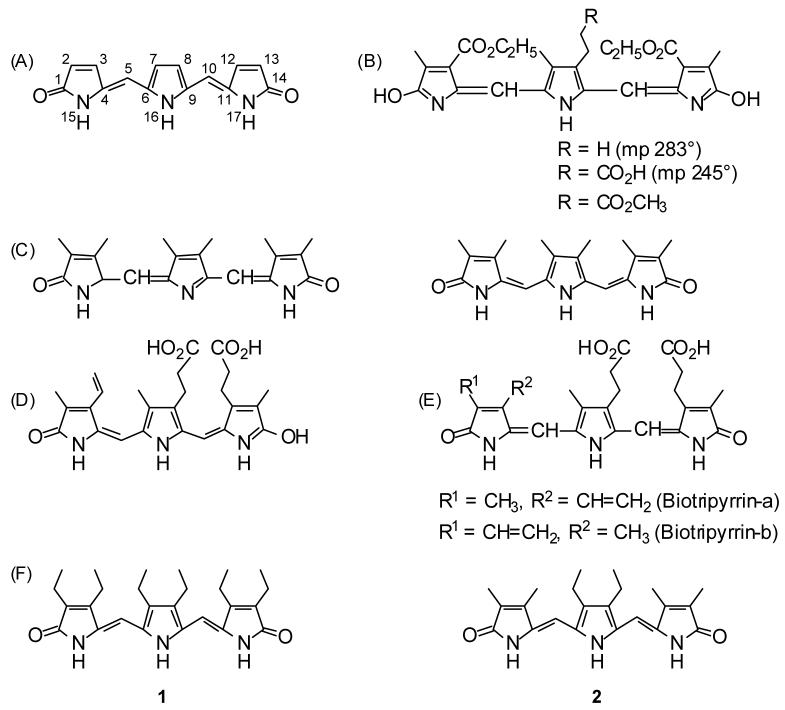
Figure 1.
(A) Tripyrrindione carbon framework and numbering system. (B) Fischer and Adler’s brown-violet-colored synthetic tripyrrindiones (ref. 1) in the Fischer representations as hydroxypyrroles with no C=C stereochemistry denoted. (C) Hexamethyl tripyrrindione of von Dobeneck (ref. 2) as formulated in 1966 (left) and revised in 1971 (right) with the E stereochemistry as drawn by von Dobeneck. (D) The reddish urinary pigment, uroerythrin as represented in ref. 3. (E) Nakajima’s tripyrrolic pigments from urine, redrawn as represented (ref. 4). (F) The synthetic targets of the current work.