Abstract
Iron is an essential element of living cells and organisms as a component of numerous metabolic pathways. Hemoglobin and ferric-transferrin in vertebrate host blood are the two major iron sources for female mosquitoes. We used inductively coupled plasma mass spectrometry (ICP-MS) and radioisotope-labeling to quantify the fate of iron supplied from hemoglobin or as transferrin in Aedes aegypti. At the end of the first gonotrophic cycloe, ~87% of the ingested total meal heme iron was excreted, while 7% was distributed into the eggs and 6% was stored in different tissues. In contrast, ~8% of the iron provided as transferrin was excreted and of that absorbed, 77% was allocated to the eggs and 15% distributed in the tissues. Further analyses indicate that of the iron supplied in a blood meal, ~7% appears in the eggs and of this iron 98% is from hemoglobin and 2% from ferric-transferrin. Whereas of iron from a blood meal retained in body of the female, ~97% is from heme and <1 % is from transferrin. Evaluation of iron-binding proteins in hemolymph and egg following intake of 59Fe-transferrin revealed that ferritin is iron loaded in these animals, and indicate that this protein plays a critical role in meal iron transport and iron storage in eggs in A. aegypti.
Keywords: Blood Meal, Heme Iron, Transferrin Iron, Ferritin, Iron Transport, Egg Iron Reserve
Introduction
Iron is an essential element in living cells as a cofactor of proteins involved in numerous metabolic pathways including DNA synthesis and repair, energy metabolism and immunity. Blood feeding is a unique life phenomenon of hematophagous arthropods including mosquitoes. Mosquitoes feed on blood to provide the nutrients for egg production. Among many nutrients in the blood meal, iron is required for optimal egg development and viable offspring. The high transmission rates of mosquito-borne diseases reflect the great numbers of these vectors. Our work is directed toward discovering and interfering with iron delivery and usage in the yellow fever vector, Aedes aegypti.
The blood meal provides high levels of iron as hemoglobin in erythrocytes, and as ferric-transferrin. To date, all experimental studies in mosquito iron metabolism have addressed iron-related proteins including ferritin (Geiser et al., 2006, 2003; Pham & Chavez, 2005; Pham et al., 2005, 2003, 2000, 1999; Dunkov et al., 2002, 1995), transferrin (Harizanova et al., 2005; Yoshiga et al., 1997), ribonucleotide reductase (Pham et al., 2002, 2006), iron-regulatory protein I (IRP-I, Zhang et al., 2002) and the divalent metal transporter1 (DMT1, Martinez-Barnetche et al., 2007). Although these studies provide valuable information about proteins involved in mosquito iron metabolism, one area that has been neglected is quantitative evaluation of the fate of blood meal iron. Such information could serve to indicate the importance of iron to egg development and mosquito biology and to increase our understanding of how the female mosquito transports and utilizes iron from the blood meal constituents. This, in turn, would reveal unique aspects of the physiology of meal iron metabolism and could provide clues to guide our future research.
We used iron inductively coupled plasma mass spectrometry (ICP-MS) and radioisotope-labeling to quantify the fate of iron from blood contituents in A. aegypt during the first gonotrophic cycle. We report that at the end of the first gonotrophic cycle, the majority of the heme iron provided by a blood meal is excreted. Iron provided as ferric-transferrin is highly absorbed and distributed primarily to the ovaries and eggs, while that from hemoglobin is less well absorbed and distributed to other tissues in addition to the ovaries and eggs. Because iron provided from heme in blood is in much greater quantity than that provided by transferrin, the majority of iron absorbed from the blood meal comes from heme. Iron provided as 59Fe-transferrin in a blood meal is found in hemolymph ferritin and in ferritin in eggs indicating that secreted ferritin plays a critical role in meal iron transport in hemolymph and subsequent retention in eggs in mosquitoes.
2. Materials and Methods
2.1. Mosquitoes
A. aegypti (Rockefeller strain) larvae were fed on a mixture of brewer’s yeast (USB, Cleveland, OH), lactalbumin hydrolysate (USB, Cleveland, OH) and finely ground rat chow (Sunburst Pet Foods, Phoenix, AZ) (1:1:1). Adult mosquitoes were routinely maintained at 27°C, 70-80% relative humidity with a photoperiod of 16:8 h (L:D) on 10% sucrose solution ad libitum.
2.2. Pig blood feeding and sampling of mosquitoes for ICP-MS measurement
Two hundred female A. aegypti four-days old were fasted from 10% sucrose solution for 12-24 h prior to the feeding experiments and were then allowed to feed on pig blood for 30 min. Engorged animals were collected at 0, 24 and 72 h post feeding. Body iron content was determined for three animals and the head, midgut, fat body and ovaries were dissected from four animals at each time interval to determine iron content of these tissues. Hemolymph was collected from four animals at each time interval using the method described by Beerntsen and Christensen (1990). Briefly, the mosquitoes were kept in a petri dish on ice prior to perfusion. The distal abdomen was gently separated at the last 2 segments. The hemolymph was obtained by perfusion from the hemocoel with Aedes saline (0.6 mM MgCl2, 4 mM KCl, 1.8 mM NaHCO3, 150 mM NaCl, 25 mM Hepes, 1.7 mM CaCl2, pH 7, Hagedorn et al., 1977) via displacement from a microinoculation needle inserted through the neck membrane and into the hemocoel. The first 2 drops of perfusate (hemolymph and saline) from each mosquito were collected and centrifuged to remove hemocytes (2 min, 7200×g); the supernatant then was stored at -70°C until used. In addition, the waste from 3 females in each group was collected at 120 h post blood meal as previously described (Zhou et al., 2004). At each of the time points indicated, the accumulated excretory waste and the spent females were analyzed by iron ICP-MS, respectively.
For egg collection, three fed mosquitoes were placed in a plastic water cup containing a 30-ml plastic beaker with water lined with a small piece of paper towel. The cup was sealed with a piece of mesh. Eggs were collected at 120 h post feeding, counted and washed in water twice. The spent females were dissected to collect any eggs retained in ovaries. Of 10 groups used for egg production, the three that contained the highest egg numbers were selected for iron ICP-MS analysis of mosquito whole body and eggs.
In order to determine the contribution of meal iron alone, we determined the body and tissue iron content of mosquitoes fed minimal iron diet. For this diet, we removed the hemoglobin from Kogan’s meal (10% pig albumin (Sigma, St. Louis, MO), 1.5% pig γ-globulin (Sigma), 0.8% pig hemoglobin (Sigma), and 5mM ATP (Sigma) in feeding buffer (100mM NaHCO3, 150mM NaCl, pH7.0, Kogan, 1990). In Kogan’s meal, the γ-globulins were thought to initiate the hormonal responses necessary for normal egg development, hemoglobin was used as a visual marker of feeding and albumin as a concentrated source of protein to achieve egg yields equivalent to those from blood-fed controls (Kogan, 1990). Heme is 3.43% of the mass of hemoglobin; the remainder is globin (Vinogradov et al., 1982). In order to compensate for the removal of globin, we added an equivalent amount of protein as albumin and γ-globulin based on the mass ratio of both components in the original Kogan’s meal (20:3).
2.3. ICP-MS for iron in mosquito samples
Two milliliters of 50% HNO3 (V/V) were added to each sample of mosquito tissues. The inside wall of the tube was rinsed 3 times, the sample collected by centrifugation into the bottom of the tube. The samples were transferred into an XP-500 closed digestion vessel (Lakefield Research Limited, Lakefield, ON, Canada) and placed into a CEM Corporation microwave laboratory unit (CEM Mars X, Mathews, NC). The digestion program was set as follows: energy (1200W), power (100%), temperature (100°C), time (5-15 min) and cooling time (2-2.5min). After digestion, the vessels were placed on ice to bring them to room temperature. The samples were separated into two new 1.5-ml tubes for iron ICP-MS.
Iron ICP-MS was conducted as previously described (Geiser et al., 2006). The pretreated samples were diluted by placing 20 μl of each sample in 5 ml of 1% HNO3. About 5 ml MilliQ H20 and 20 μl of 50% HNO3 were mixed to form 5 ml of 1% HNO3 and used as negative controls for iron contamination of diluted samples and equipment. The iron content of the samples was quantified with the Elan Dynamic Reaction Cell (DRC) II (Perkin ElmerSCIEX, Shelton, CT). The DRC compartment was purged with NH3 to avoid polyatomic complexes formed by the presence of argon, which gives the most sensitive detection limit by avoiding interfering oxides. To further reduce oxide formation, a hotter plasma than normal was used. The temperature of the argon plasma is a function of the flow rate of the nebulizer gas. If the flow is increased (i.e. 1 L/ min or higher) the situation is referred to as a “cool plasma”, though it can still be close to 6000°C. The lower flow rates (i.e. 0.9 L/ min or less) is referred to as “hotter plasma”. The samples were run at 0.82 L/ min, but the rate can vary slightly from run to run and thus, needs to be optimized before each set of samples are analyzed. The oxide formation was monitored by the CeO/Ce ratio. The ICP-MS readings were expressed as ng Fe per animal.
2.4. Preparation of 59Fe-transferrin
59Fe-transferrin was prepared according to Pintor et al. (1993) and Simonson et al.(1982) using human apo-transferrin (Sigma). Briefly, apo-transferrin was diluted (1 mg protein /ml) in acidic feeding buffer (100 mM NaHCO3, 150 mM NaCl, pH 5.0), transferred onto the Centricon YM-50 Centrifugal Filter Device (Millipore, Ltd., Watford, UK) and centrifuged (3000×g) at room temperature (RT) for 2 min. The protein was washed with 2 ml of acidic feeding buffer (pH 5.0), and then with neutral (pH 7.0) feeding buffer. Concentrated apo-transferrin was collected into a microfuge tube and the volume brought to 2 ml with neutral feeding buffer. 59FeCl3 (64 μCi, 32 μCi/μl, specific activity: 185 GBq/g ferric chloride, PerkinElmer Life and Analytical Sciences, Boston, MA) was added to the apo-transferrin solution and the reaction allowed to proceed for 30 min at RT. The reaction mixture was loaded onto the Centricon YM-50 and centrifuged at RT for 15 min. The iron-transferrin was washed twice with 2 ml of neutral feeding buffer (pH 7.0). The 59Fe-transferrin was concentrated to 80 μl; one μl was counted in Gamma Counter (Model: 1282 CompuGamma CS, Turku, Finland) and the recovery and total acquired radioactivity were calculated. The remaining 59Fe-transferrin was added into 1 ml of pig blood meal and mixed well by pipet and fed to mosquitoes.
2.5. 59Fe-transferrin Feeding Experiments
Pig blood meal containing 59Fe-transferrin was pre-warmed at 37°C for 5 min. Four days old female A. aegypti (100 animals) were allowed to feed for 30 min. At 24 h post feeding, hemolymph was collected from 4 groups of 4 fed females. The hemolymph was collected into 1.5-ml microfuge tubes containing 2 μl of protease inhibitor cocktail (50×, 800 μg/ml benzamidine, 500μg/ml phenanthroline, 500μg/ml aprotinin, 500μg/ml leupeptin, 500μg/ml pepstatin A, and 50mM PMSF in 100% ethanol, Ausubel et al., 2001) using the perfusion technique described, frozen in liquid nitrogen, and stored at -80 °C until use. The remaining fed females were maintained in a convection incubator at 28°C, RH 80% and photoperiod 16:8 h (L:D), as described by Zhou et al. (2004). At the end of the gonotrophic cycle (120 h post feeding), oviposited eggs were counted and collected into 1.5-ml microtubes, washed three times with water and dried in a hood. Radioactivity of the eggs and the spent females was measured with the Gamma Counter. After radioactive measurement, the eggs were pooled and stored at -80°C for protein extraction. All radioactive procedures were carried out in a designated area with adequate protection as approved by the Radiation Control Office at The University of Arizona.
2.6. Hemolymph and egg analyses
Eggs were homogenized with 0.1% Triton X-100 containing 2 μl of protease inhibitor cocktail (Ausubel et al., 2001) in a 1.5-ml Eppendorf tube using a motorized pellet pestle (Kontes Glass Company, Vineland, NJ; Sigma, St. Louis, MO).
Immunoprecipitation of ferritin from 59Fe-labeled hemolymph and egg extracts was performed using the Seize Classic (A) Immunoprecipitation Kit (Pierce, Rockford, IL). Anti-A. aegypti ferritin polyclonal antibody (100 μl, 1mg/ml, Dunkov et al., 1995) was added to 59Fe-labeled mosquito hemolymph or protein extracted from eggs and incubated (RT, 30 min). Purified Manduca sexta ferritin (15 μg) immunoprecipitated with anti-M. sexta ferritin rabbit serum was used as control. Ferritin was obtained by Protein A-antibody affinity chromagraphy (Pro-Chem Inc. Littleton, MA) conducted according to the manufacturer’s instructions. Homogenized egg samples or hemolymph were centrifuged at 5000 rpm for 5 min. The supernatant was mixed with the same volume of native sample buffer for final concentration of 43.75 mM Tris-HCl (pH 6.8), 15% glycerol, 0.0125% bromophenol blue. The proteins were resolved by PAGE at 150 V for 2.5 h on 4-20% gradient polyacrylamide native gels, prepared by the buffer system of Laemmli (1970) without SDS, according to the method of Davis (1964). The separated proteins were stained with the SYPRO Ruby protein gel staining kit (Bio-Rad, Hercules, CA). Proteins were transferred onto a PVDF membrane (Millipore, Eschborn, Germany) and membranes were blocked with 5% skim milk (Difco Lab. Detroit, MI) in 50 mM Tris/HCl (pH 7.6), 0.15 M NaCl (TBS). The membranes were incubated with anti-A. aegypti ferritin (1:4000) at 25 C for 2h in TBS containing 0.1% Tween-20. Alkaline phosphatase-conjugated goat anti-mouse IgG was used as the second antibody at a 1:5000 dilution in the same buffer. The membranes were then washed twice with TBS containing 0.1% Tween-20 and the protein visualized with BCIP and NBT (Promega, Madison, WI). Molecular markers (Bio Rad, Hercules, CA) were resolved under identical conditions. Membranes were exposed to a Kodak Biomax Light Film (Kodak, New Haven, CT) in a Kodak BioMax Cassette with intensifying screen mounted inside (Kodak) at -80°C overnight and developed with a Mini-Med/90 X-ray film processor (AFP Imaging, Elmsford, NY).
2.7. Data Expression and Statistics
Data are expressed as ng Fe per animal or as cpm per animal. Each group of experiments was repeated at least three times. In the pig blood meal feeding experiments, a cohort fed with modified Kogan’s meal without hemoglobin served as controls and the iron ICP-MS experimental data was determined by subtracting the mean of the control data for each experimental treatment. Data are presented as mean ± SEM. The significance level is α<0.05. All the statistical analyses and graphs were prepared by using GraphPad Prism 3 (Graph-Pad Prism Software, Inc., San Diego, CA).
3. Results
3.1. Absorbed meal iron is distributed primarily to the ovaries following blood feeding
We evaluated the distribution of iron absorbed by ICP-MS at different time intervals for various tissues of females following a pig blood meal. In order to control for endogenous iron present in the female at the time of blood feeding, values were obtained for a control cohort fed the modified Kogan’s meal without added iron and subtracted from those determined for animals fed pig blood. Some residual iron was present in the control meal as compared with a sugar meal; however, iron in pig blood was significantly greater than iron provided by the albumin, gamma globulin (AG) meal (p<0.0001, Fig. 1A). Meal iron in the gut tissues increased dramatically immediately post blood feeding, and then dropped precipitously by 72 hours (Fig. 1B). Hemolymph iron also increased by 24 hours and declined by 72 hours, while iron levels of fat body and head were relatively stable during the gonotrophic cycle (Fig. 1B). Iron in the ovary increased progressively with time. From these data we conclude that although a high load is consumed in the blood meal, only a fraction of the iron is absorbed and the majority of this iron is found in the ovaries and eggs.
Fig.1.
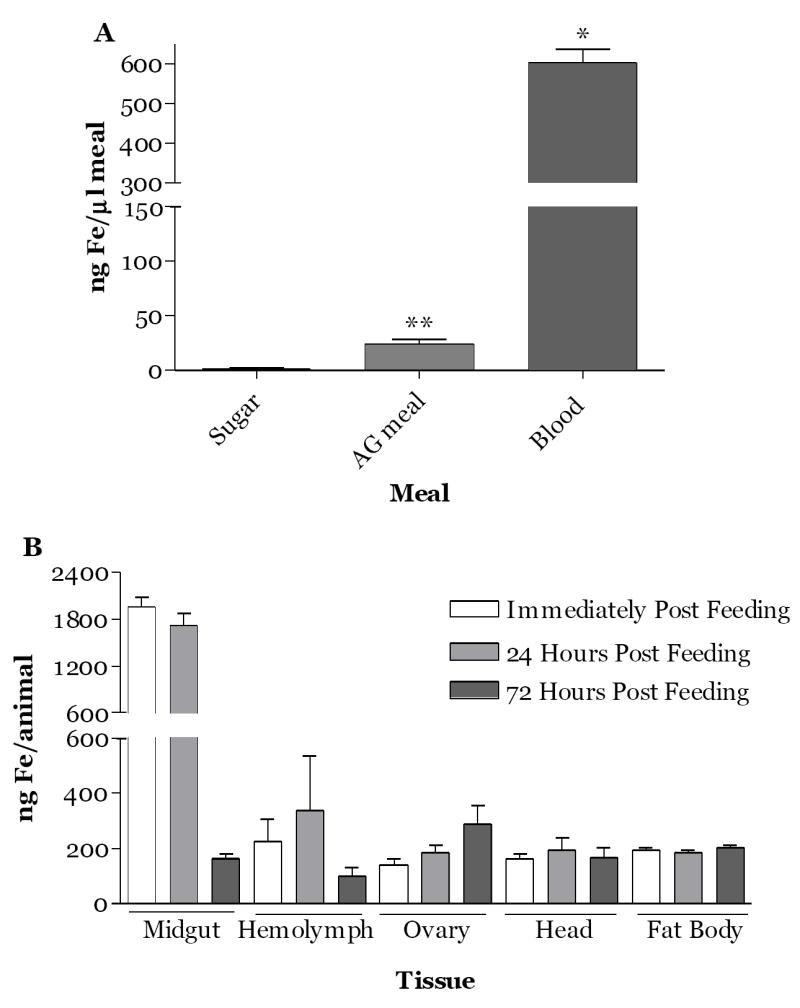
Temporal and tissue distribution of blood meal iron in A.aegypti mosquitoes during the first gonotrophic cycle. (A) Pig blood contains significantly more iron than the meal of albumin and gamma globulin (AG) or sugar. Meal iron was measured by ICP-MS as described in the methods. *=p<0.0001 and **= p≤0.01. (B) Distribution of iron from a blood meal among tissues. Female A.aegypti (four-days old) were fed on pig blood meal for 30 min. At the time intervals indicated tissues were dissected from four animals and hemolymph from 8 animals was collected. Iron was measured by ICP-MS as described in the Methods. Mean values obtained for animals fed a modified Kogan’s artificial meal without hemoglobin were subtracted from those obtained for blood fed animals. Data represent the mean ± SEM of three independent experiments.
3.2. The majority of meal iron is distributed to the ovaries and eggs or excreted at the end of the gonotrophic cycle
In order to further explore the distribution and loss of meal iron post blood feeding, we evaluated net meal iron in tissues, eggs, waste and spent females at the end of the first gonotrophic cycle. We found that 87% of meal iron was excreted as waste (1644.6 ng meal iron/female). Of the 13% retained, 7% was transferred to the eggs (124.7 ng iron/female) and the remainder was distributed to fat body (3%, 51.2 ng/female), ovaries (1 %, 19.9 ng/female), midgut (1 %, 20.8 ng/female) and head (1%, 23.6 ng/female, Fig. 2). These data support our conclusion that the majority of the iron in a blood meal is excreted, while ~ 60% of the iron that is absorbed is transferred to the ovaries and eggs. The remainder is retained in various body tissues indicating that females accumulate body iron over their life cycle.
Fig.2.
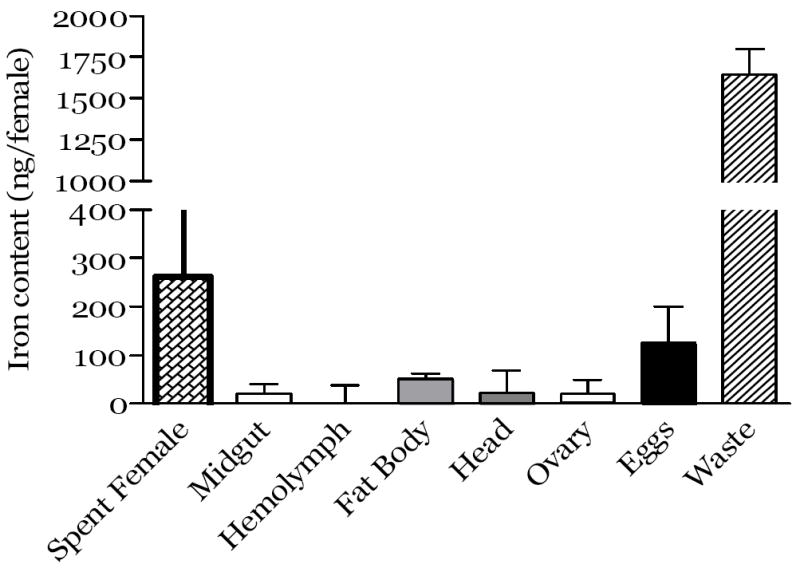
Iron in tissues, spent females, eggs and waste at the end of the first gonotrophic cycle for mosquitoes fed a pig blood meal. The mosquitoes fed with a pig blood meal (treatment group) or with a modified artificial meal (control group) were maintained till the eggs were laid. The spent females were measured, midgut, fat body, head and ovaries tissues were collected by dissection or the hemolymph was obtained. Eggs were collected and washed as described in the Methods. Iron was measured as described in the Methods by ICP-MS. The results are expressed as ng iron per female for pig blood results minus the mean values respectively for the control cohort. Data from each point represent the mean ± SEM of at least 3 independent experiments.
3.3. Iron from transferrin in the blood meal is absorbed and transferred to eggs
In order to evaluate the iron absorbed from transferrin in the blood meal, we fed animals 59Fe-transferrin as part of the blood meal and determined the levels of 59Fe in eggs and spent females at the end of the gonotrophic cycle. The animals consumed an average 3.0±0.2 μl of blood (cpm per female/cpm/μl blood, Table 1). Following oviposition, spent females retained ~15% of the ingested radio-label (2777 cpm/female). Eggs contained ~77% of the ingested 59Fe (14172 cpm/female), and surprisingly, only 8% of ingested 59Fe from transferrin was excreted as waste (Table 1). From these data we conclude that iron bound to transferrin in blood is more bioavailable than that provided as heme. Further, iron from transferrin is transferred primarily to the eggs and little iron in this form is lost as waste in these animals.
Table 1.
Location of meal transferrin iron at the end of the gonotrophic cycle in A.aegypti.
| Pig blood meal (cpm/μl): | 6140±140 | ||
| Total 59Fe consumed (cpm/♀): | 18522±980 | ||
| Meal size in 59Fe-Tf feeding (μl/♀): | 3.0±0.2 | ||
| Body | Eggs | Waste | |
| Location of 59Fe at 120 h (cpm/♀): | 2777±544 | 14172±520 | 1574±520 |
| Meal Tf-iron utilized (%): | 15% | 77% | 8% |
3.4. Relative contributions of meal iron from heme or transferrin to the female and to eggs
Serum transferrins are highly conserved among mammals and we would expect that the data obtained for 59Fe-transferrin added to pig blood would be similar for a blood meal taken from humans. We used these data to estimate the relative contribution of iron from heme and transferrin to mosquito eggs and waste for a human blood meal as follows (Tables 2a-2c). Iron in human serum is exclusively bound to transferrin under normal physiological conditions and has a mean value of 1.2 ng Fe/μl blood (Reilly, 2004). Thus, we reasoned that in 3 μl of human blood, a mosquito ingests ~3.6 ng of Fe from transferrin (Table 2a, Tf-Fe) that would be distributed to eggs (2.8 ngs, 77%) and lost as waste (0.3 ng, 8%). We subtracted these values from those obtained by iron ICP-MS (Figure 2) for eggs, waste and spent females (Table 2a, Heme-Fe+Tf-Fe) to obtain the relative contribution of heme iron (Table 2a, Heme-Fe). As noted above, 87% of the iron from heme would be lost as waste, while ~13% would be absorbed (Table 2a). In contrast to Fe-transferrin, heme-derived iron is distributed about equally to the tissues (49%, Table 2b) and eggs (51%) and substantial iron from heme (114.9 ngs) remains in the spent female supporting that these animals accumulate iron over their lifecycle as a result of blood feeding. Despite the lower bioavailability of iron from heme relative to that provided by transferrin, the majority of iron retained by the spent female and transferred to eggs (~124.7 ngs/female) comes from heme (Table 2c) because of the much greater levels of heme in blood.
Table 2A.
Comparison of utilization of the ingested meal iron from blood in A.aegypti.
| Transferrin-Fe | 1Heme-Fe+Transferrin-Fe | Heme-Fe* | ||||
|---|---|---|---|---|---|---|
| Fe (ng/♀) | (%) | Fe (ng/♀) | (%) | Fe (ng/♀) | (%) | |
| Body | 0.5 | 15 | 115.4 | 6 | 114.9 | 6 |
| Eggs | 2.8 | 77 | 124.7 | 7 | 121.9 | 7 |
| Waste | 0.3 | 8 | 1644.6 | 87 | 1644.3 | 87 |
| Total | 3.6 | 100 | 1884.7 | 100 | 1881.1 | 100 |
Total iron distribution taken from data from Figure 2.
Heme-Fe is determined by subtracting Transferrin-Fe from Heme-Fe+Transferrin-Fe.
Table 2C.
The majority of meal iron retained by A. aegypti at the end the first gonotrophic cycle originates from heme.
| Body | Eggs | |||
|---|---|---|---|---|
| Iron (ng/♀) | (%) | Iron (ng/♀) | (%) | |
| Heme-Fe | 114.9 | 99.6 | 121.9 | 98 |
| Transferrin-Fe | 0.5 | 0.4 | 2.8 | 2 |
| Total | 115.4 | 100 | 124.7 | 100 |
Table 2B.
Iron derived from heme or transferrin is distributed differently at the end of the first gonotrophic cycle.
| Heme-Fe | Transferrin-Fe | |||
|---|---|---|---|---|
| Iron (ng/♀) | (%) | Iron (ng/♀) | (%) | |
| Body | 114.9 | 49 | 0.5 | 15 |
| Eggs | 121.9 | 51 | 2.8 | 85 |
| Total | 236.8 | 100 | 3.3 | 100 |
3.5. Ferritin in hemolymph and eggs is the primary protein that retains meal iron
We were interested to know what proteins in eggs and hemolymph bound radio-labeled iron. We fed mosquitos pig blood with 59Fe-transferrin added and analyzed the proteins in hemolymph for bound radiolabeled iron. In addition, we immuno-precipitated ferritin from hemolymph and eggs of animals (Dunkov et al., 1995) and analyzed the original and precipitated samples. Figure 3a and 3b document that the mass of native mosquito approximates that of thyroglobin (660 kDa). As expected numerous proteins are present in eggs and hemolymph (Figure 3c, lanes 1 and 2); ferritin is partially removed from these samples by immuno-precipitation with our antiserum (Figure 3c and 3d, lanes 4 and 5). Autoradiography of the western blot indicates that ferritin present in the samples as well as that obtained by immuno-precipitation contain radio-labeled iron and confirm that hemolymph ferritin is loaded with iron from the diet (Figure 3e). Further these data support that ferritin is the primary protein in hemolymph and eggs that contains iron provided in the diet as Fe-transferrin. We anticipated that more of the radio-labeled iron would be precipitated by our antiserum. However, a second immuno-precipitation of the sample showed similar results. Our antiserum was obtained from a rabbit injected with ferritin purified from iron-treated larvae. Possibly, larval ferritin differs from that of adults. There is some support for this notion from studies that indicate that hemolymph ferritin consists primarily of the heavy chain subunit, while larval ferritin contains both the heavy and light chain subunits. Nonetheless, from these data we conclude that iron from transferrin taken in a meal is absorbed and transferred to ferritin that is either taken into the eggs or delivered to the eggs for storage in egg ferritin.
Fig.3.
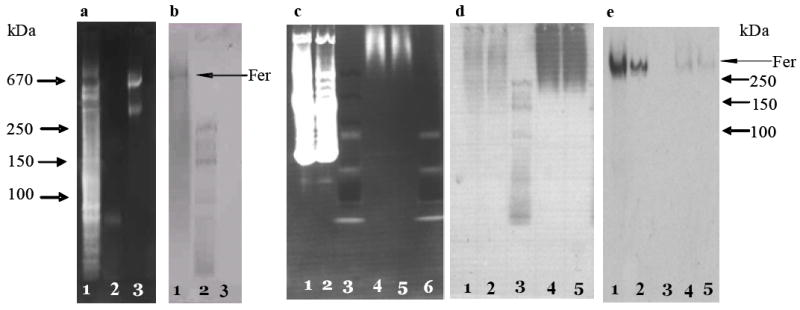
Protein profiles and ferritin of mosquito hemolymph and eggs after a pig blood meal supplemented with 59Fe-transferrin. 59Fe-transferrin was prepared as described in the Methods, then supplemented to a regular pig blood meal and fed to 100 female (4 days old) mosquitoes. The 59Fe-labeled hemolymph at 24 h post feeding and the 59Fe-labeled egg protein at the end of gonotrophic cycle were collected and extracted as described in the Methods. Immuno-precipitation of ferritin was done with rabbit specific anti-A.aegypti ferritin serum (Dunkov et al., 1995). All proteins are resolved by 4-20% gradient native-PAGE. Western blots were done as described in the methods and ferritin identified by by the same antiserum. Panel a, native PAGE of A. aegypti larval extract stained using the SYPRO Ruby Protein Gel Staining Kit. Panel b, Western blot of a side-by-side gel corresponding to that of panel a. Lane 1, A. aegypti larval extract (7.5 μg), lane 2, mass standards (6 μl, Bio-Rad), and lane 3, thyroglobulin (10 μg). Panel c, native-PAGE of [59Fe]-labeled hemolymph and egg extracts stained using the SYPRO Ruby Protein Gel Staining Kit as described in the Methods. Panel d, Western blot of a side-by-side native-PAGE gel corresponding to panel c. Panel e, autoradiography of the Western blot shown in panel d. Lane 1, egg protein (22 μl, representing 83 eggs from one female), lane 2, hemolymph (10 μl, representing 4 females), lane 3, mass standards (15 μl, Bio-Rad), lane 4: immuno-precipitated egg protein (22 μl), lane 5, immuno-precipitated hemolymph protein (10 μl). Fer = Ferritin.
Discussion
The mosquito receives iron as part of the blood meal; she must have mechanisms that allow her to prevent iron-mediated oxidative stress, to absorb and rapidly transport the iron required for development to the ovaries and to excrete any iron excess. We are interested in mapping the iron metabolism pathway in these insects and in discovering how females deliver iron to eggs.
Within 72 hours of blood feeding, they absorb the desired iron, transfer more than half to the ovaries and eggs, distribute the remainder primarily to various tissues and excrete the excess. Iron distribution to the ovaries and eggs predominates supporting that blood meal iron is a critical factor for mosquito reproductive success and essential for egg development. Further, if iron absorption and transfer in the first gonotrophic cycle can be extended to other cycles, the female accumulates iron in her body over her life cycle.
The great majority of iron consumed as part of the blood meal is excreted as waste within 72 h of blood feeding. This appears to be the primary mechanism for removal of an iron load from these animals. This is similar to vertebrates where iron homeostasis is maintained by controlling iron absorption and hemochromatosis occurs when proteins of these pathways are defective (Arredondo et al., 2001; Fleming and Bacon, 2005). The form of iron excreted by A. aegypti is not known, but our data indicate that the majority of iron excreted by these animals originates from heme. Another laboratory recently reported a protein that binds heme in a saturated process on the peritrophic matrix of A. aegypti (Devenport et al., 2004, 2005, 2006). Levels of this protein increased in parallel with the progression of digestion and reach a maximum 48 h after feeding (Pascoa et al., 2002). The role of this protein in mosquito iron metabolism remains unclear. It could bind heme from hemoglobin and render it available for absorption by the gut cells. Alternatively, it could bind heme to protect gut tissues from potential heme-mediated oxidative stress and to promote iron excretion. If the latter notion proves to be the case, then this protein might be a key factor that allows the females to excrete the high levels of heme iron we note here.
Iron is present in host blood as Fe-transferrin and hemoglobin (Winzerling and Pham, 2006). Hemoglobin is found almost exclusively inside red blood cells, while the affinity of the iron-binding pockets of transferrin virtually precludes the presence of free iron in blood under normal conditions (Kurokawa et al., 1999; Zak and Aisen 2003). Our data support that iron provided as transferrin is rapidly processed and appears in the hemolymph and eggs within 24 h post blood feeding. Thus, iron from this blood constituent could serve a role in meeting iron demands during the early events of mosquito oogenesis. Our data also indicate that the bioavailability of iron provided as transferrin is much greater than that of iron provided as heme and suggest that iron from transferrin and iron from heme are absorbed by different mechanisms. This is the case for mammals where heme and non-heme iron are absorbed by different pathways. In mammals, dietary non-heme iron is absorbed via DMT1 (Mackenzie and Hediger, 2004; Fleming, 2005; Mackenzie et al., 2006; Wessling-Resnick, 2006), while heme iron is absorbed, at least in part, by the heme carrier protein 1(HCP1, Shayeghi et al., 2005; Sharma et al., 2007). We have cloned and sequenced cDNAs for both proteins from mosquitoes (Geiser and Winzerling, unpublished) and reported that DMT1 of mosquito cells is responsive to iron exposure (Geiser et al., 2005). More recently, Martinez-Barnetche et al (2007) reported that a DMT1/NRAMP homolog, AnaNRAMP, from Anopheles albimanus is highly expressed in the head, midgut and Malpighian tubules. However, this protein is down regulated in the midgut post blood feeding. The differences in the bioavailability and distribution of iron originating from hemoglobin verses transferrin also could reflect that time is needed to acquire iron from hemoglobin. Heme in mammals is released from the ring by heme oxygenase (HO1), a rate-limiting step in the iron recovery process (Elbirt and Bonkovsky, 1999; Ponka, 1999; Ryter et al., 2002). If a similar process is required for mosquitoes, then iron acquisition from heme also could reflect the time needed for iron delivery.
Our work documents that iron from the blood meal is absorbed and found in hemolymph ferritin and in ferritin in eggs. To our knowledge this is the first direct evidence that mosquitoes secrete ferritin loaded with meal iron into hemolymph and provides strong evidence that ferritin functions as a meal iron transporter in mosquitoes. We can not, however, conclude that ferritin is the only protein involved in meal iron transport in these animals because iron from meal heme could be transported differently than that from Fe-transferrin. A hemolymph heme-transport protein is present in Rhodnius and is involved in embryogenesis (Braz et al., 2002; Paiva-Silva et al., 2002). Although we found no homologue for this protein in the available mosquito databases (Winzerling and Pham, 2006), studies beyond the scope of this report would be required to determine if heme is absorbed and transported as such in the hemolymph of mosquitoes. We attempted as part of these studies to obtain a dose response analysis for heme taken as part of the artificial blood meal, but we were unable to get the animals to sufficiently feed on diets with high heme levels to obtain accurate data.
We were surprised that we did not detect 59Fe bound to mosquito transferrin in hemolymph, despite autoradiography of several gels for hemolymph obtained from several animals (data not shown). In mammals, blood transferrin is responsible for iron transport to tissues (Sargent et al., 2005; Taketani, 2005). Mosquitoes have at least two transferrins, TSF1 and TSF2. TSf1 was first identified by Yoshiga et al. (1997); the deduced amino acid sequence contains a secretion signal and message levels are up regulated by iron, but down regulated by blood feeding and by compounds mimicking juvenile hormone (Harizanova, et al., 2005). Perhaps, our failure to detect 59Fe-TSF1 in hemolymph indicates that we were beyond the levels of detection for this protein. Alternatively, TSF1 could serve a different role in these animals as suggested by Yoshiga, et al (1997), who hypothesized that it sequesters iron from invading organisms as necessary. Other reports support this latter notion and have shown that TSF1 functions as one of the immune peptides in mosquito cell lines infected with bacteria (Nasr and Fallon, 2003; Fallon and Sun, 2001; Lowenberger, 2001a,b).
Taken together, the data we present here and other available evidence supports that meal iron is released from hemoglobin and transferrin and loaded into ferritin in midgut epithelial cells, subsequently secreted into hemolymph as holoferritin, and then delivered to other organs and tissues. Iron transport in secreted ferritin in hemolymph would define a unique role for this molecule in insects. Many years ago, Locke and colleagues reported visualization by electron microscopy of iron-loaded ferritin in the secretory pathway in insect cells (Locke and Leung, 1984; Nichol and Locke, 1990; Locke et al., 1991). Their studies led these authors to suggest that in insects, dietary iron is loaded into ferritin in the endoplasmic reticulum, from which it is subsequently secreted into hemolymph (Locke and Leung, 1984; Nichol and Locke, 1990, 1999). More recently, Dunkov et al. (2002) reported high levels of ferritin message and protein in the midgut tissues of blood-fed mosquitoes and suggested that this tissue is the primary producer of hemolymph ferritin post blood feeding (Dunkov et al., 2002). We have found that iron taken up by mosquito larval epithelial cells is transferred primarily to the membrane fractions and loaded into membrane ferritin that is subsequently secreted into culture medium (Geiser et al., 2006; Geiser et al., 2007a,b).
Our results also show that meal iron appears in the eggs stored in ferritin and supports an important role for iron in the development and early life cycle of mosquitoes. Our findings agree with those of others who have reported ferritin in the ovaries and eggs of insects (Dunkov et al., 2002; Georgieva et al., 2002; Kurama et al., 1995; Kim et al., 2001; Bottke, 1982). However, to our knowledge, this is the first documentation that egg ferritin is loaded with iron, and further, with iron obtained in a meal. Ferritin in eggs could serve at least two functions, that of sequestering excess meal iron to protect the embryo from the formation of iron-derived reactive oxygen species and that of maintaining and concentrating yolk iron in a form that is biologically accessible and readily mobilized to meet development requirements. Although it is clear that the iron in egg ferritin originates from the meal, we did not determine if egg holo-ferritin originated from the hemolymph or if the iron was delivered and loaded into ferritin synthesized within these tissues. If egg ferritin originates from the hemolymph, then how might this protein enter the eggs? Ferritin receptors have been detected on some types of mammalian cells, but no sequence is available. Alternatively, uptake could occur as in Xenopus laevis oogenesis, where ferritin penetrates the basement membrane on the distal surface of the follicle cells and passes through channels between adjacent follicle cells into the vitelline envelope, and then to the surface of developing oocytes where it is incorporated by endocytosis (Dumont, 1978). Others have described uptake of large proteins into developing eggs in insects (Ziegler and Van Antwerpen, 2006). Further studies will be required to determine the origin of egg ferritin in mosquitoes.
We have shown that mosquitoes control the consequences of the iron load of the blood meal by 1) blocking absorption, 2) excreting excess iron, 3) storing absorbed iron in ferritin that is secreted into hemolymph, and 4) distributing the iron to the eggs and multiple tissues. If the findings for the first gonotrophic cycle are applicable to successive cycles, then our data suggest that a low, but consistent, increase in body iron could occur over the life cycle of female mosquitoes. Does this impact mosquito longevity? Is the iron stored primarily in the fat body? Will knockout of the ferritin subunits be lethal in these animals as they are in mammals? Will knockdown result in failure of iron transport following blood feeding and hinder fecundity, egg development, hatch or larval viability? Will eggs developed in the absence of iron influence larval numbers? The answers to these questions as well as determination of the roles of the various tissues in mosquito iron biology await further studies.
Acknowledgments
We thank Dr. Michael Reihle, Department of Entomology, University of Arizona, for providing mosquito facilities for regular mosquito rearing. This work was supported by Centro de Investigación en Alimentación y Desarrollo, Hermosillo, CP 83000, México, the National Institutes of Health (GM056812), the Center for Insect Science of the Arizona Research Laboratories, Bio5 Institute for Collaborative Research, and the Agricultural Experiment Station and the College of Agriculture and Life Sciences at the University of Arizona, Tucson, AZ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arredondo M, Munoz P, Mura CV, Nunez MT. HFE inhibits apical iron uptake by intestinal epithelial (Caco-2) cells. The FASEB Journal. 2001;15:1276–1278. doi: 10.1096/fj.00-0578fje. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. Vol. 3. John Wiley & Sons, Inc., Massachusetts General Hospital, Harvard Medical School; USA: 2001. p. 16.11.10. [Google Scholar]
- Beerntsen BT, Christensen BM. Dirofilaria immitis: effect on hemolymph polypeptide synthesis in Aedes aegypti during melanotic encapsulation reactions against microfilariae. Experimental Parasitology. 1990;71:406–414. doi: 10.1016/0014-4894(90)90066-l. [DOI] [PubMed] [Google Scholar]
- Bottke W. Isolation and properties of vitellogenic ferritin from snails. Journal of Cell Science. 1982;58:225–240. doi: 10.1242/jcs.58.1.225. [DOI] [PubMed] [Google Scholar]
- Braz GR, Moreira MF, Masuda H, Oliveira PL. Rhodnius heme-binding protein (RHBP) is a heme source for embryonic development in the blood-sucking bug Rhodnius prolixus (Hemiptera, Reduviidae) Insect Biochemistry & Molecular Biology. 2002;32:361–367. doi: 10.1016/s0965-1748(01)00163-1. [DOI] [PubMed] [Google Scholar]
- Davis BJ. Disc electrophoresis II. Method and application to human serum proteins. Annuals of New York Academic Sciences. 1964;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Devenport M, Alvarenga PH, Shao L, Fujioka H, Bianconi ML, Oliveira PL, Jacobs-Lorena M. Identification of the Aedes aegypti peritrophic matrix protein AeIMUCI as a heme-binding protein. Biochemistry. 2006;45:9540–9549. doi: 10.1021/bi0605991. [DOI] [PubMed] [Google Scholar]
- Devenport M, Fujioka H, Donnelly-Doman M, Shen Z, Jacobs-Lorena M. Storage and secretion of Ag-Aper14, a novel peritrophic matrix protein, and Ag-Muc1 from the mosquito Anopheles gambiae. Cell & Tissue Research. 2005;320:175–185. doi: 10.1007/s00441-004-1067-3. [DOI] [PubMed] [Google Scholar]
- Devenport M, Fujioka H, Jacobs-Lorena M. Storage and secretion of the peritrophic matrix protein Ag-Aper1 and trypsin in the midgut of Anopheles gambiae. Insect Molecular Biology. 2004;13:349–358. doi: 10.1111/j.0962-1075.2004.00488.x. [DOI] [PubMed] [Google Scholar]
- Dumont JN. Oogenesis in Xenopus laevis (Daudin). VI. The route of injected tracer transport in the follicle and developing oocyte. Journal of Experimental Zoology. 1978;204:193–217. doi: 10.1002/jez.1402040208. [DOI] [PubMed] [Google Scholar]
- Dunkov BC, Georgieva T, Yoshiga T, Hall M, Law JH. Aedes aegypti ferritin heavy chain homologue: feeding of iron or blood influences message levels, lengths and subunit abundance. Journal of Insect Science. 2002;2:1–9. doi: 10.1093/jis/2.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkov BC, Zhang D, Choumarov K, Winzerling JJ, Law JH. Isolation and characterization of mosquito ferritin and cloning of a cDNA that encodes one subunit. Archives of Insect Biochemistry and Physiology. 1995;29:293–307. doi: 10.1002/arch.940290307. [DOI] [PubMed] [Google Scholar]
- Elbirt KK, Bonkovsky HL. Heme oxygenase: recent advances in understanding its regulation and role. Proceedings of the Association of American Physicians. 1999;111:438–447. [PubMed] [Google Scholar]
- Fallon AM, Sun D. Exploration of mosquito immunity using cells in culture. Insect Biochemistry and Molecular Biology. 2001;31:263–278. doi: 10.1016/s0965-1748(00)00146-6. [DOI] [PubMed] [Google Scholar]
- Fleming RE. Advances in understanding the molecular basis for the regulation of dietary iron absorption. Current Opinion in Gastroenterology. 2005;21:201–206. doi: 10.1097/01.mog.0000153362.98276.db. [DOI] [PubMed] [Google Scholar]
- Fleming RE, Bacon BR. Orchestration of iron homeostasis. The New England Journal of Medicine. 2005;352:1741–1744. doi: 10.1056/NEJMp048363. [DOI] [PubMed] [Google Scholar]
- Geiser DL, Chavez CA, Flores-Munguia R, Winzerling JJ, Pham DQ. Aedes aegypti ferritin. European Journal of Biochemistry. 2003;270:3667–3674. doi: 10.1046/j.1432-1033.2003.03709.x. [DOI] [PubMed] [Google Scholar]
- Geiser DL, Mayo JJ, Winzerling JJ. Experimental Biology. San Diego, CA: Federation of American Societies for Experimental Biology; 2005. Pilot Study: The regulation by iron of a putative divalent metal transporter in Anopheles gambiaelarval cells, MOS55. [Google Scholar]
- Geiser DL, Zhang D, Winzerling JJ. Secreted ferritin: mosquito defense against iron overload? Insect Biochemistry and Molecular Biology. 2006;36:177–187. doi: 10.1016/j.ibmb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Geiser DL, Mayo JJ, Winzerling JJ. The unique regulation of Aedes aegypti larval cell ferritin by iron. Insect Biochemistry and Molecular Biology. 2007a doi: 10.1016/j.ibmb.2007.01.003. In press. [DOI] [PubMed] [Google Scholar]
- Geiser DL, Shen M, Mayo J, Winzerling J. Mosquito cells regulate iron by secreting iron-loaded ferritin. Insect Biochemistry and Molecular Biology. 2007b Submitted. [Google Scholar]
- Georgieva T, Dunkov BC, Dimov S, Ralchev K, Law JH. Drosophila melanogaster ferritin: cDNA encoding a light chain homologue, temporal and tissue specific expression of both subunit types. Insect Biochemistry and Molecular Biology. 2002;32:295–302. doi: 10.1016/s0965-1748(01)00090-x. [DOI] [PubMed] [Google Scholar]
- Hagedorn HH, Turner S, Hagedorn EA, Pontecorvo F, Greenbaum P, Pfeiffer D, Flanagan TR, Wheelock G. Postemergence growth of the ovarian follicles of Aedes aegypti. Journal of Insect Physiology. 1977;23:203–206. doi: 10.1016/0022-1910(77)90030-0. [DOI] [PubMed] [Google Scholar]
- Harizanova N, Georgieva T, Dunkov BC, Yoshiga T, Law JH. Aedes aegypti transferrin: gene structure, expression pattern, and regulation. Insect Molecular Biology. 2005;14:79–88. doi: 10.1111/j.1365-2583.2004.00533.x. [DOI] [PubMed] [Google Scholar]
- Kim BS, Lee CH, Yun CY, Yeo SM, Park WM, Kim HR. Characterization and immunological analysis of ferritin from the hemolymph of Galleria mellonella. Comparative Biochemistry and Physiology. 2001;A129:501–509. doi: 10.1016/s1095-6433(01)00287-2. [DOI] [PubMed] [Google Scholar]
- Kogan PH. Substitute blood meal for investigating and maintaining Aedes aegypti (Diptera: Culicidae) Journal of Medical Entomology. 1990;27:709–712. doi: 10.1093/jmedent/27.4.709. [DOI] [PubMed] [Google Scholar]
- Kurama T, Kurata S, Natori S. Molecular characterization of an insect transferrin and its selective incorporation into eggs during oogenesis. European Journal of Biochemistry. 1995;228:229–235. [PubMed] [Google Scholar]
- Kurokawa HJC, Dewan JC, Mikami B, Sacchettini JC, Hirose M. Crystal structure of hen apo-ovotransferrin. Both lobes adopt an open conformation upon loss of iron. Journal of Biological Chemistry. 1999;274:28445–28452. doi: 10.1074/jbc.274.40.28445. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Locke M, Ketola-Pirie C, Nichol H. Vacuolar apoferritin synthesis by the fat body of an insect (Calpodes ethlius) Journal of Insect Physiology. 1991;37:297–309. [Google Scholar]
- Locke M, Leung H. The induction and distribution of an insect ferritin-a new function for the endoplasmic reticulum. Tissue and Cell. 1984;16:739–766. doi: 10.1016/0040-8166(84)90007-7. [DOI] [PubMed] [Google Scholar]
- Lowenberger CA. Form, function and phylogenetic relationships of mosquito immune peptides. Advanced Experimental Medicine and Biology. 2001a;484:113–129. doi: 10.1007/978-1-4615-1291-2_11. [DOI] [PubMed] [Google Scholar]
- Lowenberger CA. Innate immune response of Aedes aegypti. Insect Biochemistry and Molecular Biology. 2001b;31:219–229. doi: 10.1016/s0965-1748(00)00141-7. [DOI] [PubMed] [Google Scholar]
- Mackenzie B, Hediger MA. SLC11 family of H+-coupled metal-ion transporters NRAMP1 and DMT1. Pflugers Archiv - European Journal of Physiology. 2004;447:571–579. doi: 10.1007/s00424-003-1141-9. [DOI] [PubMed] [Google Scholar]
- Mackenzie B, Ujwal ML, Chang MH, Romero MF, Hediger MA. Divalent metal-ion transporter DMT1 mediates both H+ -coupled Fe2+ transport and uncoupled fluxes. Pflugers Archiv - European Journal of Physiology. 2006;451:544–558. doi: 10.1007/s00424-005-1494-3. [DOI] [PubMed] [Google Scholar]
- Martinez-Barnetche J, Solache MG, Lecona AN, Tello AT, Rodriguez MDC, Gamba G, Vazquez N, Rodriguez MH, Lanz-Mendoza H. Cloning and functional characterization of the Anopheles albimanus DMT1/NRAMP homolog: implications in iron metabolism in mosquitoes. Insect Biochemistry and Molecular Biology. 2007 doi: 10.1016/j.ibmb.2007.02.009. in press. [DOI] [PubMed] [Google Scholar]
- Nasr NM, Fallon AM. Detection of lysozyme-like enzymatic activity secreted by an immune-responsive mosquito cell line. Journal of Invertebrate Pathology. 2003;82:162–166. doi: 10.1016/s0022-2011(03)00030-2. [DOI] [PubMed] [Google Scholar]
- Nichol H, Locke M. The localization of ferritin in insects. Tissue and Cell. 1990;22:767–777. [Google Scholar]
- Nichol H, Locke M. Secreted ferritin subunits are of two kinds in insects: molecular cloning of cDNAs encoding two major subunits of secreted ferritin from Calpodes ethlius. Insect Biochemistry and Molecular Biology. 1999;29:999–1013. doi: 10.1016/s0965-1748(99)00076-4. [DOI] [PubMed] [Google Scholar]
- Paiva-Silva GO, Sorgine MH, Benedetti CE, Meneghini R, Almeida IC, Machado EA, Dansa-Petretshi M, Yepiz-Plascencia G, Law JH, Oliveira PL, Masuda H. On the biosynthesis of Rhodnius prolixus heme-binding protein. Insect Biochemistry & Molecular Biology. 2002;32:1533–1541. doi: 10.1016/s0965-1748(02)00074-7. [DOI] [PubMed] [Google Scholar]
- Pascoa V, Oliveira PL, Dansa-Petretski M, Silva JR, Alvarenga PH, Jacobs-Lorena M, Lemos FJ. Aedes aegypti peritrophic matrix and its interaction with heme during blood digestion. Insect Biochemistry and Molecular Biology. 2002;32:517–523. doi: 10.1016/s0965-1748(01)00130-8. [DOI] [PubMed] [Google Scholar]
- Pham DQ, Brown SE, Knudson DL, Winzerling JJ, Dodson MS, Shaffer JJ. Structure and location of a ferritin gene of the yellow fever mosquito Aedes aegypti. European Journal of Biochemistry. 2000;267:3885–3890. doi: 10.1046/j.1432-1327.2000.01428.x. [DOI] [PubMed] [Google Scholar]
- Pham DQ, Blachuta BJ, Nichol H, Winzerling JJ. Ribonucleotide reductase subunits from the yellow fever mosquito, Aedes aegypti: cloning and expression. Insect Biochemistry and Molecular Biology. 2002;32:1037–1044. doi: 10.1016/s0965-1748(02)00041-3. [DOI] [PubMed] [Google Scholar]
- Pham DQ, Chavez CA. The ferritin light-chain homologue promoter in Aedes aegypti. Insect Molecular Biology. 2005;14:263–270. doi: 10.1111/j.1365-2583.2005.00556.x. [DOI] [PubMed] [Google Scholar]
- Pham DQ, Douglass PL, Chavez CA, Shaffer JJ. Regulation of the ferritin heavy-chain homologue gene in the yellow fever mosquito, Aedes aegypti. Insect Molecular Biology. 2005;14:223–236. doi: 10.1111/j.1365-2583.2004.00550.x. [DOI] [PubMed] [Google Scholar]
- Pham DQ, Kos PJ, Mayo JJ, Winzerling JJ. Regulation of the ribonucleotide reductase small subunit (R2) in the yellow fever mosquito. Aedes aegypti Gene. 2006;372:182–190. doi: 10.1016/j.gene.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Pham DQ, Winzerling JJ, Dodson MS, Law JH. Transcriptional control is relevant in the modulation of mosquito ferritin synthesis by iron. European Journal of Biochemistry. 1999;266:236–240. doi: 10.1046/j.1432-1327.1999.00849.x. [DOI] [PubMed] [Google Scholar]
- Pham DQ, Shaffer JJ, Chavez CA, Douglass PL. Identification and mapping of the promoter for the gene encoding the ferritin heavy-chain homologue of the yellow fever mosquito Aedes aegypti. Insect Biochemistry and Molecular Biology. 2003;33:51–62. doi: 10.1016/s0965-1748(02)00167-4. [DOI] [PubMed] [Google Scholar]
- Pintor M, Ferreiros CM, Criado MT. Characterization of the transferrin-iron uptake system in Neisseria meningitides. FEMS Microbiol Lett. 1993;112:159–165. doi: 10.1111/j.1574-6968.1993.tb06442.x. [DOI] [PubMed] [Google Scholar]
- Ponka P. Cell biology of heme. American Journal of the Medical Sciences. 1999;318:241–256. doi: 10.1097/00000441-199910000-00004. [DOI] [PubMed] [Google Scholar]
- Reilly C. Chapter 2. Iron. In: Reilly C, editor. The Nutritional Trace Metals. Vol. 2004. Blackwell Publishing Ltd; Oxford, UK: 2004. pp. 35–81. [Google Scholar]
- Ryter SW, Otterbein LE, Morse D, Choi AM. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Molecular & Cellular Biochemistry. 2002:234–235. 249–263. doi: 10.1023/A:1015957026924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent PJ, Farnaud S, Evans RW. Structure/function overview of proteins involved in iron storage and transport. Current Medicinal Chemistry. 2005;12:2683–2693. doi: 10.2174/092986705774462969. [DOI] [PubMed] [Google Scholar]
- Sharma S, Dimasi D, Broer S, Kumar R, Della NG. Heme carrier protein 1 (HCP1) expression and functional analysis in the retina and retinal pigment epithelium. Experimental Cell Research. 2007;313:1251–1259. doi: 10.1016/j.yexcr.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, Khan Y, Warley A, McCann FE, Hider RC, Frazer DM, Anderson GJ, Vulpe CD, Simpson RJ, McKie AT. Identification of an intestinal heme transporter. Cell. 2005;122:789–801. doi: 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Simonson C, Brener D, DeVoe IW. Expression of a high-affinity mechanism for acquisition of transferrin iron by Neisseria meningitides. Infect Immun. 1982;36:107–113. doi: 10.1128/iai.36.1.107-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketani S. Aquisition, mobilization and utilization of cellular iron and heme: endless findings and growing evidence of tight regulation. Tohoku Journal of Experimental Medicine. 2005;205:297–318. doi: 10.1620/tjem.205.297. [DOI] [PubMed] [Google Scholar]
- Vinogradov SN, Kosinski TF, Zak B. Complexometric determination of iron in heme proteins with 4-(2-pyridylazo) resorcinol. Analytic Biochemistry. 1982;120:111–112. doi: 10.1016/0003-2697(82)90325-6. [DOI] [PubMed] [Google Scholar]
- Wessling-Resnick M. Iron imports. III. Transfer of iron from the mucosa into circulation. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2006;290:G1–G6. doi: 10.1152/ajpgi.00415.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzerling JJ, Pham DQ. Iron metabolism in insect disease vectors: mining the Anopheles gambiae translated protein database. Insect Biochemistry and Molecular Biology. 2006;36:310–321. doi: 10.1016/j.ibmb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Yoshiga T, Hernandez VP, Fallon AM, Law JH. Mosquito transferrin, an acute-phase protein that is up-regulated upon infection. The Proceedings of the National Academy of Sciences in the United States of America. 1997;94:12337–12342. doi: 10.1073/pnas.94.23.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak O, Aisen P. Iron release from transferrin, its C-lobe, and their complexes with transferrin receptor: presence of N-lobe accelerates release from C-lobe at endosomal pH. Biochemistry. 2003;42:12330–12334. doi: 10.1021/bi034991f. [DOI] [PubMed] [Google Scholar]
- Zhang D, Dimopoulos G, Wolf A, Minana B, Kafatos FC, Winzerling JJ. Cloning and molecular characterization of two mosquito iron regulatory proteins. Insect Biochemistry and Molecular Biology. 2002;32:579–589. doi: 10.1016/s0965-1748(01)00138-2. [DOI] [PubMed] [Google Scholar]
- Zhou G, Flowers M, Friedrich K, Horton J, Pennington JE, Wells MA. Metabolic fate of [14C]-labeled meal protein amino acids in Aedes aegypti mosquitoes. Journal of Insect Physiology. 2004;50:337–349. doi: 10.1016/j.jinsphys.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Ziegler R, Van Antwerpen R. Lipid uptake by insect oocytes. Insect Biochemistry and Molecular Biology. 2006;36:264–272. doi: 10.1016/j.ibmb.2006.01.014. [DOI] [PubMed] [Google Scholar]


