Abstract
We show here that the nitric oxide (NO)-detoxifying Hmp flavohemoprotein increases by 3-fold the transcription of the Salmonella pathogenicity island 2 (SPI2) in macrophages expressing a functional inducible NO synthase (iNOS). However, Hmp does not prevent NO-related repression of SPI2 transcription in IFNγ-primed phagocytes, despite preserving intracellular transcription of sdhA sdhB subunits of Salmonella succinate dehydrogenase within both control and IFNγ-primed phagocytes. To shed light into the seemingly paradoxical role that Hmp plays in protecting intracellular SPI2 expression in various populations of macrophages, N2O3 was quantified as an indicator of the nitrosative potential of Salmonella-infected phagocytes in different states of activation. Hmp was found to prevent the formation of 300 nM N2O3/h/bacteria in IFNγ-primed macrophages, accounting for about a 60% reduction of the nitrosative power of activated phagocytes. Utilization of the vacuolar ATPase inhibitor bafilomycin indicates that a fourth of the ~200 nM N2O3/h sustained by IFNγ-primed macrophages is generated in endosomal compartments via condensation of HNO2. In sharp contrast, control macrophages infected with wild-type Salmonella produce as little N2O3 as iNOS-deficient controls. Collectively, these findings indicate that the NO-metabolizing activity of Salmonella Hmp is functional in both control and IFNγ-primed macrophages. Nonetheless, a substantial amount of the NO generated by IFNγ-primed macrophages gives rise to N2O3, a species that not only enhances the nitrosative potential of activated phagocytes but also avoids detoxification by Salmonella Hmp.
Keywords: Hmp, IFNγ, macrophages, N2O3, nitric oxide, nitrosative stress, Salmonella
Introduction
NO is an integral part of host defense. Mice rendered pharmacologically or genetically deficient in their ability to sustain high NO synthesis are hypersusceptible to experimentally-induced systemic salmonellosis (Alam et al., 2002; Mastroeni et al., 2000; Shiloh et al., 1999; White et al., 2005). The contribution of NO to resistance to this enteric pathogen strongly correlates with the iNOS-dependent anti-Salmonella activity of macrophages (Vazquez-Torres et al., 2000a). Toll-like receptor-4 agonists, such as Salmonella lipopolysaccharide (Vazquez-Torres et al., 2004), stimulate innate transcription of iNOS through direct activation of NF-κB and indirect induction of IRF-1 upon autocrine release of IFNβ (Fujihara et al., 1994; Gao et al., 1998; Ohmori and Hamilton, 2001; Xie et al., 1994; Zhang et al., 1994). The pro-inflammatory cytokine IFNγ, possibly through further activation of IRF-1 (Kamijo et al., 1994; Martin et al., 1994), synergizes with innate responses to Salmonella ligands to augment iNOS transcription (Vazquez-Torres et al., 2000a; Vazquez-Torres et al., 2004). Involvement of NO in the anti-Salmonella activity of IFNγ-treated macrophages is well accepted (Vazquez-Torres et al., 2000a; Vazquez-Torres et al., 2004; Webb et al., 2001); however, the role that this diatomic radical plays in the anti-Salmonella arsenal of macrophages has proven to be a contentious topic (Chakravortty et al., 2002; Ekman et al., 1999; Saito et al., 1991; Shiloh et al., 1999; Shiloh et al., 1997; Vazquez-Torres et al., 2000a; Vazquez-Torres et al., 2004).
The ability of Salmonella to cause disseminated disease greatly depends on the type III secretion system encoded within the SPI2 pathogenicity island 2 (Hensel et al., 1995; Ochman et al., 1996; Shea et al., 1996). SPI2 enhances Salmonella intracellular fitness by remodeling the phagosome, and thus it minimizes the cytotoxicity of lysosomal hydrolytic enzymes and oxyradicals generated by NADPH oxidase enzymatic complexes (Gallois et al., 2001; Suvarnapunya and Stein, 2005; Uchiya et al., 1999; Vazquez-Torres et al., 2000b). In addition, SPI2 boosts antinitrosative defenses of Salmonella by preventing close interactions of phagosomes with iNOS-containing vesicles (Chakravortty et al., 2002). However, we have shown that nitrogen oxides produced by IFNγ-primed macrophages render this type III secretion system nonfunctional, ultimately leading to progression of Salmonella phagosomes along the degradative pathway (McCollister et al., 2005). It is seemingly paradoxical that NO can selectively mediate strong anti-Salmonella activity of IFNγ-primed macrophages, but exerts negligible or minimal effects in populations of phagocytes that had not been stimulated by IFNγ. We have used herein a combination of bacterial genetics and biochemical assays to study the nitrosative potential of control and IFNγ-primed macrophages in response to Salmonella infection.
Material and Methods
Bacterial strains
Salmonella enterica serovar Typhimurium strain ATCC 14028s was used throughout this study as wild-type and as background for the construction of mutants (Table 1). S. Typhimurium strain AV0615 (ΔspiC::FRT Δhmp::km) was constructed after P22-mediated transduction of the Δhmp::km allele from strain AV0468 into strain AV0201 carrying a ΔspiC::FRT mutant allele (McCollister et al., 2005). The ΔspiC::lacZ transcriptional fusion was transduced from strain AV0207 into AV0468 (McCollister et al., 2005), generating strain AV0539 (ΔspiC::lacZ Δhmp::FRT). Pseudolysogens were eliminated by streaking on Evans blue uranine agar plates. The green-fluorescent protein expressed under the control of the Ptac promoter was amplified from pRSET::gfp using the primer pair F-5′-AGC TGT TGA CAA TTA ATC ATC GGC TCG TAT AAT GTG TGG AAT TGT GAG CGG ATA ACA ATT TCA CAC AGG AAC AGA AAT GAG TAA AGG AGA AGA ACT TTT C-3′ (the Ptac promoter is underlined) and R-5′-TAA TAC GAC TCA CTA TAG GG-3′. The PCR product was ligated into pCR®-Blunt (Invitrogen, Carlsbad, CA), and the resulting plasmid was transformed into Salmonella to generate strain AV0101.
Table 1.
Bacterial strains and plasmids.
| Strains | Genotype | Parent strain | Source |
|---|---|---|---|
| S. Typhimurium 14028s | Wild-type | ATCC | |
| AV0101 | pCRGFP | 14028s | This study |
| AV0201 | ΔspiC::FRT | 14028s | (McCollister et al., 2005) |
| AV0207 | φ(spiC′-lac+) | AV0201 | (McCollister et al., 2005) |
| AV0305 | Δhmp::lacZ | AV0468 | (McCollister et al., 2005) |
| AV0468 | Δhmp::FRT | 14028s | (McCollister et al., 2005) |
| AV0539 | φ(spiC′-lac+) Δhmp::FRT | AV0207/AV0468 | This study |
| AV0615 | ΔspiC::FRT Δhmp::km | AV0201/AV0468 | This study |
|
| |||
| Plasmid | |||
|
| |||
| PCRGFP | PCR®-Blunt::Ptac gfp+ | This study | |
Macrophages
Macrophages were collected from C57BL/6 and congenic gp91phox−/−, iNOS−/− and doubly immunodeficient gp91phox−/− iNOS−/− (MacMicking et al., 1995; Pollock et al., 1995; Shiloh et al., 1999) mice by peritoneal lavage 4 d after intraperitoneal inoculation of 1 mg/ml sodium periodate as described (De Groote et al., 1997). The peritoneal exudate cells were resuspended in RPMI 1640 medium (Cellgro, Herndon, VA) supplemented with 10% heat-inactivated fetal bovine serum (BioWhittaker, Walkersville, MD), 15 mM Hepes, 2 mM L-glutamine, 1 mM sodium pyruvate (Sigma-Aldrich, St. Louis, MO) and 100U·ml−1/100mg·ml−1 of penicillin/streptomycin (Cellgro). Peritoneal exudate cells were seeded at 2×105 cells/well in 96-well plates for macrophage killing assays, or 106 cells/well in 24-well plates (Falcon, Franklin Lakes, NJ) for RNA transcriptional studies. Peritoneal macrophages were selected by adherence following 48 h of culture at 37°C in a 5% CO2 incubator. IFNγ (Life Technologies, St. Paul, MN) was added at a final concentration of 200 U/ml to selected groups of macrophages 20 h prior to Salmonella infection.
Macrophage killing assay
Macrophages were challenged at an MOI of 2 with stationary phase Salmonella opsonized with 10% normal mouse serum for 20 min. Extracellular bacteria were removed from the monolayers 25 min after challenge by washing with pre-warmed RPMI medium containing 6 μg/ml of gentamicin (Sigma) (De Groote et al., 1997). Salmonella-infected macrophages were lysed at the indicated time points after challenge, and the surviving bacteria were enumerated on Luria-Bertani agar plates. The results are expressed as % survival.
Synthesis of cDNA
Total RNA was isolated from Salmonella-infected macrophages as previously described (McCollister et al., 2005) using a combination of the TRIzol reagent (Invitrogen, Carlsbad, CA) and an RNAeasy kit (Qiagen, Valencia, CA). Complementary cDNA was synthesized at 42°C for 30 min using MMLV reverse transcriptase (Promega), RNasin (Promega), dNTPs, and random primers or a poly-dT oligomer (Promega). The synthesized cDNA was then used as template for quantitative real-time PCR and standard or nested PCR.
Transcriptional profiling
Real-time PCR reactions contained cDNA, Takara OmniMix™ HS (Takara Bio Inc., Otsu, Shiga, Japan), 200 nM forward and reverse primers and 240 nM fluorescent-labeled DNA probes. Oligonucleotide primers and probes for transcriptional analysis of Salmonella spiC and rpoD expression were used as previously described (McCollister et al., 2005). Real-time PCR analysis of macrophage iNOS and GAPDH expression was performed using primers and probes described in supplementary Table 2. Real-time PCR reactions consisted of a cycle of 94°C for 45 s followed by 45 cycles of 94°C for 5 s and 59°C for 30 s. The resulting fluorescence was recorded using the SmartCycler®II thermocycler (Cepheid, Sunnyvale, CA). Salmonella spiC and murine iNOS transcripts were normalized with respect to house-keeping Salmonella RNA polymerase sigma factor rpoD or eukaryotic GAPDH, respectively. Transcription of rpoD and sdhA and sdhB Salmonella genes in macrophages isolated from gp91phox- or gp91phox iNOS-deficient mice was assessed by nested PCR using primers described in Table 2. Nested PCR consisted of an initial 15 cycle amplification using the long primers. The number of amplification cycles used in the nested PCR were adjusted according to rpoD transcript levels determined by real-time PCR.
Table 2.
Oligonucleotides used for RT-PCR, real-time RT-PCR and nested PCR.
| Gene amplified | Primer/probea sequence |
|---|---|
| F:5′-CTTGGATCCGGATTCATGCTGGCAGTTTT | |
| spiC | R:5′-TGGAAGCTTTCCAGGTCATTTAAGAACAAAGAA |
| Probe: 5′-6-FAM™-CATCCTGCCAGAGGAGAAATTTTCTCA-BHQ™-1 | |
| F:5′-GTGGCTTGCAATTCCTTGAT real time and nested primer | |
| R:5′-AGCATCTGGCGAGAAATACG real time and nested primer | |
| rpoD | F:5′-GCGAACTTGCGTCTGGTTAT long primer |
| R:5′-TTTTATCTTCCGGCATCAGC long primer | |
| Probe: 5′-6-FAM™-ATAAGTTCGAATACCGTCGCGGCTACA-BHQ™-1 | |
| hmpA | F:5′-CTTGGATCCTTAATGCTATCGCGGCCTAC |
| R:5′-TGGAAGCTTTCAAAGCTGGTGATCAGTGC | |
| F:5′-GTTGTGGTGTGGGGTGTGTA long primer | |
| sdhA | R: 5′-GACCCCTTAACGGTGTCGTA long primer |
| F:5′-GATGCTGTTGTGATTGGTGC nested primer | |
| R:5′-CTTCATGGGTATTGCCGAGC nested primer | |
| F: 5′-AATTGCGGAGACAGGATGAT long primer | |
| sdhB | R: 5′-GGGTAATACAGGCCAGACCA long primer |
| F: 5′-TCGTTATAACCCGGATGTCG nested primer | |
| R: 5′-TCAGAACCGCACACACCTTC nested primer | |
| iNOS | F-5′-CTTGGATCCGTGGTGACAAGCACATTTGG |
| R-5′-CAGCAATGGGCAGACTCTGAAGAAAT | |
| Probe: 5′-6-FAMTM-CCAGCAATGGGCAGACTCTGAAGAAAT-BHQTM-1 | |
| F-5′-AACTTTGGCATTGTGGAAGG | |
| GAPDH | R-5′-GGATGCAGGGATGATGTTCT-3′ |
| Probe: 5′-6-FAMTM-ACTGCCACCCAGAAGACTGTGGAT-BHQTM-3′ |
Dual-labeled oligonucleotide probes contain both the fluorescent dye 6-carboxyfluorescein (6-FAM™) and Black Hole Quencher™ 1 (BHQ™-1).
Expression of spiC::lacZ transcriptional fusions
SPI2 expression was induced in vitro by culturing Salmonella in low osmolarity N salts medium as described (Deiwick et al., 1999). Briefly, S. Typhimurium strains harboring a spiC::lacZ transcriptional fusion were grown overnight in high Mg++ N salts medium [5 mM KCl, 7.5 mM (NH4)2SO4, 0.5 mM K2SO4, 1 mM KH2PO4, 38 mM glycerol, 0.1% casamino acids] supplemented with 10 mM MgCl2 and 100 mM Tris-HCl, pH 7.6. The bacteria were subcultured in high Mg++ N salts medium and grown at 37°C in a shaker incubator to OD600 of 0.5. SPI2 expression was induced by switching the bacteria to 8 μM MgCl2 N salts medium, pH 6.9. The expression of spiC::lacZ was quantified spectrophotometrically as β-galactosidase enzymatic activity using the substrate o-nitrophenyl-β-D-galactopyranoside (Sigma) (Miller, 1972). β-galactosidase activity is expressed in Miller units according to the equation 1000 × [(OD420 −1.75 × OD550)]/(T(min) × V(ml) × OD600). The NO donor spermine NONOate (Cayman Chemical, Ann Arbor, MI) was used to determine the effects of RNS on SPI2 transcription. Spermine NONOate dissolved in 10 mM Tris-HCl, pH 8.0 was quantified spectrophotometrically using ε252 = 8500 M−1 cm−1. The NO donor was added to the cultures after Salmonella had been grown for 1 h in 8 μM MgCl2 N salts medium, a time at which the bacterial cells had reached early stationary phase.
Intracellular hmp::lacZ expression was determined 20 h after wild-type and iNOS-deficient macrophages were infected with strain AV0305 at an MOI of 2. Selected populations of macrophages were activated with 200 U/ml IFNγ 20 h prior to infection. β-galactosidase was monitored in an Lmax luminometer (Molecular Devices, Sunnyvalle, CA) following directions of the Galacton-Plus® kit (Applied Biosystems, Foster City, CA). Intracellular hmp::lacZ expression is represented as β-galactosidase/106 bacteria.
NOx determination
Rates of macrophage NO synthesis were estimated over time as previously described (Vazquez-Torres et al., 2000a) by measuring the accumulation of nitrite (NO2−) and nitrate (NO3−), which are stable metabolites of the reaction of NO with oxygen. Salmonella-challenged macrophages were washed and resuspended in prewarmed IMDM medium (Sigma) supplemented with 0.3% wt/vol sodium bicarbonate (Sigma), 2 mM L-glutamine, 1% Nutridoma-SP (Boehringer), and 6 μg/ml gentamicin 1 h prior to the designated time points, untreated or IFNγ-treated. NOx were allowed to accumulate for 1 h prior to collection. Enzymatic reduction of NO3− to NO2− was performed as described before (Vazquez-Torres et al., 2000a) using nitrate reductase (Sigma). NOx were then estimated from total NO2− concentrations measured spectrophotometrically at 550 nm using the Griess reagent (0.5% sulfanilamide and 0.05% N-1-naphthylethylenediamide hydrochloride in 2.5% phosphoric acid). Standard curves were prepared with NaNO2.
Analysis of nitrosative stress
Nitrosative stress can be associated with a variety of RNS, including peroxynitrite, dinitrosyl iron complexes and N2O3. N2O3 was quantified as an indicator of the nitrosative capacity of control and IFNγ-treated macrophages. N2O3 was indirectly visualized as the N-nitroso 2,3-naphthotriazole (NAT) derivative of 2,3-diaminonaphthalen (DAN) (Sigma) as described (Espey et al., 2000). A 100 mM stock of DAN prepared in dimethylformamide was used at a final concentration of 200 μM in MEM medium supplemented with 1% nutridoma and 6 μg/ml gentamicin. Accumulation of NAT was recorded for 1 h at the indicated times after infection. Fluorescence was measured on a Synergy HT fluorometer (BioTek, Winooski, Vermont) set at λex=375 nm, λem=460 nm. Concentration of N2O3 released by the macrophages was estimated by linear regression analysis of serially diluted NAT prepared after 30 min incubation of 25 μM DAN with 1 mM spermine NONOate.
Immunofluorescence microscopy
Macrophages plated on 13 mm glass coverslips in a 24-well plate at a density of 2.5×105 cells per well were infected as described above. Cells were fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) 20 h post-infection, washed with PBS, permeabilized with 0.1% Triton X-100 in PBS (Sigma), and blocked at 37°C with PBS containing 4% donkey serum. Cells were incubated with an anti-iNOS polyclonal rabbit antibody (Upstate, Charlottesville, VA) for 45 min at 37°C followed by a Cy3-conjugated donkey anti-rabbit secondary antibody (Jackson Immunoresearch, West Grove, PA) and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; 2 μg/ml, Sigma) for 45 min at 37°C. After washing with PBS, coverslips were mounted onto glass slides with Prolong Antifade (Molecular Probes, Eugene, OR) and viewed on a Zeiss Axiophot fluorescence microscope.
Statistical analysis
Data are expressed as mean ± SEM. The data were analyzed using a paired Student’s t test.
Results
Hmp protects SPI2 expression from NO produced during the innate response of macrophages
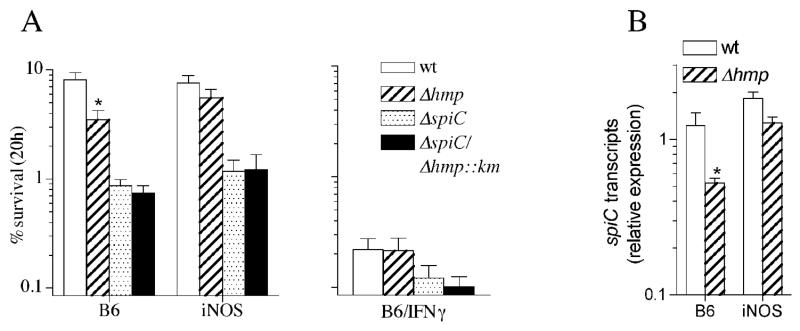
We have previously shown that the expression of SPI2 by intracellular Salmonella residing in macrophages is repressed in an iNOS-dependent manner upon activation of the phagocytes with IFNγ (McCollister et al., 2005). Therefore, we examined whether the NO-metabolizing capacity of Hmp (Gardner et al., 1998; Hausladen et al., 2001; Hausladen et al., 1998) preserves SPI2-dependent Salmonella survival in macrophages that have not been treated with IFNγ. Hmp significantly (p<0.05) enhanced wild-type but not spiC-deficient Salmonella survival in macrophages that have not been treated with IFNγ (Fig. 1A, left panel). The protective effects of Hmp appear to be related to its NO-consuming activity because survival of hmpA-deficient Salmonella was restored to wild-type levels in iNOS-deficient macrophages (Fig. 1A, left panel). The contribution of Hmp to Salmonella survival in macrophages was related to protection of SPI2 transcription as spiC transcripts were significantly (p<0.05) reduced by NO in the absence of Hmp (Fig. 1B). Accordingly, the expression of spiC by hmpA-deficient Salmonella returned to wild-type levels in iNOS-deficient macrophages (Fig. 1B). Remarkably, the protective role of Hmp was lost in IFNγ-activated macrophages as bacterial survival was equally reduced in the presence or absence of hmpA (Fig. 1A, right panel).
Fig. 1.
The flavohemoprotein Hmp protects SPI2-dependent Salmonella survival. (A) The % of wild-type (WT) Salmonella and its isogenic ΔhmpA-, ΔspiC-, and ΔspiC/ΔhmpA::km-deficient controls recovered from untreated (left panel) or IFNγ-treated (right panel) macrophages from immunocompetent (B6) or iNOS-deficient (iNOS) mice was determined 20 h after infection. (B) spiC transcripts were measured by real-time PCR of samples isolated from B6 or iNOS-deficient macrophages 20 h after challenge. *, p<0.05 compared to wild-type Salmonella controls.
Quantification of hmpA expression by intracellular Salmonella
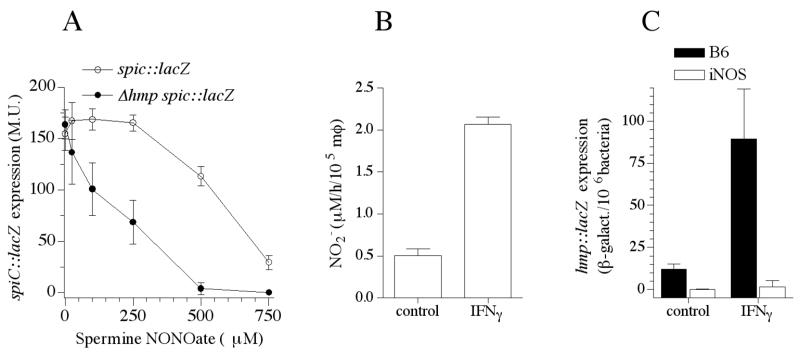
Hmp is the most important antinitrosative defense of Salmonella (Bang et al., 2006). We calculated the degree by which Hmp protects SPI2 expression against nitrosative stress and the extent of hmpA expression during innate and acquired responses of macrophages. An IC50 value of 563 μM was estimated for the inhibition of SPI2 transcription by the NO donor spermine NONOate as measured by β-galactosidase activity of a spiC::lacZ transcriptional fusion expressed by wild-type Salmonella (Fig. 2A). In the absence of Hmp, the spermine NONOate IC50 was, however, reduced to 176 μM. In comparison, we have estimated an IC50 value of 538 μM for the inhibition of respiration by the NO donor spermine NONOate in wild-type Salmonella (data not shown) indicating that the electron transport chain may be as susceptible as SPI2 to the inhibitory effects of NO. These data support a role for the NO-consuming activity of Hmp in protecting SPI2 transcription in macrophages expressing iNOS. In contrast, SPI2 expression has been shown to be inhibited in IFNγ-activated macrophages. The inability of Hmp to protect SPI2 expression against the 4-fold increased NOx rates sustained by IFNγ-primed macrophages (Fig. 2B) is surprising since we found that 7-fold more hmpA was expressed by Salmonella in activated macrophages (Fig. 2C).
Fig. 2.
Hmp lessens NO-mediated inhibition of SPI2 transcription. (A) Expression of SPI2 was estimated as β-galactosidase activity of a spiC::lacZ transcriptional fusion expressed in hmpA-proficient or –deficient Salmonella. Bacterial samples were collected 2 h after culture in 8 μM MgCl2 N salts medium in the presence of increasing concentrations of the NO donor spermine NONOate. (B) The NO2--producing capacity of control and IFNγ-primed macrophages was measured by the Griess reaction 12 h after challenge. (C) Intracellular hmpA expression was quantified following expression of the hmpA::lacZ transcriptional fusion 20 h after infection.
Distribution of iNOS in IFNγ-activated macrophages
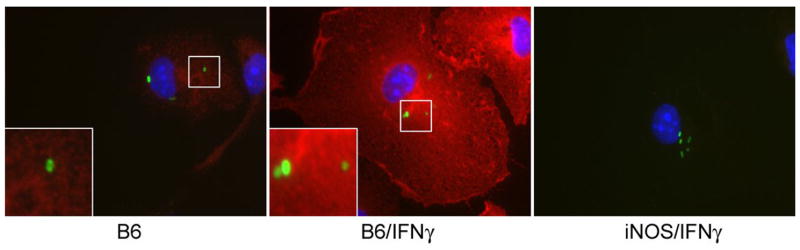
The iNOS enzymatic complex can partition in vesicular as well as cytosolic and cortical cytoskeletal fractions (Vodovotz et al., 1995; Webb et al., 2001). Because IFNγ enhances killing of nonfusogenic microbes such as Mycobacterium and Chlamydia by enhancing vesicular trafficking (MacMicking et al., 2003; Nelson et al., 2005), we tested whether abrogation of SPI2-dependent Salmonella survival manifested in IFNγ-activated macrophages may be related to the redistribution of iNOS-containing vesicles. Fluorescence microscopy at 20 h post Salmonella infection revealed punctate iNOS staining consistent with localization of the hemoprotein within membrane-bound vesicles as suggested in left insert of Fig. 3. In agreement with previous investigations (Chakravortty et al., 2002; Webb et al., 2001), no obvious clustering of iNOS-containing vesicles was observed around Salmonella within macrophages. No apparent differences were seen for the cellular distribution of iNOS in control and IFNγ-primed macrophages. Nonetheless, IFNγ induced a marked increase in iNOS staining throughout Salmonella-infected macrophages (Fig. 3, middle panel), consistent with the synergistic effects of IFNγ and bacterial ligands on iNOS transcription (Ding et al., 1988; Kamijo et al., 1993; Lorsbach et al., 1993; Xie et al., 1993). Staining for iNOS protein was enriched in both control and IFNγ-activated macrophages in perinuclear areas occupied by Salmonella (Fig. 3, panel insets).
Fig. 3.
IFNγ increases the expression of iNOS but does not stimulate the intracellular redistribution of iNOS. Distribution of iNOS (red) within untreated (B6) or IFNγ-treated (B6/IFNγ) macrophages was visualized 20 h post-infection with gfp-expressing Salmonella (green). Insets show detailed intracellular distribution of iNOS and Salmonella. IFNγ-treated macrophages isolated from iNOS-deficient mice (iNOS/IFNγ) were used as controls. Original magnification of 1000×.
IFNγ enhances nitrosative chemistry of Salmonella-infected macrophages
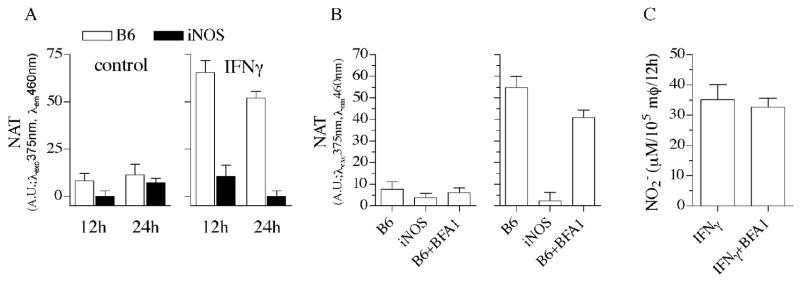
We explored the ability of control and IFNγ-primed macrophages to generate the strong nitrosating species N2O3. Similar to iNOS-deficient controls, periodate-elicited macrophages synthesized very little NAT during the innate response to Salmonella (Fig. 4A). In contrast, IFNγ-primed macrophages generated significant amounts of NAT after 12 h of infection. DAN can be nitrosated to NAT by N2O3 produced in the autooxidation of NO or by the condensation of HNO2 arising from acidified NO2−. Since the Salmonella phagosome acidifies to pH 5.0 (Rathman et al., 1996), it is possible that the N2O3 detected in IFNγ-primed macrophages represents a mixture of these two chemistries. To quantify the contribution of acidified NO2− to global N2O3 engendered by IFNγ-primed macrophages, the vacuolar ATPase inhibitor bafilomycin was added at the time of infection. Fig. 4B indicates that about one fourth of the N2O3 produced by macrophages can be accounted for by condensation of HNO2 in the acidified lumen of the Salmonella phagosome. Bafilomycin did not, however, reduce the overall formation of NO2− (Fig. 4C), demonstrating that reduction on N2O3 by bafilomycin does not reflect indiscriminate toxicity of the cell.
Fig. 4.
Effects of IFNγ treatment on the nitrosative capacity of Salmonella-infected macrophages. (A) Nitrosative stress was measured as the N-nitroso (NAT) derivative of diaminonaphthalene by wild-type and iNOS-deficient macrophages infected with Salmonella at an MOI of 2. Contribution of acidified NO2- to the nitrosative capacity of Salmonella-infected macrophages was estimated by studying the effects of the vacuolar ATPase inhibitor bafilomycin A1 (BFA1) on the generation of NAT (B) and NO2− (C).
Hmp prevents generation of N2O3 intracellularly
We tested the extent by which Hmp inhibits generation of N2O3 in the cytoplasm of Salmonella residing in control and IFNγ-activated macrophages (Fig. 5). Because the bacterial burden recovered from control and IFNγ-primed wild-type macrophages from C57BL/6 mice is so disparate (Fig. 1), the ability of Hmp to prevent N2O3 synthesis was studied in gp91phox−/−macrophages that are known to inhibit SPI2 transcription in an iNOS-dependent manner, while they unable to reduce Salmonella viability (McCollister et al., 2005; Vazquez-Torres et al., 2000a). To increase the sensitivity of the assay, N2O3 generation was measured in macrophages cultured in 24 well plates. Macrophages generated 3 times more N2O3 when infected with hmpA-deficient bacteria. A functional Hmp was calculated to prevent formation of ~40 nM N2O3/bacteria/h in macrophages that had not been treated with IFNγ, whereas this enzyme blocked generation of ~300 nM N2O3 in IFNγ-treated macrophages. Despite this robust diminution in N2O3 synthesis, Hmp-expressing bacteria were exposed to ~200 nM/h N2O3, which represents about nine times the amount of N2O3 encountered by Salmonella in control macrophages not treated with IFNγ.
Fig. 5.
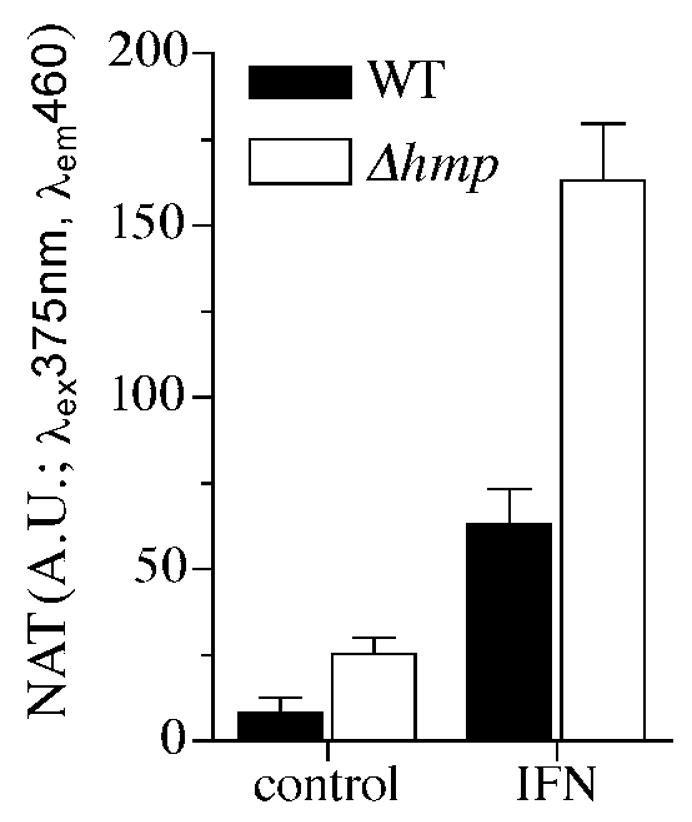
Contribution of N2O3 to the nitrosative capacity of IFNγ-primed macrophages. The ability of Hmp to diminish N-nitrosation by macrophages from C57BL/6 and gp91phox-deficient mice was studied 12 h after infection by following the generation of triazol (NAT) from DAN. Selected groups of macrophages were treated in vitro with 200 U/ml IFNγ 12-16 h before infection with wild-type (WT) or ΔhmpA-mutant Salmonella. To increase sensitivity of the assay, NAT formation was monitored in macrophages cultured in 24 well plates at a density of 5×105 cells/well and challenged with hmpA-proficient or -deficient Salmonella at an MOI of 5. After 12 h of infection, macrophages were incubated for 1 h in MEM+ medium supplemented with 200 μM DAN and 6 μg/ml gentamycin and the amount of NAT formed measured as above.
Hmp protects intracellular transcription of sdhA-encoding succinate dehydrogenase of the electron transport chain
Repression of SPI2 transcription in activated macrophages despite the abundant expression of Hmp represents an apparent paradox. To assess whether the Hmp expressed in IFNγ-primed macrophages is functionally active, we followed transcription of the respiratory sdhA and sdhB genes, which are tightly controlled by the ArcB sensor of the electron transport chain (Georgellis et al., 2001; Shen and Gunsalus, 1997). Expression of sdhA and sdhB was studied in gp91phox-deficient macrophages, because these phagocytes produce nitrosative chemistry inhibitory for SPI2 expression (McCollister et al., 2005) while preserving bacterial viability (Vazquez-Torres et al., 2000a). Hmp protected sdhA and sdhB expression in both control and IFNγ-primed macrophages (Fig. 6). Inhibition of sdhA and sdhB transcription in ΔhmpA::FRT Salmonella within gp91phox-deficient macrophages is NO-mediated, because transcription of these genes was restored in doubly immunodeficient phagocytes lacking both gp91phox and iNOS (Fig. 6).
Fig. 6.
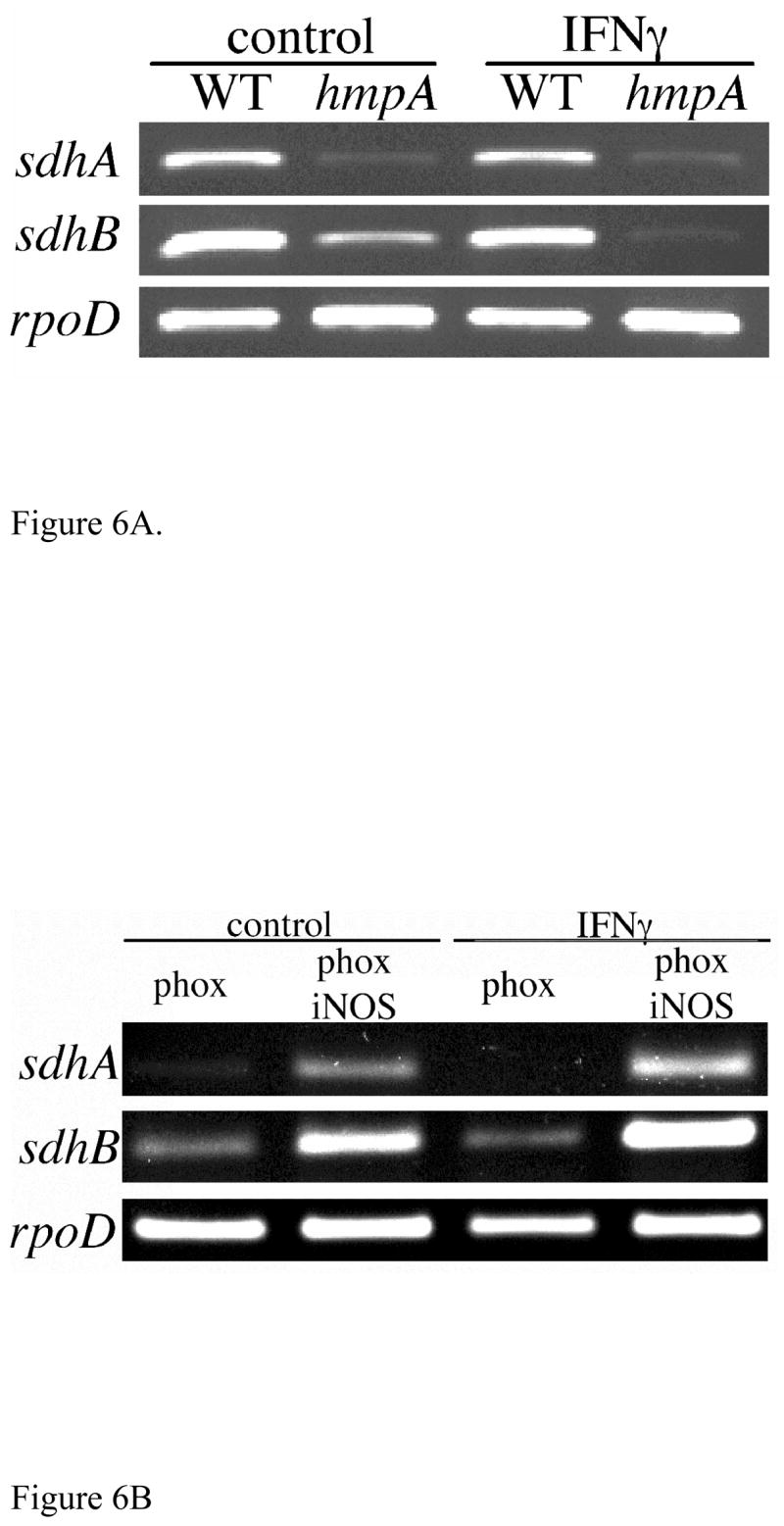
HmpA protects the Salmonella electron transport chain during innate and acquired responses of macrophages. (A) Expression of the sdhA and sdhB-encoding subunits of succinate dehydrogenase was studied by nested PCR in WT and Δhmp::FRT Salmonella RNA samples isolated from gp91phox-deficient macrophages 12 h after Salmonella infection. (B) Expression of sdhA and sdhB Δhmp::FRT Salmonella in gp91phox- or doubly gp91phox iNOS-deficient macrophages. Intracellular expression of the rpoD housekeeping sigma factor was used as a control.
Discussion
The enzymatic activity of iNOS is an integral component of the antimicrobial arsenal of macrophages; however, the biological chemistry mediating host defense associated with this hemoprotein is very little understood. We have used here a combination of bacterial genetics and biochemical assays to shed light into the nature of the RNS differentially expressed by populations of macrophages exhibiting strong iNOS-dependent antimicrobial activity against the intracellular pathogen Salmonella. Transcriptional analysis of sdhA and sdhB genes encoding subunits of the complex II of the electron transport chain indicates that Salmonella Hmp is functional in both control and IFNγ-primed macrophages. The 4-fold increases in NO synthesis in IFNγ-primed macrophages are met by a 7-fold up-regulation in transcription of hmpA. This probably explains the sustained expression of sdhA and sdhB in control and IFNγ-activated macrophages. Transcription of sdhA and sdhB is tightly regulated by ArcB (Georgellis et al., 2001; Shen and Gunsalus, 1997), which senses the reduced pool of quinones in the electron transport chain. Transcription of sdhA and sdhB in macrophages sustaining disparate levels of nitrosative stress is probably an indication of preserved respiratory activity by the NO-consuming activity of Hmp. These findings suggest that a functional Hmp detoxifies NO quite effectively at the various rates generated by professional phagocytes in various states of activation.
Hmp also protects SPI2 transcription against nitrosative stress generated by resting macrophages. Salmonella lacking Hmp experienced three times more N2O3 in resting macrophages than in wild-type controls, resulting in comparable reductions in SPI2 expression and intracellular survival. Because SPI2 is critical for intracellular survival of Salmonella in a variety of phagocytic and nonphagocytic cells and is essential for the development of systemic salmonellosis (Ochman et al., 1996; Shea et al., 1996; Vazquez-Torres et al., 2000b), protection of SPI2 transcription against NO toxicity represents a novel mechanism by which Hmp may contribute to Salmonella virulence (Bang et al., 2006). It should be noted, nonetheless, that SPI2-dependent survival in resting macrophages was not completely abrogated in hmpA-deficient Salmonella. In the absence of Hmp, the low yields of nitrosating species invoked by innate LPS-TLR4 host signaling are likely to be antagonized by the small thiol-containing molecules homocysteine and glutathione (De Groote et al., 1996; Hausladen et al., 1996). In addition, the vacuolar-remodeling capacity of SPI2 may also lessen NO-mediated toxicity in resting populations of macrophages (Chakravortty et al., 2002). Together, the ability of Salmonella to avoid, scavenge and detoxify NO lessen nitrosative stress evoked by the innate expression of iNOS.
Hmp fails, however, to protect SPI2 transcription from the toxicity associated with nitrogen oxides produced by IFNγ-activated macrophages. Repression of SPI2 in view of Hmp-protected sdhA sdhB transcription is even more remarkable when one considers that the spermine NONOate IC50 values for inhibition of SPI2 expression and respiration are quite similar in vitro. These data suggest that IFNγ-primed macrophages generate a variety of nitrogen oxides with distinct biological chemistries. We therefore evaluated the ability of IFNγ-primed macrophages to generate N2O3 in response to hmpA-proficient and -deficient Salmonella. Hmp prevented formation of 300 nM N2O3/h in IFNγ-primed macrophages. This activity indicates that at least 60% of the total nitrosative capacity of activated macrophages is directed towards Salmonella. Activated macrophages produced 200 nM N2O3/h that is not susceptible to detoxification by Hmp, an amount of N2O3 that represents nine times that produced by control phagocytes. Studies with the vacuolar inhibitor bafilomycin indicate that at least 50 nM/h are generated in the vacuoles by the condensation of HNO2. Once N2O3 is formed by either the autooxidation of NO or the condensation of HNO2, the NO-detoxifying activity of Hmp cannot protect Salmonella against the cytotoxicity derived from these RNS. N2O3 is a potent nitrosative species that could increase the anti-Salmonella potential of IFNγ-primed macrophages.
In summary, Hmp detoxifies NO produced by control and IFNγ-primed macrophages, thus effectively preserving the function of NO-sensitive targets in disparate intracellular conditions. However, high NO fluxes sustained by IFNγ-primed macrophages generate nitrogen oxides such as N2O3 for which Hmp offers no protection. Further investigations will be needed to determine the extent by which products of the autooxidation of NO abrogate SPI2-transcription, thereby mediating the anti-Salmonella activity of IFNγ-primed macrophages.
Acknowledgments
We would like to thank Dr. R.Y. Tsien for kindly providing plasmid pRSET::gfp. Support of this work was provided by the National Institutes of Health (AI54959, AI053213, AI07447 and RR16082).
Abbreviations
- IFNγ
interferon-γ
- iNOS
inducible nitric oxide synthase
- N2O3
dinitrogen trioxide
- NO
nitric oxide
- RNS
reactive nitrogen species
- SPI2
Salmonella pathogenicity island 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alam MS, Akaike T, Okamoto S, Kubota T, Yoshitake J, Sawa T, Miyamoto Y, Tamura F, Maeda H. Role of nitric oxide in host defense in murine salmonellosis as a function of its antibacterial and antiapoptotic activities. Infect Immun. 2002;70:3130–3142. doi: 10.1128/IAI.70.6.3130-3142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang IS, Liu L, Vazquez-Torres A, Crouch ML, Stamler JS, Fang FC. Maintenance of nitric oxide and redox homeostasis by the salmonella flavohemoglobin hmp. J Biol Chem. 2006;281:28039–28047. doi: 10.1074/jbc.M605174200. [DOI] [PubMed] [Google Scholar]
- Chakravortty D, Hansen-Wester I, Hensel M. Salmonella pathogenicity island 2 mediates protection of intracellular salmonella from reactive nitrogen intermediates. J Exp Med. 2002;195:1155–1166. doi: 10.1084/jem.20011547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groote MA, Testerman T, Xu Y, Stauffer G, Fang FC. Homocysteine antagonism of nitric oxide-related cytostasis in Salmonella typhimurium. Science. 1996;272:414–417. doi: 10.1126/science.272.5260.414. [DOI] [PubMed] [Google Scholar]
- De Groote MA, Ochsner UA, Shiloh MU, Nathan C, McCord JM, Dinauer MC, Libby SJ, Vazquez-Torres A, Xu Y, Fang FC. Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc Natl Acad Sci USA. 1997;94:13997–14001. doi: 10.1073/pnas.94.25.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiwick J, Nikolaus T, Erdogan S, Hensel M. Environmental regulation of Salmonella pathogenicity island 2 gene expression. Mol Microbiol. 1999;31:1759–1773. doi: 10.1046/j.1365-2958.1999.01312.x. [DOI] [PubMed] [Google Scholar]
- Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- Ekman P, Saarinen M, He Q, Virtala M, Salmi M, Granfors K. Human monocytic U937 cells kill Salmonella in vitro by NO-independent mechanisms. Infect Immun. 1999;67:3670–3673. doi: 10.1128/iai.67.7.3670-3673.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espey MG, Miranda KM, Pluta RM, Wink DA. Nitrosative capacity of macrophages is dependent on nitric-oxide synthase induction signals. J Biol Chem. 2000;275:11341–11347. doi: 10.1074/jbc.275.15.11341. [DOI] [PubMed] [Google Scholar]
- Fujihara M, Ito N, Pace JL, Watanabe Y, Russell SW, Suzuki T. Role of endogenous interferon-β in lipopolysaccharide-triggered activation of the inducible nitric-oxide synthase gene in a mouse macrophage cell line, J774. J Biol Chem. 1994;269:12773–12778. [PubMed] [Google Scholar]
- Gallois A, Klein JR, Allen LA, Jones BD, Nauseef WM. Salmonella pathogenicity island 2-encoded type III secretion system mediates exclusion of NADPH oxidase assembly from the phagosomal membrane. J Immunol. 2001;166:5741–5748. doi: 10.4049/jimmunol.166.9.5741. [DOI] [PubMed] [Google Scholar]
- Gao JJ, Filla MB, Fultz MJ, Vogel SN, Russell SW, Murphy WJ. Autocrine/paracrine IFN-αβ mediates the lipopolysaccharide-induced activation of transcription factor Stat1α in mouse macrophages: pivotal role of Stat1α in induction of the inducible nitric oxide synthase gene. J Immunol. 1998;161:4803–4810. [PubMed] [Google Scholar]
- Gardner PR, Gardner AM, Martin LA, Salzman AL. Nitric oxide dioxygenase: an enzymic function for flavohemoglobin. Proc Natl Acad Sci USA. 1998;95:10378–10383. doi: 10.1073/pnas.95.18.10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgellis D, Kwon O, Lin EC. Quinones as the redox signal for the arc two-component system of bacteria. Science. 2001;292:2314–2316. doi: 10.1126/science.1059361. [DOI] [PubMed] [Google Scholar]
- Hausladen A, Privalle CT, Keng T, DeAngelo J, Stamler JS. Nitrosative stress: activation of the transcription factor OxyR. Cell. 1996;86:719–729. doi: 10.1016/s0092-8674(00)80147-6. [DOI] [PubMed] [Google Scholar]
- Hausladen A, Gow AJ, Stamler JS. Nitrosative stress: metabolic pathway involving the flavohemoglobin. Proc Natl Acad Sci USA. 1998;95:14100–14105. doi: 10.1073/pnas.95.24.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausladen A, Gow A, Stamler JS. Flavohemoglobin denitrosylase catalyzes the reaction of a nitroxyl equivalent with molecular oxygen. Proc Natl Acad Sci USA. 2001;98:10108–10112. doi: 10.1073/pnas.181199698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel M, Shea JE, Gleeson C, Jones MD, Dalton E, Holden DW. Simultaneous identification of bacterial virulence genes by negative selection. Science. 1995;269:400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- Kamijo R, Shapiro D, Le J, Huang S, Aguet M, Vilcek J. Generation of nitric oxide and induction of major histocompatibility complex class II antigen in macrophages from mice lacking the interferon gamma receptor. Proc Natl Acad Sci USA. 1993;90:6626–6630. doi: 10.1073/pnas.90.14.6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo R, Harada H, Matsuyama T, Bosland M, Gerecitano J, Shapiro D, Le J, Koh SI, Kimura T, Green SJ, Mak TW, Taniguchi T, Vilcek J. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science. 1994;263:1612–1615. doi: 10.1126/science.7510419. [DOI] [PubMed] [Google Scholar]
- Lorsbach RB, Murphy WJ, Lowenstein CJ, Snyder SH, Russell SW. Expression of the nitric oxide synthase gene in mouse macrophages activated for tumor cell killing. Molecular basis for the synergy between interferon-γ and lipopolysaccharide. J Biol Chem. 1993;268:1908–1913. [PubMed] [Google Scholar]
- MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie QW, Sokol K, Hutchinson N, Chen H, Mudgett JS. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- MacMicking JD, Taylor GA, McKinney JD. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science. 2003;302:654–659. doi: 10.1126/science.1088063. [DOI] [PubMed] [Google Scholar]
- Martin E, Nathan C, Xie QW. Role of interferon regulatory factor 1 in induction of nitric oxide synthase. J Exp Med. 1994;180:977–984. doi: 10.1084/jem.180.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroeni P, Vazquez-Torres A, Fang FC, Xu Y, Khan S, Hormaeche CE, Dougan G. Antimicrobial actions of the NADPH Phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II Effects on microbial proliferation and host survival in vivo. J Exp Med. 2000;192:237–247. doi: 10.1084/jem.192.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollister BD, Bourret TJ, Gill R, Jones-Carson J, Vazquez-Torres A. Repression of SPI2 transcription by nitric oxide-producing, IFNγ-activated macrophages promotes maturation of Salmonella phagosomes. J Exp Med. 2005;202:625–635. doi: 10.1084/jem.20050246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH. Experiments in molecular genetics. Plainview, NY: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- Nelson DE, Virok DP, Wood H, Roshick C, Johnson RM, Whitmire WM, Crane DD, Steele-Mortimer O, Kari L, McClarty G, Caldwell HD. Chlamydial IFN-γ immune evasion is linked to host infection tropism. Proc Natl Acad Sci USA. 2005;102:10658–10663. doi: 10.1073/pnas.0504198102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H, Soncini FC, Solomon F, Groisman EA. Identification of a pathogenicity island required for Salmonella survival in host cells. Proc Natl Acad Sci USA. 1996;93:7800–7804. doi: 10.1073/pnas.93.15.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori Y, Hamilton TA. Requirement for STAT1 in LPS-induced gene expression in macrophages. J Leukoc Biol. 2001;69:598–604. [PubMed] [Google Scholar]
- Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- Rathman M, Sjaastad MD, Falkow S. Acidification of phagosomes containing Salmonella typhimurium in murine macrophages. Infect Immun. 1996;64:2765–2773. doi: 10.1128/iai.64.7.2765-2773.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Onozuka K, Shinomiya H, Nakano M. Sensitivity of bacteria to NaNO2 and to L-arginine-dependent system in murine macrophages. Microbiol Immunol. 1991;35:325–329. doi: 10.1111/j.1348-0421.1991.tb01561.x. [DOI] [PubMed] [Google Scholar]
- Shea JE, Hensel M, Gleeson C, Holden DW. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:2593–2597. doi: 10.1073/pnas.93.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Gunsalus RP. Role of multiple ArcA recognition sites in anaerobic regulation of succinate dehydrogenase (sdhCDAB) gene expression in Escherichia coli. Mol Microbiol. 1997;26:223–236. doi: 10.1046/j.1365-2958.1997.5561923.x. [DOI] [PubMed] [Google Scholar]
- Shiloh MU, Ruan J, Nathan C. Evaluation of bacterial survival and phagocyte function with a fluorescence-based microplate assay. Infect Immun. 1997;65:3193–3198. doi: 10.1128/iai.65.8.3193-3198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiloh MU, MacMicking JD, Nicholson S, Brause JE, Potter S, Marino M, Fang F, Dinauer M, Nathan C. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- Suvarnapunya AE, Stein MA. DNA base excision repair potentiates the protective effect of Salmonella Pathogenicity Island 2 within macrophages. Microbiology. 2005;151:557–567. doi: 10.1099/mic.0.27555-0. [DOI] [PubMed] [Google Scholar]
- Uchiya K, Barbieri MA, Funato K, Shah AH, Stahl PD, Groisman EA. A Salmonella virulence protein that inhibits cellular trafficking. EMBO J. 1999;18:3924–3933. doi: 10.1093/emboj/18.14.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Torres A, Jones-Carson J, Mastroeni P, Ischiropoulous H, Fang FC. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I Effects on microbial killing by activated peritoneal macrophages in vitro. J Exp Med. 2000a;192:227–236. doi: 10.1084/jem.192.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Torres A, Xu Y, Jones-Carson J, Holden DW, Lucia SM, Dinauer MC, Mastroeni P, Fang FC. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science. 2000b;287:1655–1658. doi: 10.1126/science.287.5458.1655. [DOI] [PubMed] [Google Scholar]
- Vazquez-Torres A, Vallance BA, Bergman MA, Finlay BB, Cookson BT, Jones-Carson J, Fang FC. Toll-like receptor 4 dependence of innate and adaptive immunity to Salmonella: importance of the Kupffer cell network. J Immunol. 2004;172:6202–6208. doi: 10.4049/jimmunol.172.10.6202. [DOI] [PubMed] [Google Scholar]
- Vodovotz Y, Russell D, Xie QW, Bogdan C, Nathan C. Vesicle membrane association of nitric oxide synthase in primary mouse macrophages. J Immunol. 1995;154:2914–2925. [PubMed] [Google Scholar]
- Webb JL, Harvey MW, Holden DW, Evans TJ. Macrophage nitric oxide synthase associates with cortical actin but is not recruited to phagosomes. Infect Immun. 2001;69:6391–6400. doi: 10.1128/IAI.69.10.6391-6400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JK, Mastroeni P, Popoff JF, Evans CA, Blackwell JM. Slc11a1-mediated resistance to Salmonella enterica serovar Typhimurium and Leishmania donovani infections does not require functional inducible nitric oxide synthase or phagocyte oxidase activity. J Leukoc Biol. 2005;77:311–320. doi: 10.1189/jlb.0904546. [DOI] [PubMed] [Google Scholar]
- Xie QW, Whisnant R, Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J Exp Med. 1993;177:1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- Zhang X, Alley EW, Russell SW, Morrison DC. Necessity and sufficiency of beta interferon for nitric oxide production in mouse peritoneal macrophages. Infect Immun. 1994;62:33–40. doi: 10.1128/iai.62.1.33-40.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]