Summary
Background
Constraint-Induced Movement therapy (CIMT) uses a variety of treatment components, including restricted use of the better upper extremity, to promote increased use of the contralesional limb for many hours each weekday over two consecutive weeks. The EXCITE Trial demonstrated the efficacy of this intervention for patients 3-9 months post-stroke who were followed for the next 12 months. We assessed the retention of improvements through 24 months.
Method
Measurements were made every four months for impaired upper extremity function (Wolf Motor Function Test - WMFT and Motor Activity Log - MAL) and health related quality of life (Stroke Impact Scale - SIS) amongst 106/222 participants randomized into one arm of the EXCITE Trial in which they received CIMT rather than usual and customary care.
Findings
There was no observed regression from the treatment effects observed at 12 months after treatment during the next 12 months for the primary outcome measures of WMFT and MAL. In fact, the additional changes were in the direction of increased therapeutic effect. For the strength components of the WMFT the changes were significant (P < .05) Secondary outcome variables, including the SIS, exhibited a similar pattern.
Interpretation
Mild to moderately impaired patients who are 3-9 months post-stroke demonstrate substantial improvement in functional use of the paretic upper extremity and quality of life 2 years after receiving a 2-week CIMT intervention. Thus this intervention has persistent benefits.
Introduction
Constraint Induced Movement Therapy (CIMT) requires patients with stroke to undergo functionally relevant repetitive task practice with the paretic limb that includes shaping procedures for up to 6 hours each week day while the less affected wrist and hand are restrained during most waking hours.1, 2 This signature form of CIMT differs from “forced use”3, 4 in which the patient must use the impaired limb during restraint of the better limb, but without formalized training, and from modified CIMT5 representing a distributed form of practice with the patient wearing a restraint for 5 hours each week day for 10 weeks combined with periodic rehabilitation sessions.
CIMT has been applied to patients immediately after stroke6 and among chronic patients1, 7-13 with considerable success, provided that these patients demonstrated an ability to initiate extension movements at the wrist and fingers.11, 14 The Extremity Constraint Induced Movement Therapy Evaluation (EXCITE) demonstrated that CIMT administered 3-9 months post-stroke, resulted in statistically significant and clinically relevant improvement in upper extremity function during the first year compared to those achieved by participants undergoing usual and customary care.15 This study is the first randomized clinical trial to examine retention and improvements for the 24 month period following CIMT therapy in a subacute sample.
Methods
Study participants and procedures
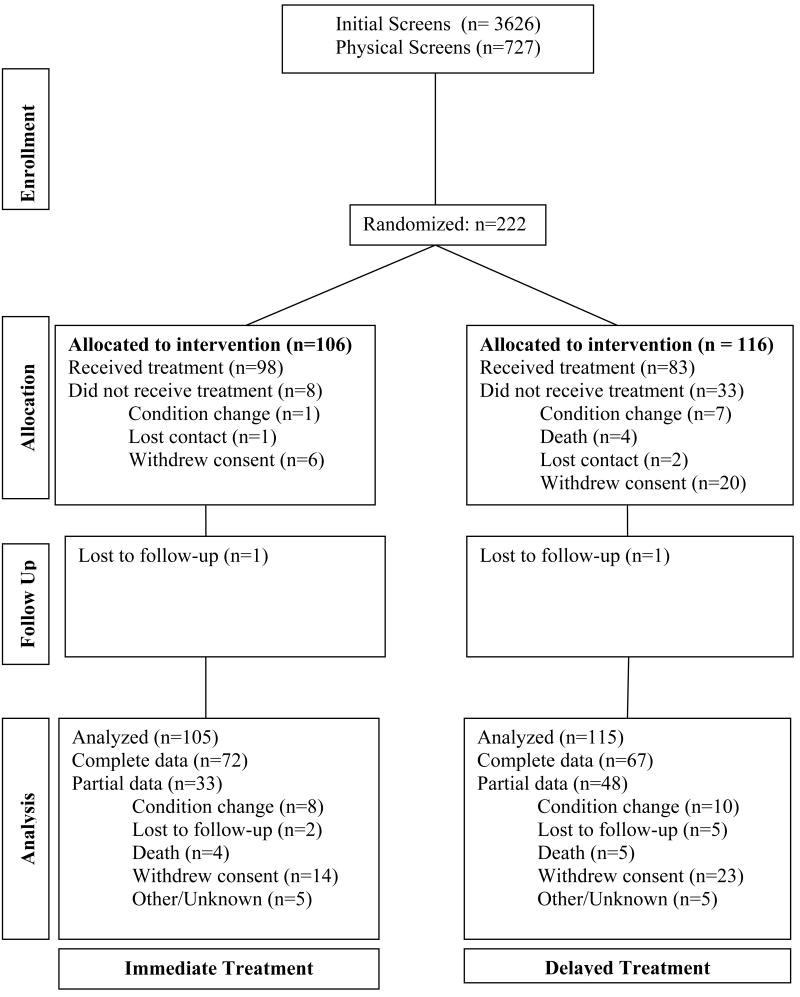
Methodological details pertaining to the multisite EXCITE Trial have been published previously.16 Briefly, seven sites participated in this study. At each site, participants first underwent telephone based initial screens (Figure 1) to assess eligibility. On-site evaluations of 727 prospective participants allowed determination of whether they met higher or lower functioning status based upon established criteria.14,17 Higher functioning (HF) patients were required to actively extend the wrist at least 20° and the metacarpophalangeal and interphalangeal joints of each digit by at least 10° starting at a resting position with the wrist hanging over the edge of a table. Lower functioning (LF) participants were required to demonstrate at least 10° of active wrist extension, at least 10° of thumb abduction/extension, and at least 10° of extension in at least two additional digits. These movements had to be repeated 3 times within 1 minute.17 Participants were required to demonstrate adequate balance while wearing the restraint and during transfers to and from the toilet, and stand for 2 minutes, without additional support. Patients were also assessed using other functional and neuromuscular measures, which, along with exclusion and inclusion criteria are detailed elsewhere.16 Potential participants were also required to score at least a 24 on the Mini-Mental State Examination18 and have medical clearance.
Figure1.
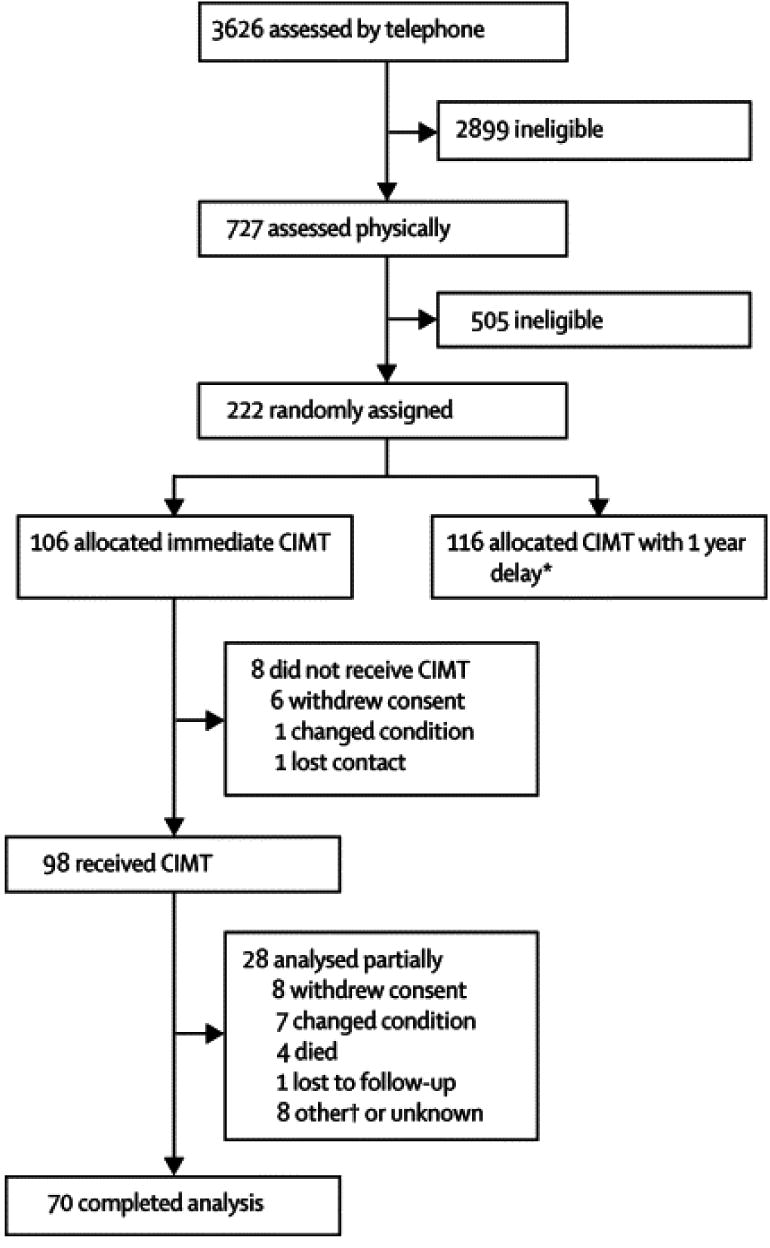
Consort diagram showing enrollment, allocation, follow-up and analyses for EXCITE Trial participants. Note that the data presented for this paper address only those participants in the immediate group (left column).
Study Design
The EXCITE Trial used a single masked cross-over design, with participants undergoing adaptive randomization to balance gender, prestroke dominant side, side of stroke, and level of paretic arm function across sites. Following baseline assessment, participants were randomized into groups receiving CIMT or usual and customary care. Usual and customary care was quite varied, ranging from minimal (no treatment) to varying amounts (application of orthotics; home and clinic-based occupational therapy; physiotherapy), but always exclusive of any CIMT. Provision of usual and customary care treatment options was tracked on a regular basis through periodic phone calls. Following a baseline evaluation, CIMT was delivered up to 6 hours per day, 5 days per week for 2 weeks. Subsequent evaluations were made after the two week period, and at 4, 8, and 12 months. Because the control group was crossed over to receive CIMT after one year, data from additional evaluations were gathered only with the participants receiving CIMT first at 12.5, 16, 20 and 24 months. While the primary outcome measures (see below) were assessed at each of these time intervals, the Stroke Impact Scale (SIS) was administered only at baseline, 4, 12, 16 and 24 month evaluations.
During CIMT, participants wore a padded, protective mitt that covered their less impaired wrist and hand, both in and out of the laboratory, thus preventing use of that limb to manipulate objects in the environment, while allowing voluntary or protective limb responses to unexpected loss of balance or simple support. The mitt was to be worn for 90% of waking hours for the two weeks of CIMT. During that time, patients underwent adaptive task practice (arm training based upon shaping principles19) or repetitive practice of a specific task, such as grooming or eating, performed continuously for 15-20 minutes. Behavioral techniques to promote adherence to mitt use and to assure safety while way from the research laboratory included provision of a participant behavioral contract, caregiver behavioral contract, monitoring compliance using a mitt-based compliance device, and homework assignments (for details see20).
Primary outcome measures
The Wolf Motor Function Test (WMFT) is a laboratory-based measure of upper extremity motor function, and consists of 15 timed movement tasks and 2 strength-based tasks. Improvements mean less time to complete tasks (or greater strength in the two force tasks). The timed tasks are arranged progressively to engage more upper extremity joints. The strength tasks involve upper extremities (raising a weight strapped on the arm to a box [WMFT-WTB]; hand dynamometer for grip strength [WMFT-GS]). The WMFT was administered at all 9 clinical assessment points. Each WMFT task is also assessed by masked raters from video records on a 6–point Functional Ability Scale (FAS) for quality of movement before and after the intervention and twice at two week intervals after one year. Summary measures for statistical analysis include the mean of timed tasks (WMFT-T), the number of items with times exceeding 120 seconds (WMFT-GT120) indicating that the task could not be completed, mean of the FAS (WMFT-FAS), WMFT-WTB, and WMFT-GS. Clinimetric properties of the WMFT have been determined21, 22 as have their attributes for the EXCITE Trial.23
The Motor Activity Log (MAL) is a structured participant interview assessing Amount of Use (AOU) and How Well (HW) participants perform 30 activities of daily living using the paretic arm outside the laboratory. Assessment is done on a 6-point scale. The MAL was administered to participants and available caregivers independently. Participants were evaluated at all 9 clinical assessment points. Among EXCITE participants, the MAL has shown reliability and convergent validity (r = 0.68)24 with the Hand Function domain of the Stroke Impact Scale (SIS)25 and with an accelerometer-based measure of arm activity outside the laboratory (r = .52).26 Summary measures for statistical analysis were the mean for both the AOU and HW components (MAL-AOU, MAL-HW).
Secondary outcome measures
The SIS, a full-spectrum health status and quality of life assessment, was administered at baseline and at months 4, 12, 16 and 24. This interview instrument measures changes in 8 impairment, function, and quality of life domains following stroke, plus an overall measure of physical function.25 Of the 9 domains, results for 4 (Domains 1, 2, 5, 8 and physical function) are presented in tables. Other secondary outcome measures include the MAL caregiver mean (MAL-C-AOU, MAL-C-HW) and ratio of number of items out of 30 with ratings greater than 3, indicating independent use of the limb and limb use exceeding the 50 % pre-stroke level (MAL-AOU-GT3, MAL-HW-GT3). Although analyses for these variables were performed, they are not presented since they are entirely consistent with MAL-AOU and MAL-HW results (results are available upon request).
Moderator factors
Key moderators were examined to determine the extent to which they mediated outcome measures over time. These variables included functional level, gender, and concordance of stroke. Functional level is defined above in Patients and Participants. Concordance of stroke is defined as either concordant (dominant hand is also stroke-affected hand) or discordant (dominant hand is not stroke-affected).
Statistical analysis
This paper examined data from participants randomized within 3-7 days of enrollment to receive CIMT immediately and followed their outcomes through 24 months to determine the extent to which improvements in primary outcome measures one year after CIMT 15 were retained through the 24 month endpoint. Mixed-model methods using PROC MIXED (SAS Institute, Cary, North Carolina, USA, 2006) were undertaken for the repeated measures analysis of values over time, using planned contrasts to address specific questions. Dependencies between values measured at different times were modeled with an AR(1) covariance structure. Means are least-square means from PROC MIXED. The long-term impact of training was evaluated using a multiple degrees of freedom (df) contrast comparing the pre-CIMT score (Baseline) against the mean of all post-CIMT scores (Mean Post-Training), which defines “improvement”. Significance of the difference is done by repeated measures F test. Directionality of the difference, if significant, will be indicated by looking at relevant columns in Table 1. To determine “retention”, an estimate of the difference between the M12 (Month 12) and M24 (Month 24) values was computed from the repeated measures mixed model ANOVA. Retention means that either no change (maintaining the improvement post-training) or a significant further improvement between M12 and M24 occurred. A decrease in function from M12 to M24 meant there was no retention. A 95 % CI for the mean of all post-training time points was also included to further clarify plausible values. To examine the effects of the three moderating factors, the “improvement” and “retention” tests were repeated for each level of the moderator variable (simple main effect analysis). WMFT-T was analyzed both untransformed and with a log transformation to reduce skewness. All analyses used significance level α = .05. To clearly convey uncertainty, 95 % confidence intervals were included on tables as appropriate.
Table 1.
Overall results, including means and tests
| Least-squares Means (Single Time Points) | Improvement: Baseline v All Post-Training | Retention: M12 - M24 | Post-Training Mean | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | Pre-CIMT | Post-CIMT | Month-12 | Month-24 | F value | p > F | Mean (95 % CI) | Mean (95 % CI) |
| WMFT Sample Size | 105 | 98 | 80 | 68 | ||||
| WMFT Mean Performance Time (log) | 2.66 | 2.09 | 2.02 | 1.94 | 69.54 | <.0001 | 0.07(-0.11,0.26) | 2.03(1.86,2.21) |
| Mean Performance Time, sec | 22.72 | 12.59 | 13.57 | 13.88 | 44.43 | <.0001 | -0.32(-3.70,3.06) | 13.57(10.15,16.99) |
| WMFT-WTB, lbs | 5.12 | 6.98 | 8.91 | 10.30 | 42.57 | <.0001 | -1.39(-2.74,-0.04) | 8.60(7.50,9.70) |
| WMFT–GS, kg | 8.86 | 10.71 | 14.37 | 18.75 | 37.57 | <.0001 | -4.39(-6.91,-1.86) | 14.63(12.99,16.28) |
| Functional Ability Sample Size | 103 | 98 | 80 | 0 | ||||
| Functional Ability, 0-5 scale | 2.55 | 2.84 | 2.89 | 101.11 | <.0001 | 2.88(2.78,2.98) | ||
| MAL Sample Size | 105 | 98 | 80 | 70 | ||||
| MAL AOU, 0-5 scale | 1.37 | 2.53 | 2.40 | 2.57 | 177.46 | <.0001 | -0.17(-0.38,0.04) | 2.49(2.30,2.68) |
| MAL QOL, 0-5 scale | 1.46 | 2.50 | 2.48 | 2.62 | 174.49 | <.0001 | -0.14(-0.34,0.06) | 2.53(2.35,2.71) |
| SIS Sample Size | 105 | 88 | 78 | 65 | ||||
| SIS1 Strength | 54.02 | 57.55 | 56.57 | 62.27 | 9.69 | 0.0020 | -5.71(-9.72,-1.70) | 58.78(56.37,61.19) |
| SIS2 Memory-Thinking | 82.83 | 83.88 | 83.59 | 87.54 | 3.27 | 0.0716 | -3.95(-7.43,-0.47) | 85.34(82.70,87.99) |
| SIS5ADL/IADL | 63.52 | 69.76 | 71.20 | 76.06 | 37.88 | <.0001 | -4.87(-8.70,-1.03) | 72.86(70.10,75.63) |
| SIS8 Social Participation | 49.62 | 63.30 | 63.05 | 72.12 | 66.56 | <.0001 | -9.08(-14.41,-3.74) | 66.02(63.01,69.04) |
| SIS Physical Domain | 59.39 | 65.79 | 67.09 | 70.75 | 52.09 | <.0001 | -3.66(-6.67,-0.65) | 68.22(65.69,70.74) |
Abbreviations:
Log Performance Time: Log mean time to complete the WMFT tasks
WMFT-T: Mean time to complete the WMFT tasks
WMFT-WTB: weight to box task for shoulder flexion strength
WMFT-GS: WMFT grip strength
MAL AOU: MAL amount of use for 30 tasks
MAL HW: MAL how well limb used for 30 tasks
Role of funding sources
The granting agency for this study had no role in study design, data collection, analyses or interpretation, or in the writing of this report. The corresponding author had full access to all the data in this study as well as responsibility for the decision to submit for publication.
Results
The consort diagram (Figure 1) shows patient flow through the two years of the EXCITE program for those individuals receiving CIMT immediately and one year after enrollment (delayed) and reasons for withdrawal from the trial. This paper addresses only one arm of the EXCITE trial, immediate participants of whom 21.7% had dropped out at one year and 33.9% at trial's end (24 months). These rates were 15.1% and 23.6% for 12 and 24 months, respectively if drop outs caused by death or deteriorating medical status are not included.
Table 1 shows least-square means for individual time points (Pre-CIMT, Post-CIMT, Month 12, Month 24). It also presents results for “Improvement” and “Retention”. Finally, the mean for all post-training points, as well as a 95 % confidence bound around this value are shown. The tests of “improvement” indicate that, in all cases except for SIS Memory-Thinking, improvement occurs as a result of CIMT training. The tests for “Retention” are presented as 95 % CI in Table 1. For the WMFT time values and the MAL items, the treatment effect observed at 12 months was not eroded; moreover, the average change was in the direction of an additional treatment effect. For the Weight to Box (WMFT-WTB) and Grip Strength (WMFT-GS) tasks, the difference is significant. For the SIS domains, all 5 of the presented scales show continued improvement. Thus, “retention” is shown for all terms. The final column in Table 1 is the mean of all post-training values, with associated 95 % CI.
Impact of Moderating Factors
In general, results for each of the moderating factors in Table 2 were similar to those of the entire group. In most cases, “Improvement” is significant while “Retention” is not significant (thus supporting retention). Differences are detailed below.
Table 2.
Tests of significance Broken Down by Functional Level, Concordance of Affected Side with Pre-stroke Dominant Side and Gender
| Partial Effect | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Functional Level | Concordance | Gender | ||||||||||
| Improvement: Pre-CIMT v All Post-Training | Retention: M12 v M24 | Improvement: Pre-CIMT v All Post-Training | Retention: M12 v M24 | Improvement: Pre-CIMT vAll Post-Training | Retention: M12 v M24 | |||||||
| Variable | High | Low | High | Low | Concord | Discord | Concord | Discord | Male | Female | Male | Female |
| Log Performance Time | <.0001 | <.0001 | 0.4578 | 0.7959 | <.0001 | <.0001 | 0.9810 | 0.3017 | <.0001 | <.0001 | 0.6287 | 0.5474 |
| Performance Time, sec | <.0001 | <.0001 | 0.9275 | 0.6798 | <.0001 | <.0001 | 0.6100 | 0.8836 | <.0001 | 0.0001 | 0.9799 | 0.7998 |
| WMFT-WTB, lbs | <.0001 | 0.1125 | 0.0874 | 0.3501 | <.0001 | 0.0006 | 0.1605 | 0.1522 | <.0001 | 0.0100 | 0.1152 | 0.2101 |
| WMFT-GS, kg | <.0001 | 0.0012 | 0.0203 | 0.0009 | <.0001 | <.0001 | 0.0191 | 0.0164 | <.0001 | 0.0032 | 0.0246 | 0.0080 |
| MAL AOU, 0-5 scale | <.0001 | <.0001 | 0.2454 | 0.2216 | <.0001 | <.0001 | 0.2476 | 0.2643 | <.0001 | <.0001 | 0.3377 | 0.1748 |
| MAL QOL, 0-5 scale | <.0001 | <.0001 | 0.2878 | 0.3238 | <.0001 | <.0001 | 0.3486 | 0.2886 | <.0001 | <.0001 | 0.4767 | 0.1702 |
| SIS1 Strength | 0.0098 | 0.1000 | 0.0294 | 0.0535 | 0.0040 | 0.1290 | 0.0060 | 0.2400 | 0.0096 | 0.0714 | 0.0006 | 0.8996 |
| SIS2 Memory-Thinking | 0.0804 | 0.4084 | 0.0162 | 0.7895 | 0.2437 | 0.1593 | 0.1162 | 0.1087 | 0.0260 | 0.9940 | 0.1130 | 0.0945 |
| SIS5 ADL/IADL | <.0001 | 0.0877 | 0.0127 | 0.6328 | 0.0002 | <.0001 | 0.1576 | 0.0299 | <.0001 | 0.0084 | 0.0061 | 0.5296 |
| SIS8 Social Participation | <.0001 | 0.0026 | 0.0078 | 0.0191 | <.0001 | <.0001 | 0.0161 | 0.0205 | <.0001 | <.0001 | 0.0096 | 0.0319 |
| Physical Domain | <.0001 | 0.0245 | 0.0263 | 0.4546 | <.0001 | <.0001 | 0.0807 | 0.0940 | <.0001 | 0.0008 | 0.0092 | 0.5382 |
Abbreviations:
Pre-CIMT v All PT: Change between baseline measurements and all subsequent evaluations (Post-training), “Improve”
Retention M12 v M24: Mean values at 12 versus 24 months, “Retention”
Log WMFT-T: Log mean time to complete the WMFT tasks
WMFT-T: Mean time to complete the WMFT tasks
WMFT-WTB: WMFT weight to box task for shoulder flexion strength
WMFT-GS: WMFT grip strength
MAL AOU: MAL amount of use for 30 tasks
MAL HW: MAL how well limb used for 30 tasks
Functional Level
For the High Functioning participants, SIS scales show significant differences in the Retain terms, and this observation is due to continued improvement among these individuals. For the Low Function group, a number of the SIS factors fail to show significant “improvement” (SIS Typical Activities, SIS Physical Problems), while SIS Meaningful Activities shows significance for the “retention” test.
Concordance
For non-dominant hand paresis (Discordant) and for dominant hand paresis (Concordant), improvement is seen for all variables except SIS Physical Problems and Memory Thinking domains. For the test of “Retention,” SIS domains show significant further improvements among Concordant participants in the Strength and Social Participation domains. For Discordant participants, only SIS Social Participation and SIS ADL/IADL Activities show significant “Retention” results, again based on continued improvement.
Gender
For Male and Female participants, results for “Improvement” are similar, save for a significant difference in the SIS Physical Problems and Memory-Thinking domains among Females. For “Retention,” there are significant results for Males on SIS domains, but not for Females.
Discussion
Results from the EXCITE Trial demonstrated that CIMT produced statistically and clinically meaningful improvements in the WMFT, MAL and Hand Function domain of the SIS compared to those seen in participants receiving usual and customary care after the intervention and 1 year later.15 When examined by functional level, concordance or gender, results did not change on the WMFT and the MAL. While the number of randomized clinical trials in stroke neurorehabilitation is growing, to date none of them has documented the retention of CIMT past one year. Therefore, the present findings extend previous observations by showing that the improvements in function noted at 12 months following a 2-week CIMT intervention are retained or improve even further WMFT strength tasks, SIS domains 1,2,5, 8 and physical domain. Collectively these observations highlight the possibility of further improvements in the upper extremities of mild to moderately impaired stroke survivors beyond one year following a 2-week CIMT intervention.
Grip strength improved over 24 months among all participants irrespective of concordance or gender. The weight-to-box (WTB) UE strength task improved in higher functioning participants without respect to concordance or gender and these improvements were retained.
The mean WMFT time approached 11 seconds during the follow-up year, which fulfills a prediction of a greater than 50% level of recovery following CIMT.27 Grip strength is a reasonable proxy for overall UE strength,28 and the continued improvement observed in HF participants, who were stronger than LF participants at baseline,23 may reflect continued use of the more affected UE in activities of daily living to a greater extent than did LF participants. WTB strength is specific for shoulder musculature. The literature on strength changes in hemiparetic upper extremity muscles is equivocal with indications that while improved strength is possible,29 the functional ramifications of such changes are uncertain.30 Given that LF participants demonstrated half the strength of their HF counterparts prior to treatment23, their lack of comparable improvement in strength on this task would not be surprising. During the first 12 months, higher and lower functioning patients receiving CIMT did not have significantly different treatment effects.15 However over the next 12 months, while both groups improved, the significant interaction seen in WMFT times and grip strength, was caused by more substantial improvements in the higher functioning cohort. Such differences might be manifest in the potential for cortical plasticity to be more profound among higher functioning patients, a notion that is supported by enlarged cortical representation following CIMT in higher functioning, chronic stroke survivors.9 Alternatively, among higher functioning patients manifestations of improved upper extremity capacity may take several months before becoming apparent.31 Recently we have presented evidence indicating that the intensity of CIMT training or the extent to which training was delivered through repetition of movement or shaping can impact WMFT outcomes.32 Despite these observations, higher functioning participants may well have benefited more than lower functioning participants given the fact that most significant long term changes in the SIS were seen exclusively among them.
MAL scores that had improved following CIMT showed consistency throughout the following year and hovered about a mean score of 2.5, a value associated with a patient perception of more than 50% recovery.27 This finding suggests that patients demonstrated consistency in how they rated their amount of use or quality of movement through the second year.
The additional improvements in SIS domains observed over the 24 month interval for Strength (domain 1), ADL/IADL (domain 5) and Social Participation (domain 8) suggest that initial gains in hand function and use may contribute to secondary changes in functional use of the hand, resulting in increased strength and improved ability to perform activities of daily living and social participation. This observation complements findings by Dettmers et al33 who reported initial changes in hand function after distributed CIMT with subsequent improvement in social participation, measured by the SIS. The relationship between dexterity (Hand Function), strength and overall function is complex. Strength and dexterity contribute to improved function34, 35 and activities of daily living36; yet, upper extremity function, and not strength or dexterity independently, seems to have the strongest link to health related quality of life or participation.35 In this regard the improvements in physical function may have had extended benefits in improving social participation. This possibility is particularly important, because gains in participation reflect active engagement in activities for one's overall well being.37
The inter-relationships of strength, dexterity, and function are even more convoluted when moderating factors (concordance, gender) are examined. Here, changes in the strength subscale of the SIS were greater for higher functioning concordant subjects, and gains in ADL/IADL continued to improve in the second year following the intervention in men. In a sub-study of EXCITE participants that evaluated factors impacting quality of life, concordance affected overall quality of life across the SIS domains at baseline.38 With respect to strength changes, Matsuoka and colleagues39 have shown that among right-handed stroke survivors those with left hemispheric lesions (concordant) demonstrated a trend toward decreased strength subsequent to 6 weeks of bilateral arm training, compared to an increase in strength for right hemispheric lesions (discordant), which differs from the present study; however that investigation involved both genders and did not preferentially target hemiparetic hand training. Male gender has also been previously reported to have a three fold impact on recovery of ADL skills, which was thought to be associated with the greater strength levels in men or the increased willingness of women to solicit help.40 Clearly, the role of concordance and gender on quality of life activities bears further scrutiny.
The fact that memory-thinking domain of the SIS did not change after administration of CIMT is consistent with observations made by Dettmers et al33 who applied the same amount of CIMT over a longer time period and at a six month follow up. The lack of change in this domain is not surprising given that CIMT does not target cognitive function. In general, our stroke survivors achieved a higher quality of life that persisted over time, which is an ultimate goal of rehabilitation.
In conclusion, we found that the improvements in functional gains following the provision of a standardized 2-week CIMT program that were still present at 12 months were retained for an additional year. Significant differences post-training indicate continued improvement over that period. Specific gains in upper extremity strength improved further during the second year. The original gains in the Hand Function domain of the SIS were still present two years following the intervention (data not shown), but improvements in Strength, ADL/IADL, and Social Participation domains were now apparent as well. These results emphasize the importance of long-term follow-up in rehabilitation clinical trials to determine more adequately the full extent of effects from therapeutic interventions.
Acknowledgments
EXCITE (Extremity Constraint-Induced Therapy Evaluation) Steering Committee: The Principal Investigator is Steven L. Wolf, Ph.D., PT, FAPTA (Emory University) and the Co-Principal Investigator is Carolee Winstein, Ph.D., PT, FAPTA (University of Southern California). Other site Principal Investigators include: Gitendra Uswatte and Edward Taub, Ph.D., (University of Alabama at Birmingham), Kathye Light, Ph.D., PT (University of Florida, Gainesville), Carol Giuliani, Ph.D., PT (University of North Carolina), David Good, M.D. (Wake Forest University), and Deborah Nichols-Larsen, Ph.D., PT (The Ohio State University). The Training Core is co-directed by David Morris, Ph.D., PT and Edward Taub, Ph.D. (University of Alabama at Birmingham). The Data Management Center is located at Washington University (St. Louis) and is directed by J. Philip Miller. The three-member Data Safety and Monitoring Committee are: Dr. Bruce Dobkin, Chair (University of California at Los Angeles), Rebecca Craik, Ph.D., P.T., FAPTA (Arcadia University), and Terrance Therneau, Ph.D., (Mayo Clinic). We wish to thank our project coordinators, evaluators and trainers at all the EXCITE locations for their tireless and dedicated efforts throughout this trial. The project was funded by National Institutes of Health Grant No HD37606 from the Center for Medical Rehabilitation Research and the National Institute of Neurology Diseases and Stroke.
Footnotes
SLW made primary contributions to the drafting and approval of the final manuscript as well as to the conceptualization and rationale of the study and interpretation of the study. ET made primary contributions to the conceptualization and rationale of the study as well as drafting and approval of the manuscript. JPM and PT made primary contributions to data analyses and drafting of the manuscript. CW, GU, DM, SB, DL and PC contributed primarily to the interpretation of the results and drafting and approval of the manuscript.
Conflict of interest statement: We have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Steven L. Wolf, EXCITE Trial, Principal Investigator, Department of Rehabilitation Medicine, Emory University School of Medicine, Atlanta, GA 30322.
Carolee J. Winstein, Co-Principal Investigator, winstein@usc.edu, Division Biokinesiology and Physical Therapy, Dept. Neurology, Keck School of Medicine, University of Southern California, Los Angeles, CA 90089.
J Phillip Miller, Director EXCITE Data Management Center, phil@wubios.wustl.edu, Division of Biostatistics, Washington University School of Medicine, St. Louis, MO 63110.
Paul A. Thompson, EXCITE Data Management Center, paul@wubios.wustl.edu, Division of Biostatistics, Washington University School of Medicine, St. Louis, MO 63110
Edward Taub, etaub@uab.edu, Department of Psychology, University of Alabama at Birmingham, Birmingham, AL 35294
Gitendra Uswatte, guswatte@uab.edu, Department of Psychology, University of Alabama at Birmingham, Birmingham, AL 35294
David Morris, morrisd@uab.edu, Department of Physical Therapy, University of Alabama at Birmingham, Birmingham, AL 35294.
Sarah Blanton, sblanto@emory.edu, Department of Rehabilitation Medicine, Emory University School of Medicine, Atlanta, GA 30322.
Deborah Nichols-Larsen, DLarsen@amp.osu.edu, School of Allied Medical Professions, The Ohio State University, Columbus, Ohio 43210
Patricia C. Clark, pclark@gsu.edu, Byrdine F. Lewis School of Nursing, Georgia State University, Atlanta, GA 30303.
References
- 1.Taub E, Miller NE, Novack TA, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993 Apr;74(4):347–354. [PubMed] [Google Scholar]
- 2.Taub E, Uswatte G, Elbert T. New treatments in neurorehabilitation founded on basic research. Nat Rev Neurosci. 2002 Mar;3(3):228–236. doi: 10.1038/nrn754. [DOI] [PubMed] [Google Scholar]
- 3.Ostendorf CG, Wolf SL. Effect of forced use of the upper extremity of a hemiplegic patient on changes in function. A single-case design. Phys Ther. 1981 Jul;61(7):1022–1028. doi: 10.1093/ptj/61.7.1022. [DOI] [PubMed] [Google Scholar]
- 4.Wolf SL, Lecraw DE, Barton LA, Jann BB. Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke and head-injured patients. Exp Neurol. 1989 May;104(2):125–132. doi: 10.1016/s0014-4886(89)80005-6. [DOI] [PubMed] [Google Scholar]
- 5.Page SJ, Sisto S, Johnston MV, Levine P. Modified constraint-induced therapy after subacute stroke: a preliminary study. Neurorehabil Neural Repair. 2002 Sep;16(3):290–295. doi: 10.1177/154596830201600307. [DOI] [PubMed] [Google Scholar]
- 6.Dromerick AW, Edwards DF, Hahn M. Does the application of constraint-induced movement therapy during acute rehabilitation reduce arm impairment after ischemic stroke? Stroke. 2000 Dec;31(12):2984–2988. doi: 10.1161/01.str.31.12.2984. [DOI] [PubMed] [Google Scholar]
- 7.Kunkel A, Kopp B, Muller G, et al. Constraint-induced movement therapy for motor recovery in chronic stroke patients. Arch Phys Med Rehabil. 1999 Jun;80(6):624–628. doi: 10.1016/s0003-9993(99)90163-6. [DOI] [PubMed] [Google Scholar]
- 8.Miltner WH, Bauder H, Sommer M, Dettmers C, Taub E. Effects of constraint-induced movement therapy on patients with chronic motor deficits after stroke: a replication. Stroke. 1999 Mar;30(3):586–592. doi: 10.1161/01.str.30.3.586. [DOI] [PubMed] [Google Scholar]
- 9.Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000 Jun;31(6):1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- 10.Wittenberg GF, Chen R, Ishii K, et al. Constraint-induced therapy in stroke: magnetic-stimulation motor maps and cerebral activation. Neurorehabil Neural Repair. 2003 Mar;17(1):48–57. doi: 10.1177/0888439002250456. [DOI] [PubMed] [Google Scholar]
- 11.Fritz SL, Light KE, Patterson TS, Behrman AL, Davis SB. Active finger extension predicts outcomes after constraint-induced movement therapy for individuals with hemiparesis after stroke. Stroke. 2005 Jun;36(6):1172–1177. doi: 10.1161/01.STR.0000165922.96430.d0. [DOI] [PubMed] [Google Scholar]
- 12.Tarkka IM, Pitkanen K, Sivenius J. Paretic hand rehabilitation with constraint-induced movement therapy after stroke. Am J Phys Med Rehabil. 2005 Jul;84(7):501–505. doi: 10.1097/01.phm.0000166881.71097.9d. [DOI] [PubMed] [Google Scholar]
- 13.Hakkennes S, Keating JL. Constraint-induced movement therapy following stroke: a systematic review of randomised controlled trials. Aust J Physiother. 2005;51(4):221–231. doi: 10.1016/s0004-9514(05)70003-9. [DOI] [PubMed] [Google Scholar]
- 14.Wolf SL, Binder-MacLeod SA. Electromyographic biofeedback applications to the hemiplegic patient. Changes in upper extremity neuromuscular and functional status. Phys Ther. 1983 Sep;63(9):1393–1403. doi: 10.1093/ptj/63.9.1393. [DOI] [PubMed] [Google Scholar]
- 15.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006 Nov 1;296(17):2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 16.Winstein CJ, Miller JP, Blanton S, et al. Methods for a multisite randomized trial to investigate the effect of constraint-induced movement therapy in improving upper extremity function among adults recovering from a cerebrovascular stroke. Neurorehabil Neural Repair. 2003 Sep;17(3):137–152. doi: 10.1177/0888439003255511. [DOI] [PubMed] [Google Scholar]
- 17.Taub E, Crago JE, Uswatte G. Constraint-induced movement therapy: a new approach to teatment in physical medicine. Rehabilitation Psychology. 1998;43:152–170. [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Taub E, Crago JE, Burgio LD, et al. An operant approach to rehabilitation medicine: overcoming learned nonuse by shaping. J Exp Anal Behav. 1994 Mar;61(2):281–293. doi: 10.1901/jeab.1994.61-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris DM, Taub E. Constraint-induced therapy approach to restoring function after neurological injury. Top Stroke Rehabil. 2001 Autumn;8(3):16–30. doi: 10.1310/BLJX-M89N-PTPY-JDKW. [DOI] [PubMed] [Google Scholar]
- 21.Morris DM, Uswatte G, Crago JE, Cook EW, 3rd, Taub E. The reliability of the wolf motor function test for assessing upper extremity function after stroke. Arch Phys Med Rehabil. 2001 Jun;82(6):750–755. doi: 10.1053/apmr.2001.23183. [DOI] [PubMed] [Google Scholar]
- 22.Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke. 2001 Jul;32(7):1635–1639. doi: 10.1161/01.str.32.7.1635. [DOI] [PubMed] [Google Scholar]
- 23.Wolf SL, Thompson PA, Morris DM, et al. The EXCITE trial: attributes of the Wolf Motor Function Test in patients with subacute stroke. Neurorehabil Neural Repair. 2005 Sep;19(3):194–205. doi: 10.1177/1545968305276663. [DOI] [PubMed] [Google Scholar]
- 24.Uswatte G, Taub E, Morris D, Light K, Thompson PA. The Motor Activity Log-28: assessing daily use of the hemiparetic arm after stroke. Neurology. 2006 Oct 10;67(7):1189–1194. doi: 10.1212/01.wnl.0000238164.90657.c2. [DOI] [PubMed] [Google Scholar]
- 25.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999 Oct;30(10):2131–2140. doi: 10.1161/01.str.30.10.2131. [DOI] [PubMed] [Google Scholar]
- 26.Uswatte G, Giuliani C, Winstein C, Zeringue A, Hobbs L, Wolf SL. Validity of accelerometry for monitoring real-world arm activity in patients with subacute stroke: evidence from the extremity constraint-induced therapy evaluation trial. Arch Phys Med Rehabil. 2006 Oct;87(10):1340–1345. doi: 10.1016/j.apmr.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Fritz SL, George SZ, Wolf SL, Light KE. Participant perception of recovery as criterion to establish importance of improvement for constraint-induced movement therapy outcome measures: a preliminary study. Phys Ther. 2007 Feb;87(2):170–178. doi: 10.2522/ptj.20060101. [DOI] [PubMed] [Google Scholar]
- 28.Bohannon RW. Hand-grip dynamometry provides a valid indication of upper extremity strength impairment in home care patients. J Hand Ther. 1998 Oct-Dec;11(4):258–260. doi: 10.1016/s0894-1130(98)80021-5. [DOI] [PubMed] [Google Scholar]
- 29.Ada L, Dorsch S, Canning CG. Strengthening interventions increase strength and improve activity after stroke: a systematic review. Aust J Physiother. 2006;52(4):241–248. doi: 10.1016/s0004-9514(06)70003-4. [DOI] [PubMed] [Google Scholar]
- 30.Bohannon RW. Muscle strength and muscle training after stroke. J Rehabil Med. 2007 Jan;39(1):14–20. doi: 10.2340/16501977-0018. [DOI] [PubMed] [Google Scholar]
- 31.Winstein CJ, Rose DK, Tan SM, Lewthwaite R, Chui HC, Azen SP. A randomized controlled comparison of upper-extremity rehabilitation strategies in acute stroke: A pilot study of immediate and long-term outcomes. Arch Phys Med Rehabil. 2004 Apr;85(4):620–628. doi: 10.1016/j.apmr.2003.06.027. [DOI] [PubMed] [Google Scholar]
- 32.Wolf SL, Maddy DN, H, Blanton S, Winstein C, Zhang Q. The EXCITE Trial: Relationship of intensity of constraint induced movement therapy to improvement in the Wolf Motor Function Test. Restor Neurol Neurosci. 2007 2007:In print. [PubMed] [Google Scholar]
- 33.Dettmers C, Teske U, Hamzei F, Uswatte G, Taub E, Weiller C. Distributed form of constraint-induced movement therapy improves functional outcome and quality of life after stroke. Arch Phys Med Rehabil. 2005 Feb;86(2):204–209. doi: 10.1016/j.apmr.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Canning CG, Ada L, Adams R, O'Dwyer NJ. Loss of strength contributes more to physical disability after stroke than loss of dexterity. Clin Rehabil. 2004 May;18(3):300–308. doi: 10.1191/0269215504cr715oa. [DOI] [PubMed] [Google Scholar]
- 35.Harris JE, Eng JJ. Paretic upper-limb strength best explains arm activity in people with stroke. Phys Ther. 2007 Jan;87(1):88–97. doi: 10.2522/ptj.20060065. [DOI] [PubMed] [Google Scholar]
- 36.de Groot-Driessen D, van de Sande P, van Heugten C. Speed of finger tapping as a predictor of functional outcome after unilateral stroke. Arch Phys Med Rehabil. 2006 Jan;87(1):40–44. doi: 10.1016/j.apmr.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 37.Carod-Artal J, Egido JA, Gonzalez JL, Varela de Seijas E. Quality of life among stroke survivors evaluated 1 year after stroke: experience of a stroke unit. Stroke. 2000 Dec;31(12):2995–3000. doi: 10.1161/01.str.31.12.2995. [DOI] [PubMed] [Google Scholar]
- 38.Nichols-Larsen DS, Clark PC, Zeringue A, Greenspan A, Blanton S. Factors influencing stroke survivors' quality of life during subacute recovery. Stroke. 2005 Jul;36(7):1480–1484. doi: 10.1161/01.STR.0000170706.13595.4f. [DOI] [PubMed] [Google Scholar]
- 39.Matsuoka J, Berger RA, Berglund LJ, An KN. An analysis of symmetry of torque strength of the forearm under resisted forearm rotation in normal subjects. J Hand Surg [Am] 2006 May-Jun;31(5):801–805. doi: 10.1016/j.jhsa.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 40.Paolucci S, Bragoni M, Coiro P, et al. Is sex a prognostic factor in stroke rehabilitation? A matched comparison. Stroke. 2006 Dec;37(12):2989–2994. doi: 10.1161/01.STR.0000248456.41647.3d. [DOI] [PubMed] [Google Scholar]