated with IFNγ. These experiments, (Supplemental Figure 1A–I), reveal that β-gal positive cells are normally quiescent, but that IFNγ induces their mitotic division. Furthermore, β-gal positive cells are among the few label-retaining cells detected in antral gland epithelium (Supplemental Figure 1J–M).
Marked cells are located at variable positions in isolated glands
To better assess the number and location of β-gal positive cells, we isolated whole glands and examined them under a dissecting microscope. Even after IFNγ treatment, many glands lacked β-gal staining cells. In glands with marked cells, most contained one (Figure 3E,I) and a few contained two (Figure 3F–H,J) β-gal positive cells. In one case (Figure 3F), two closely adjoined cells were seen on the long axis of the gland, the apparent products of a recent cell division. Marked cells were located at variable positions in the lower portion of the gland, between the isthmus and the gland tip (Figure 3A–E).
Figure 3. β-gal positive cells are located at or below the isthmus in isolated antral glands.
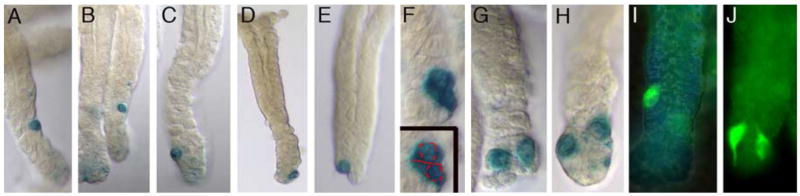
A–E) Note the distribution of β-gal marked cells at variable distances in relation to the gland base. F) Two β-gal positive cells appear to be completing mitosis along the long axis of the gland. G,H) Two glands from INFγ-treated mice; β-gal positive cells are distributed to two opposite sides of the gland tip. I,J) Labeled cells from a 12.4KVil- EGFP mouse. The two labeled cells in the gland in (J) both possess long cytoplasmic processes that are visible with the EGFP label.
In several instances, we observed two β-gal positive cells situated on opposite sides of the base of the gland, (Figure 3G,H,J; also see Figure 1D). Since gland fission has been previously shown to initiate from the base of the antral glands 6, a gland fission event would cleanly partition these cells into two different glands. In the intestine, crypt fission is believed to be a response to increased stem cell number 11, 12 and the rate of fission of colonic crypts is greatly increased in inflammatory states such as Crohn’s disease and ulcerative colitis 29. These observations are intriguing in light of the ability of IFNγ to cause amplification of these β-gal positive cells. A rapid IFNγ-mediated amplification event, followed by gland fission could explain a) the robust increase in β-gal positive cells, b) the finding that most glands still contain only one such cell and c) the clustered distribution of the expanded cell population (Figure 2D–F).
A 12.4Kb villin promoter is active in this rare cell population
We found that a 12.4Kb regulatory fragment from the mouse villin gene can drive two different transgenes (β-gal and EGFP) in this rare population of stomach cells. In both of these transgenic models, the number and distribution of marked cells as well as amplification of marked cells after IFNγ treatment were similar to that seen in the Villinβ-gal/+ model (Supplemental Figure 2). Examination of cells in the 12.4KVil-EGFP model after IFNγ treatment revealed that marked cells frequently display long cytoplasmic processes (Figure 3J). This, along with the variable position of these cells along the gland length, suggests that these cells might actively migrate along the bottom one third of the gland.
The β-gal positive cells have multilineage potential
We used a lineage tracing strategy to determine whether the marked cells have the properties of progenitor cells, i.e., to assess whether they can regenerate antral glands. 12.4KVil-Cre transgenic mice developed in our laboratory 15 were mated to ROSA indicator mice (ROSA26R) 18. The ROSA26R mice contain a floxed “neomycin stop” cassette upstream of a β-galactosidase gene at the ubiquitously expressed ROSA locus. When Cre is activated, the stop cassette is removed, and β-gal is activated by the ROSA promoter. Activation of β-gal only occurs in cells that express Cre, but progeny of those cells will also express β-gal since removal of the stop cassette is inherited. Because antral glands are monoclonal 6, we expect that if these rare 12.4KVil-Cre-expressing cells behave as gland progenitor cells, the entire gland should be β-gal positive.
Osmotic pumps containing PBS or IFNγ were implanted in four month-old 12.4KVil-CreTg/ROSA26R+/− offspring; mice were sacrificed 2, 8 or 12 weeks after pump implantation. At two weeks after pump inplantation, only single β-gal labeled cells were seen, a pattern similar to that seen in the Villinβ-gal/+ and 12.4KVil-LacZ models. This was true for both PBS-treated and IFNγ–treated animals and suggests that during this initial two week period, these cells do not contribute progeny to the gland.
In contrast, at eight and twelve weeks after pump implantation, fully labeled glands were observed in both PBS-treated (Figure 4A) and IFNγ-treated (Figure 5B-Iy) 12.4KVil-CreTg/ROSA26R+/− mice. This pattern of staining suggests that the entire gland was clonally derived from the single cell that activated the 12.4Kb villin promoter driving Cre recombinase. In six PBS-treated mice, only eight examples of entirely stained glands were found. Since the six stomachs should together contain up to 1800 cells that could potentially have given rise to labeled glands (~300 cells per stomach), the fact that only eight fully labeled glands were seen suggests that these cells are very quiescent in the absence of inflammatory stimuli.
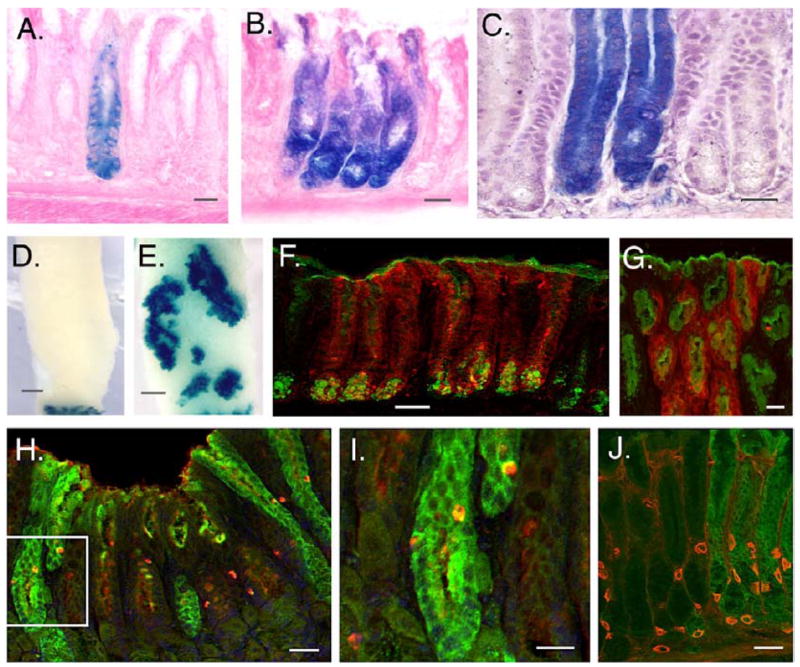
Figure 4. Lineage tracing studies - single β-gal positive cells clonally populate the gland with multiple gastric cell types.
A) Antral gland in a Villinβ-gal/+/ROSA26R control mouse treated with PBS. Few such fully stained glands are seen in control animals. B,C) Fully labeled antral glands from Villinβ−gal/+/ROSA26R mice treated with INFγ. Labelled glands tend to cluster. D,E) Whole-mount β-gal staining of the antrum in Villinβ-gal/+/ROSA26R mice treated with PBS (D) or IFN γ (E) and sacrificed after 12 weeks. The PBS-treated antrum shows no β-gal staining (D). The β-gal positive region at the bottom is duodenum. In an INFγ–treated mouse (E), large patches of contiguous β-gal positive glands are visible on the ventral side of the antrum. F–J) Fully stained glands are expressing gastric markers. For (F), anti-β-gal = red; FITC-conjugated GSII lectin = green (neck/gland cell marker). In (G), anti-β-gal = red; FITC-conjugated UAEI lectin = green (surface pit cell marker). H, I) The section is stained with anti-β-gal (green) and anti-serotonin (red), marking a common subtype of enteroendocrine cells. The image in (I) is a higher magnification of the region that is boxed in (H). J) A section from the junction of the corpus and antrum, co-stained with anti-β-gal (green) and anti-H+,K+ ATPase (red), a parietal cell marker. For D and E, Bar = 100μm; for F, Bar = 50μm; for all other panels, Bar = 20μm.
In IFNγ-treated animals, in contrast, fully labeled glands were observed frequently (Figure 4B–I). Labeled glands were found in small clusters at eight weeks after IFNγ pump placement (Figure 4B, 4C). By twelve weeks after IFNγ pump implantation, these clusters had coalesced into relatively large areas (Figure 4E) on the lesser curvature side of the stomach. This resembles the pattern produced by clonal expansion of mutant colonic crypts in the mouse 30 and the patches of cytochrome c oxidase deficient colonic crypts observed in adult humans 31. Both of these processes are believed to be mediated by repeated cycles of crypt fission.
Within β-gal positive glands, cells of typical gastric cell lineages could be found, including mucous neck cells positive for GSII (Figure 4F), mucous gland cells positive for UEAI (Figure 4G) and enteroendocrine cells positive for serotonin (Figure 4H). In addition, in β-gal staining glands located at the antral/corpus junction, parietal cells positive for H+,K+ ATPase were found (Figure 4I). Thus, the original Cre-positive cells possessed multi-lineage potential. On this basis, we propose that these cells that are prospectively identified by their robust activation of an intestine-specific promoter are gastric progenitor cells (GPC).
Importantly, fully labeled glands such as those seen in Figure 4 were never observed in the Villinβ-gal, 12.4KVil-EGFP or 12.4KVil-LacZ models, even in 18-month old mice and even when such mice were treated with IFNγ. Thus, we surmise that the villin promoter is only active in the GPC itself. That is, the villin promoter is silenced in one daughter cell concomitantly with or relatively soon after division. This division must therefore be asymmetric and the lineage trace model suggests that this division initiates the repopulation of the gland by progeny of the GPC. In contrast, the cell divisions that result in two GPC in the same gland (Figure 1D; Figure 2D; Figure 3F–H,J) may be symmetric; this increase in GPC could potentially be a stimulus for gland fission. A working model that is still speculative, but consistent with all of these data, is presented in Supplemental Figure 3.
GPC are also found at the corpus/forestomach boundary and can be detected in the fetal stomach
β-gal positive cells can also be readily detected at the boundary between the corpus and squamous forestomach (Supplemental Figure 4A,B). This region corresponds to the gastric-esophageal junction of humans, an area that, like the lesser curvature of the antrum, is a common site for tumor development23. Lee et al. have suggested that these mucous glands form a continuous “cephalic band” that separates the zymogenic glands of the corpus from the squamous forestomach32. If these mucous glands are actually continuous with the bulk of the antrum, as suggested in the reconstructions of Lee et al, this might explain the presence of the otherwise antral-predominant GPC in this more anterior location. Whether the β-gal positive cells of the cephalic band also possess progenitor cell characteristics still needs to be investigated.
The fact that GPC can be prospectively identified offers a rare opportunity to examine them during embryonic development. We therefore examined stomachs from Villinβ-gal/+ fetuses at E16.5, a time just prior to the morphogenic rearrangement of the pseudostratified stomach epithelium into antral gland-like structures. At this time, rare, widely separated, small triangular β-gal positive cells are detectable at the base of the epithelium, in contact with the basement membrane (Supplemental Figure 4C). The wide spacing of these cells is similar to the pattern seen in wild type Villinβ-gal/+ adults (see Figure 1B). Perhaps even more intriguing is the fact that in our earlier studies of the Villinβ-gal/+ model, we observed scattered β-gal positive cells on the ventral lip of the anterior intestinal portal of E8.0 embryos 21. During completion of the gut tube, cells from this area will migrate to populate the ventral side of the foregut, an area that will later become the lesser curvature of the stomach 33. Thus, the scattered distribution of GPC on the lesser curvature of the adult stomach may actually reflect their embryonic history.
Discussion
Gastric stem cell biology has been the subject of intense investigation for more than two decades, but progress in this field has been hampered by the inability to prospectively recognize progenitors and stem cells in vivo and the associated necessity to use indirect approaches for characterization of these cells. The identification of a marker for at least a subset of gastric progenitors and the establishment of a means to manipulate these cells using transgenic tools represents an important advance in this difficult field.
The GPC described here can be visualized using a villin allele containing the β-gal cDNA or a 12.4Kb villin regulatory fragment linked to a reporter gene. Paradoxically, the native villin gene is not expressed in this environment. Dissociation between the expression pattern of the Villinβ-gal/+ allele and the native villin gene has been previously documented in the embryonic choroid plexus, the endolymphatic duct and the tail gut 21. As in those tissues, β-gal expression in GPC appears to be due to fortuitous ectopic expression of the modified allele or transgene. Nevertheless, expression is reproducible and the pattern of marker gene expression for the Villinβ-gal/+, 12.4KVil-LacZ, and 12.4KVil-EGFP models appears indistinguishable, making this the first reliable marker for identification and eventual isolation of a progenitor cell population.
The GPC population is most likely not the only stem or progenitor cell type in the antrum; in fact, in the absence of pro-inflammatory signals, GPC are actually quite rare. They are also very quiescent in the unstimulated stomach and thus do not appear to be important for normal gastric epithelial homeostasis. However, considering their dramatic response to inflammation, it is tempting to consider that they could function as sensors of damage, or reservoirs with high proliferative potential that can regenerate portions of the gastric mucosa after injury. It has been suggested previously that separate populations of “actual” and “potential” stem cells may exist in the intestine 34. GPC fit the description for a type of “potential” progenitor population that could be mobilized to undergo symmetric division to amplify stem cell numbers after noxious insult. It would be interesting to test whether targeted depletion of this cell population could modulate the gastric injury response.
The position of GPC seems low in the glandular unit considering that the stem cells are thought to reside in the isthmus. In this regard, a review of classic studies of the renewal of isthmal2 and gland35 cells in the antrum is revealing. After just one day of 3H-thymidine infusion, Lee and Leblond showed that 100% of isthmal cells are labeled, indicating that all of these cells are actively cycling. Moreover, when 12 days of continuous infusion of 3H thymidine was followed by various chase periods (to allow dilution of the label in constantly dividing cells), 100% of isthmus cells were quickly depleted of label, confirming their rapid turnover2. In contrast, 23% of gland cells still retained label after 12 days of chase35. Retention of label is often cited as a stem cell attribute; these classic studies suggest that label-retaining cells are located in the gland region, not the isthmus. The idea that the isthmus is the site of the stem cell has been perpetuated by the finding of rare cells in this region that look “undifferentiated” because they are granule free. Interestingly, a significant number of these granule-free cells are labeled within one hour after a single injection of 3H thymidine2. If these are the true stem cells, they clearly cannot be described as quiescent.
Our BrdU labeling studies indicate that most GPCs, in the absence of IFNγ stimulation, are quiescent. However, after seven days of IFNγ and BrdU administration, approximately 80% of GPC are labeled, a finding consistent with the amplification of β-gal positive cells detected in sections. After seven days of labeling and IFNγ stimulation followed by seven days of chase, approximately 50% of GPCs still contain BrdU label and these are located below the isthmus. Until additional gastric progenitor cell populations are characterized, it is not possible to say with certainty whether a sub-isthmal position in the gland is an unusual characteristic unique to GPCs, or whether the historical assignment of the isthmus as the home of the stem cell is in fact incorrect.
The data presented here suggest that GPC undergo both symmetric division (to increase the number of GPC) and asymmetric division (to repopulate the glands). Confirmation of these different types of divisions must await identification of conditions that allow clonal culture of these cells. Here, we infer these types of divisions from the behavior of GPC in the various models tested. Asymmetric division is only visible in the lineage tracing experiments (12.4KVil-Cre X ROSA26R) and is “invisible” in the Villinβ-gal/+, 12.4KVil-LacZ and 12.4KVil-EGFP models since the marker is apparently silenced in one of the daughter cells soon after division. Both types of GPC division are rare in PBS-treated control mice and both types are stimulated by IFNγ treatment. The symmetric divisions that increase GPC number predominate in the first two weeks after IFNγ treatment, since all models, including the lineage tracing model, display amplification of single labeled cells during this time. The fact that robust amplification of GPC occurs within a two-week period and the observation that the majority of glands still contain only one GPC is suggestive of a period of rapid gland fission. We speculate that fission may be a direct consequence of the increase in GPC number stimulated by symmetric division. If so, these data also predict that once a fission event begins, the resolution phase at the level of the gland is quite rapid. An earlier study detailing the quantitation of fission intermediates in the antrum by morphological parameters led to the same conclusion, as fissioning intermediates were detected only rarely 2.
Our further observation that recently divided GPC may migrate to the gland base and there segregate to opposite sides of the gland suggests a strategy by which a progenitor-based trigger for gland fission could be closely tied to both a progenitor counting mechanism and a potential means to insure the even sorting of the two progenitors to the two new glands. In the intestine, PTEN phosphatase, a negative regulator of the phosphatidylinositol-3 kinase (PI3K)-Akt pathway, appears to control the number as well as the mobility of stem cells13. In PTEN deficient mice, stem cells increase in number and become mobile; the supernumerary, mislocalized stem cells apparently initiate crypt budding (from the side of the crypt) and crypt fission (from the base) since they are found at the initiation site of both of these events. It will be interesting to examine whether changes in the PTEN/Akt pathway accompany the IFNγ induced alterations in GPC number and location.
Following the period of GPC amplification, at some point between two and eight weeks after IFNγ treatment, the rate of asymmetric division of GPC must increase; multiple entire glands are replaced with GPC progeny during this six-week period. This is a relatively fast gland regeneration time, considering that long-lived gastric progenitors have been identified in gastric glands that may persist for hundreds of days 8. The rapidity of regeneration may indicate that GPC-dominated glands possess properties that differ from typical gastric glands. The fact that gland repopulation follows on the heels of a period of symmetric GPC division and potential gland fission could account for the striking clustered appearance of regenerated glands.
The study of gastric stem and progenitor cells is also directly relevant to gastric cancer. Recently, the Wang laboratory demonstrated that bone marrow-derived stem cells can engraft into gastric epithelium in the corpus of the stomach and contribute to tumor formation. Both irradiation injury and chronic Helicobacter infection were required for engraftment 36. In contrast, GPC are detectable in normal stomachs, predominantly in the antrum, where they respond robustly and quickly to acute administration of IFNγ. It is possible that GPCs are important in initial antral responses to inflammatory signals while bone marrow stem cell engraftment and transdifferentiation represent a response to chronic injury of the corpus. Both of these interesting cell populations have the potential to be a cellular source for gastric tumors.
The predominant positioning of GPC on the lesser curvature of the antrum and at the squamo-columnar junction is intriguing since these regions are common sites of tumor formation23, 27. This positioning suggests that GPC could play some role in the progression of gastric tumor formation, a process that is highly linked to chronic inflammation and to metaplastic changes. Our data suggest that GPC may not give rise directly to metaplastic lesions, but they do respond dramatically to the pro-inflammatory cytokine, IFNγ. Progenitor subpopulations in several other tissues have also been shown to proliferate in response to IFNγ. In the pancreas of transgenic mice that express IFNγ from the insulin promoter, ductular epithelial progenitors proliferate and undergo islet neogenesis; this response appears to be Bmp-dependent 37. Cerebellar granular neurons also divide in response to IFNγ 38–40. In that case, over-expression of IFNγ provokes the release of Sonic hedgehog (Shh), which acts as a powerful autocrine mitogenic stimulus; as a result, medulloblastomas are formed 38. Whether IFNγ alters Bmp and/or Shh pathway signaling in or around GPC is not yet determined, but the fact that some gastric cancers express high levels of hedgehog ligand is a compelling reason to further investigate this possibility 41.
In summary, the data presented here suggest that GPC comprise a subpopulation of resident gastric stem or progenitor cells. They are rare, quiescent cells that are located in putative stem cell niches in adult and fetal life, and they exhibit multilineage potential. Importantly, this multilineage capability is here demonstrated in vivo, rather than after manipulation in culture. This provides the first proof in situ of the Unitarian Theory proposed by Cheng and Leblond 42, that all epithelial cells of a single gland/crypt are clonal descendants of a single stem cell, holds true in the antrum. The ability to manipulate gene expression in these cells using the villin promoter will allow further analysis of their contribution to the homeostatic maintenance of stomach glands, exploration of the components that define their niche, isolation of GPC and characterization of their transcriptome as well as future investigation of the potential role of GPC in gastric tumorigenesis.
Supplementary Material
Acknowledgments
We thank Dr. Doug Engel (Department of Cell and Developmental Biology, University of Michigan) for the β-galactosidase antibody and Dr. Debra Silberg (University of Pennsylvania) for sharing the Cdx2 transgenic mice. All authors are grateful to the University of Michigan Transgenic Animal Core and the Center for Organogenesis Morphology Core for expert assistance. We are grateful for helpful manuscript pre-review from University of Michigan colleagues, Drs. Sean Morrison, Sally Camper, Doug Engel and Max Wicha.
Grant Support: The work was accomplished with support from the National Institutes of Health (P01 DK062041 and R21 CA124589) and from a Munn Idea Grant from the University of Michigan Comprehensive Cancer Center.
Footnotes
There are no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005;128:1567–78. doi: 10.1053/j.gastro.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 2.Lee ER, Leblond CP. Dynamic histology of the antral epithelium in the mouse stomach: II. Ultrastructure and renewal of isthmal cells. Am J Anat. 1985;172:205–24. doi: 10.1002/aja.1001720304. [DOI] [PubMed] [Google Scholar]
- 3.Lorenz RG, Gordon JI. Use of transgenic mice to study regulation of gene expression in the parietal cell lineage of gastric units. J Biol Chem. 1993;268:26559–70. [PubMed] [Google Scholar]
- 4.Tatematsu M, Fukami H, Yamamoto M, Nakanishi H, Masui T, Kusakabe N, Sakakura T. Clonal analysis of glandular stomach carcinogenesis in C3H/HeN<==>BALB/c chimeric mice treated with N-methyl-N-nitrosourea. Cancer Lett. 1994;83:37–42. doi: 10.1016/0304-3835(94)90296-8. [DOI] [PubMed] [Google Scholar]
- 5.Thompson M, Fleming KA, Evans DJ, Fundele R, Surani MA, Wright NA. Gastric endocrine cells share a clonal origin with other gut cell lineages. Development. 1990;110:477–81. doi: 10.1242/dev.110.2.477. [DOI] [PubMed] [Google Scholar]
- 6.Nomura S, Esumi H, Job C, Tan SS. Lineage and clonal development of gastric glands. Dev Biol. 1998;204:124–35. doi: 10.1006/dbio.1998.9055. [DOI] [PubMed] [Google Scholar]
- 7.Nomura S, Kaminishi M, Sugiyama K, Oohara T, Esumi H. Clonal analysis of isolated single fundic and pyloric gland of stomach using X-linked polymorphism. Biochem Biophys Res Commun. 1996;226:385–90. doi: 10.1006/bbrc.1996.1365. [DOI] [PubMed] [Google Scholar]
- 8.Bjerknes M, Cheng H. Multipotential stem cells in adult mouse gastric epithelium. Am J Physiol Gastrointest Liver Physiol. 2002;283:G767–77. doi: 10.1152/ajpgi.00415.2001. [DOI] [PubMed] [Google Scholar]
- 9.Nomura S, Kaminishi M, Sugiyama K, Oohara T, Esumi H. Clonal analysis of isolated intestinal metaplastic glands of stomach using X linked polymorphism. Gut. 1998;42:663–8. doi: 10.1136/gut.42.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hattori T, Fjuita S. Fractographic study on the growth and multiplication of the gastric gland of the hamster. The gland division cycle Cell Tissue Res. 1974;153:145–9. doi: 10.1007/BF00226603. [DOI] [PubMed] [Google Scholar]
- 11.Loeffler M, Birke A, Winton D, Potten C. Somatic mutation, monoclonality and stochastic models of stem cell organization in the intestinal crypt. J Theor Biol. 1993;160:471–91. doi: 10.1006/jtbi.1993.1031. [DOI] [PubMed] [Google Scholar]
- 12.Loeffler M, Bratke T, Paulus U, Li YQ, Potten CS. Clonality and life cycles of intestinal crypts explained by a state dependent stochastic model of epithelial stem cell organization. J Theor Biol. 1997;186:41–54. doi: 10.1006/jtbi.1996.0340. [DOI] [PubMed] [Google Scholar]
- 13.He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T, Wiedemann LM, Barrett TA, Hood L, Wu H, Li L. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189–98. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinson KI, Dunbar L, Samuelson L, Gumucio DL. Targeted disruption of the mouse villin gene does not impair the morphogenesis of microvilli. Dev Dyn. 1998;211:109–21. doi: 10.1002/(SICI)1097-0177(199801)211:1<109::AID-AJA10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–83. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Diaz L, Hinkle KL, Jain RN, Zavros Y, Brunkan CS, Keeley T, Eaton KA, Merchant JL, Chew CS, Samuelson LC. Parietal Cell Hyperstimulation and Autoimmune Gastritis in Cholera Toxin Transgenic Mice. Am J Physiol Gastrointest Liver Physiol. 2006 doi: 10.1152/ajpgi.00461.2005. [DOI] [PubMed] [Google Scholar]
- 17.Silberg DG, Sullivan J, Kang E, Swain GP, Moffett J, Sund NJ, Sackett SD, Kaestner KH. Cdx2 ectopic expression induces gastric intestinal metaplasia in transgenic mice. Gastroenterology. 2002;122:689–96. doi: 10.1053/gast.2002.31902. [DOI] [PubMed] [Google Scholar]
- 18.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 19.Kang W, Rathinavelu S, Samuelson LC, Merchant JL. Interferon gamma induction of gastric mucous neck cell hypertrophy. Lab Invest. 2005;85:702–15. doi: 10.1038/labinvest.3700260. [DOI] [PubMed] [Google Scholar]
- 20.Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132:279–89. doi: 10.1242/dev.01576. [DOI] [PubMed] [Google Scholar]
- 21.Braunstein EM, Qiao XT, Madison B, Pinson K, Dunbar L, Gumucio DL. Villin: A marker for development of the epithelial pyloric border. Dev Dyn. 2002;224:90–102. doi: 10.1002/dvdy.10091. [DOI] [PubMed] [Google Scholar]
- 22.Santoso A, Kaiser A, Winter Y. Individually dosed oral drug administration to socially-living transponder-tagged mice by a water dispenser under RFID control. J Neurosci Methods. 2006;153:208–13. doi: 10.1016/j.jneumeth.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 23.Odze RD. Unraveling the mystery of the gastroesophageal junction: a pathologist's perspective. Am J Gastroenterol. 2005;100:1853–67. doi: 10.1111/j.1572-0241.2005.50096.x. [DOI] [PubMed] [Google Scholar]
- 24.Zavros Y, Eaton KA, Kang W, Rathinavelu S, Katukuri V, Kao JY, Samuelson LC, Merchant JL. Chronic gastritis in the hypochlorhydric gastrin-deficient mouse progresses to adenocarcinoma. Oncogene. 2005;24:2354–66. doi: 10.1038/sj.onc.1208407. [DOI] [PubMed] [Google Scholar]
- 25.Judd LM, Alderman BM, Howlett M, Shulkes A, Dow C, Moverley J, Grail D, Jenkins BJ, Ernst M, Giraud AS. Gastric cancer development in mice lacking the SHP2 binding site on the IL-6 family co-receptor gp130. Gastroenterology. 2004;126:196–207. doi: 10.1053/j.gastro.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 26.Cai X, Carlson J, Stoicov C, Li H, Wang TC, Houghton J. Helicobacter felis eradication restores normal architecture and inhibits gastric cancer progression in C57BL/6 mice. Gastroenterology. 2005;128:1937–52. doi: 10.1053/j.gastro.2005.02.066. [DOI] [PubMed] [Google Scholar]
- 27.Correa P. Helicobacter pylori and gastric cancer: state of the art. Cancer Epidemiol Biomarkers Prev. 1996;5:477–481. [PubMed] [Google Scholar]
- 28.Schmidt PH, Lee JR, Joshi V, Playford RJ, Poulsom R, Wright NA, Goldenring JR. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79:639–46. [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng H, Bjerknes M, Amar J, Gardiner G. Crypt production in normal and diseased human colonic epithelium. Anat Rec. 1986;216:44–8. doi: 10.1002/ar.1092160108. [DOI] [PubMed] [Google Scholar]
- 30.Park HS, Goodlad RA, Wright NA. Crypt fission in the small intestine and colon. A mechanism for the emergence of G6PD locus-mutated crypts after treatment with mutagens. Am J Pathol. 1995;147:1416–27. [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald SA, Preston SL, Greaves LC, Leedham SJ, Lovell MA, Jankowski JA, Turnbull DM, Wright NA. Clonal expansion in the human gut: mitochondrial DNA mutations show us the way. Cell Cycle. 2006;5:808–11. doi: 10.4161/cc.5.8.2641. [DOI] [PubMed] [Google Scholar]
- 32.Lee ER, Trasler J, Dwivedi S, Leblond CP. Division of the mouse gastric mucosa into zymogenic and mucous regions on the basis of gland features. Am J Anat. 1982;164:187–207. doi: 10.1002/aja.1001640302. [DOI] [PubMed] [Google Scholar]
- 33.Tremblay KD, Zaret KS. Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Dev Biol. 2005;280:87–99. doi: 10.1016/j.ydbio.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt Development. 1990;110:1001–20. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 35.Lee ER, Leblond CP. Dynamic histology of the antral epithelium in the mouse stomach: IV. Ultrastructure and renewal of gland cells. Am J Anat. 1985;172:241–59. doi: 10.1002/aja.1001720306. [DOI] [PubMed] [Google Scholar]
- 36.Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–71. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 37.Gu D, Sarvetnick N. Epithelial cell proliferation and islet neogenesis in IFN-g transgenic mice. Development. 1993;118:33–46. doi: 10.1242/dev.118.1.33. [DOI] [PubMed] [Google Scholar]
- 38.Lin W, Kemper A, McCarthy KD, Pytel P, Wang JP, Campbell IL, Utset MF, Popko B. Interferon-gamma induced medulloblastoma in the developing cerebellum. J Neurosci. 2004;24:10074–83. doi: 10.1523/JNEUROSCI.2604-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Lin W, Popko B, Campbell IL. Inducible production of interferon-gamma in the developing brain causes cerebellar dysplasia with activation of the Sonic hedgehog pathway. Mol Cell Neurosci. 2004;27:489–96. doi: 10.1016/j.mcn.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Pham-Mitchell N, Schindler C, Campbell IL. Dysregulated Sonic hedgehog signaling and medulloblastoma consequent to IFN-alpha-stimulated STAT2-independent production of IFN-gamma in the brain. J Clin Invest. 2003;112:535–43. doi: 10.1172/JCI18637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, Beachy PA. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–51. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 42.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–61. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.