Abstract
Purpose
Despite extensive research, the molecular basis of hypospadias and anorectal malformations is poorly understood, likely due to a multifactorial basis. The incidence of hypospadias is increasing, thus making research in this area warranted and timely. This review presents recent molecular work broadening our understanding of these disorders.
Materials and Methods
A brief review of our recent work and the literature on the role of Eph/ephrin signaling in hypospadias and anorectal malformations is presented.
Results
Genetically engineered mice mutant for ephrin-B2 or EphB2;EphB3 manifest a variety of genitourinary and anorectal malformations. Approximately 40% of adult male heterozygous mice demonstrate perineal hypospadias. Although homozygous mice die soon after birth, 100% of homozygous males demonstrate high imperforate anus with urethral anomalies and 100% of homozygous females demonstrate persistent cloaca. Male mice compound homozygous for EphB2ki/ki;EphB3Δ/Δ/ also demonstrate hypospadias.
Conclusions
These mouse models provide compelling evidence of the role of B-class Eph/ephrin signaling in genitourinary/anorectal development and add to our mechanistic and molecular understanding of normal and abnormal embryonic development. As research on the B-class Ephs and ephrins continues, they will likely be shown to be molecular contributors to the multifactorial basis of hypospadias and anorectal malformations in humans as well.
Keywords: Hypospadias, Anorectal Malformation, Mice, Children, EphB, ephrin-B2
Hypospadias
Hypospadias is the second most common human birth defect, affecting 1 in 125 male births1. Despite the high incidence, our current understanding of the etiology of hypospadias is at best incomplete. It is well understood that the androgen signaling cascade, including testosterone (T) regulation, production and biosynthesis, the peripheral conversion of T by 5-α-reductase to dihydrotestosterone (DHT), and T/DHT–androgen receptor interactions2,3 mediate virilization of the genital tubercle, with phallic growth and urethral tubularization. Human intersex states best exemplify this signaling gone awry2,3. It is also clear that altered androgen signaling, either on a genetic or hormonal level basis, does not completely account for all human cases of hypospadias and thus is collectively not solely responsible for hypospadias4. Additional molecules likely work independently or dependently with androgen signaling, either upstream, downstream or in concert. Recent investigations into several genetic syndromes have identified additional genes which when mutated or deleted cause hypospadias (Table 1), thereby making hypospadias a multifactorial birth defect.
Table 1.
Some genes associated with hypospadias when mutated or deleted
| Human Genes known to cause Hypospadias | Human chromosome | OMIM Reference Number |
|---|---|---|
| Intersex disorders | ||
| Androgen receptor (AR) | Xq11-q12 | 313700; 312300; 300068 |
| Steroid 5-alpha-reductase-2 (SRD5A2) | 2p23 | 607306 |
| Luteinizing hormone (LH) receptor | 2p21 | 152790 |
| 3 beta-hydroxysteroid dehydrogenase gene type II | 1p13.1 | 201810 |
| 17-beta-hydroxysteroid dehydrogenase (HSD17B4) | 5q2 | 601860 |
| 3-beta-hydroxysteroid dehydrogenase | 1p13.1 | 109715 |
| 17-alpha hydroxylase deficiency | 10q24.3 | 202119 |
| Steroidogenic acute regulatory (StAR) protein | 15q23-q24, 8p11.2 | 201710; 600617 |
| Sex-determining region on Y chromosome (SRY) | Yp11.3 | 480000 |
| SRY-BOX 9 (SOX9) | 17q24.3-q25.1 | 608160 |
| Receptor tyrosine kinase-like orphan receptor 2 (ROR2) | 9q22 | 268310; 602337 |
| Wilms' tumor supressor gene-1 (WT-1) | 11p13 | 607102; 194072; 194080; 136680 |
| Midline Malformation Syndromes | ||
| Midline 1 Ring Finger Gene (MID1) (FXY) | Xp22.3 | 300000 |
| sal-like gene 1 (SALL1) | 16q12.1 | 107480; 602218 |
| Gli-Kruppel Family Member 3 (GLI3) | 7p13 | 200990; 165240; 146510 |
| Limb-Genital Syndromes | ||
| Homeobox A13 (HOXA13) | 7p15-p14.2 | 142959; 140000 |
| Homeobox D13 (HOXD13) | 2q31-q32 | 142989 |
| Ephrin-B1 (EFNB1) | Xq12 | 304110 |
| Other Miscellaneous | ||
| Paired-like homeodomain transcription factor 2 (PITX2) | 4q25-q26 | 180500; 601542 |
| Protein O-Mannosyltransferase 1 (POMT1) | 9q34.1 | 236670 |
| SMADIP1 gene aka ZFHX1B (Zinc Finger Homeobox 1B gene) | 2q22 | 235730; 605802 |
| Glypican-3 (GPC3) | Xq26 | 312870 |
| X-linked lissencephaly (ARX) | Xp22.13 | 300004; 300382 |
| Fraser 1 (FRAS1) gene | 4q21 | 607830; 219000 |
| S terol delta-7-reductase gene (DHCR7, 7-alpha dehydrocholesterol reductase) | 11q12-q13 | 270400; 602858 |
OMIM: Online Mendelian Inheritance of Man (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM)
Even with this information, most cases of hypospadias go clinically unexplained. This deficiency of knowledge is concerning given that there are several publications that suggest the incidence of hypospadias is increasing5. In addition, there is a significant controversial body of literature suggesting that prenatal exposure of male feti (animal or human) to “endocrine disruptors”, environmental compounds with antiandrogenic or estrogenic properties, may be mediating the increase in hypospadias and also cryptorchidism6,7.
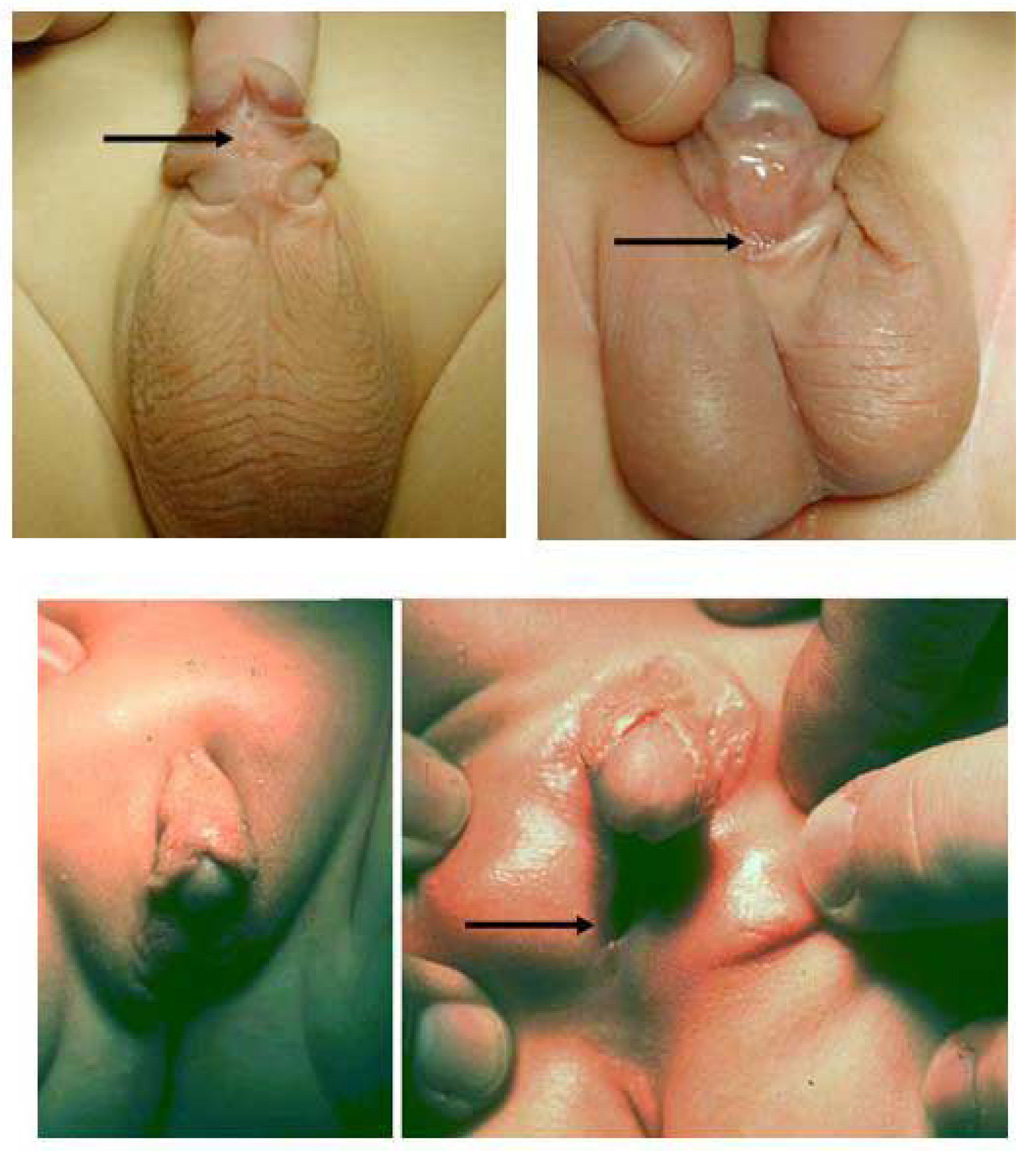
Hypospadias is clearly a human disorder with phenotypic variability, ranging from the mild distal glandular hypospadic meatus to the severe proximal perineal hypospadias (Figure 1). Fortunately, distal hypospadias is much more common, given that proximal hypospadias accounts for about 15% of cases. This information suggests that several mechanisms of maldevelopment might account for the phenotypic variability of hypospadias. In addition, proximal hypospadias cases can be associated with additional malformations, including penoscrotal transposition and anorectal malformations. Although not well recognized by urologists, the literature reports that up to 2.2–10.5% of patients with anorectal malformations also have hypospadias8–10.
Figure 1.
Three cases of human hypospadias demonstrating phenotypic variability. The arrow indicates the location of the urethral meatus in each case. (A) Subcoronal hypospadias. (B) Penoscrotal hypospadias with mild scrotal clefting. (C) Perineal hypospadias with ventral chordee and completely bifid scrotum.
Anorectal malformations
Congenital anorectal malformations are common surgical problems affecting 1 in 1500 to 1 in 5000 live births, with an even gender distribution 11 12 13 14. In humans, they present as a spectrum of abnormalities ranging from an ectopic anus, to an imperforate anus with fistula to the distal genitourinary tract, to complex cloacal abnormalities. To date, three genetic mouse models of anorectal malformations exist, namely Sonic hedgehog (Shh) 15–18, Gli2 and Gli3 18,19, and ephrin-B2lacZ/lacZ mutant mice.
Embryology
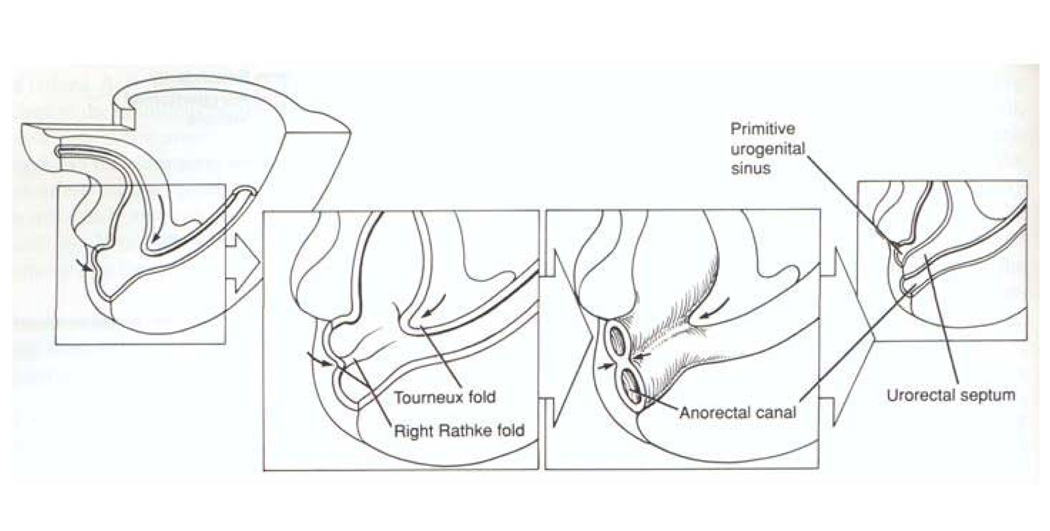
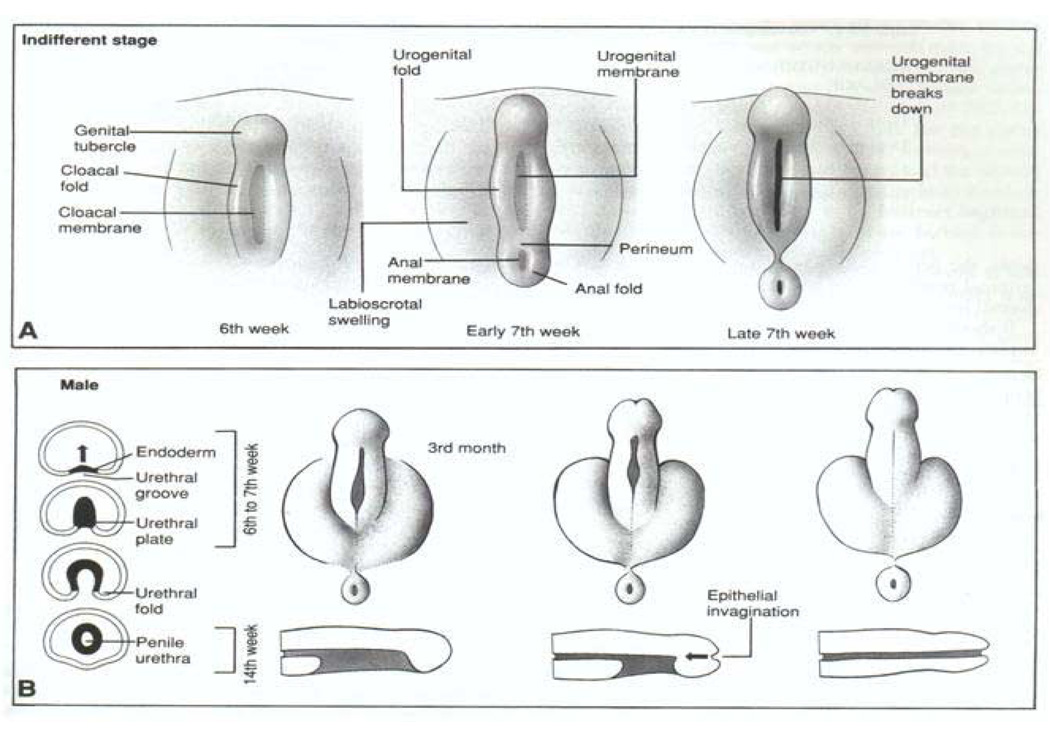
To fully understand hypospadias, anorectal malformations and how they might be linked, a review of normal embryology is warranted. In Figure 2, the process by which the cloaca (Latin – ‘sewer’) is septated is depicted, yielding separation of the urinary and fecal streams during normal embryonic development. Classic teaching describes the cloacal septation event occurring by the in growth of epithelium-covered mesenchymal folds, termed the cranial to caudal Tourneaux fold and the lateral to medial right and left Rathke folds. These folds, creating the urorectal septum, ultimately join the cloacal membrane, yielding the primitive urogenital sinus and the anorectal canal as two separate tubes. Although there has been a longstanding debate concerning the existence and nature of this septation event in human development20,21, it is true that by the early 7th week of human gestation the urogenital sinus and the anorectal canal are separate entities. Differentiation of the external genitalia (Figure 3) is not initiated until about the 8th week. This is simultaneous with when the testis or ovary differentiates depending on the activation of the SRY gene. In response to androgenic stimulation in the male, the perineum and labioscrotal folds fuse in the midline, and the genital tubercle and urethral plate undergo elongation and masculinization through the 4th month of gestation, yielding an elongated tubularized urethra. In contrast, non-virilized female external genitalia consist of a small clitoris with a short urethra, surrounded by the open labioscrotal folds. The vagina forms between the urethra and the anorectum.
Figure 2.
Depiction of human embryonic development at weeks 5–7 of gestation. The allantois and hindgut drain into the cloaca. Classic embryology teaching suggests the cloaca is septated by the caudal and ventral growth of the Tourneux fold and the lateral to medial growth of the left and right Rathke folds. These folds coalesce, forming the urorectal septum which divides the primitive urogenital sinus from the anorectal canal. This process is controversial. Reproduced with permission41.
Figure 3.
(A) Male and female human external genitalia are identical at 6–7 weeks gestation as cloacal septation occurs. (B) Under the influence of androgens produced by the differentiating male testis, the genital tubercle elongates, the urethra tabularizes, the labioscrotal folds fuse, and the male perineum forms. Female external genitalia form in the absence of androgen action. Reproduced with permission41.
The molecular mechanisms and factors which drive urethral tubularization are not fully understood. For example, it is not known whether processes (epithelial cell adhesive events, epithelial–mesenchymal interactions or mesenchymal ingrowth) that guide cloacal septation (yielding the posterior urethra in males and entire urethra in females) might also mediate male anterior urethral tubularization/formation.
Endocrine disruptor theory and hypospadias
It is well established that humans continually ingest substances with known estrogenic activity, such as insecticides utilized in crop production, natural plant estrogens, by-products of plastic production and pharmaceuticals. In fact, the canned food industry uses some chemical substances with estrogenic activity to cover the inner surface of cans. All endocrine disruptors find their way into fresh or seawater to be accumulated in higher organisms at the top of food chain. Therefore, top predators such as large fish, birds, sea mammals and humans store the highest level of environmental contaminants in their body. For example, thinning of eggshells in birds was traced to the estrogenic activity of insecticides to which birds were exposed through their diet. Thus, humans and wildlife are constantly exposed to estrogenic compounds with the ability to cause reproduction problems, the so-called estrogenic endocrine disruptors22,23.
Estrogenic contaminants are reported to disturb penile development in the American alligator24. Also, potent estrogen estradiol-17beta disrupts penile development in mice25. Diethylstilbestrol (DES) has been shown to cause urethral abnormalities in female neonatal mice26. DES-related problems are well known in human literature. Sons of DES-exposed mothers have a higher ratio of hypospadias and other urethral anomalies and difficulty in passing urine27. In mice, Kim et al. showed that treatment of pregnant mice with 17alpha ethinyl estradiol and DES between 12 and 17 days of gestation causes hypospadias in almost 50% of pups28. Another study confirmed the arrest in urethral tubularization in male mice under the effect of estrogenic compounds29.
Sexual dimorphism of the external genitalia is determined by the presence and absence of androgen receptor signaling. The fetal testes produce testosterone which is the primary serum androgen. Within the developing genital tubercle, testosterone is converted into 5 alpha-DHT by the enzyme 5alpha-reductase to create a more potent androgen. In the developing genital tubercle, 5alpha-reductase type 2 isozyme has been shown to locate at the mesenchyme surrounding the developing urethra at the particular region where midline fusion occurs for urethral tubularization30. In-utero exposure to anti-androgenic drugs inhibits the binding of testosterone and DHT to the androgen receptor to reduce the size of genital tubercle and anogenital distance. Likewise, 5 alpha-reductase inhibitors inhibit the distal development of the urethra resulting in hypospadias31.
The actions of androgens are elicited by a variety of downstream factors whose production, receptor binding and action must be intact. Unfortunately, today, we are still searching for these molecules that integrate the sex hormone balance with urethral tubularization.
B-class Eph/ephrin mutant mouse model
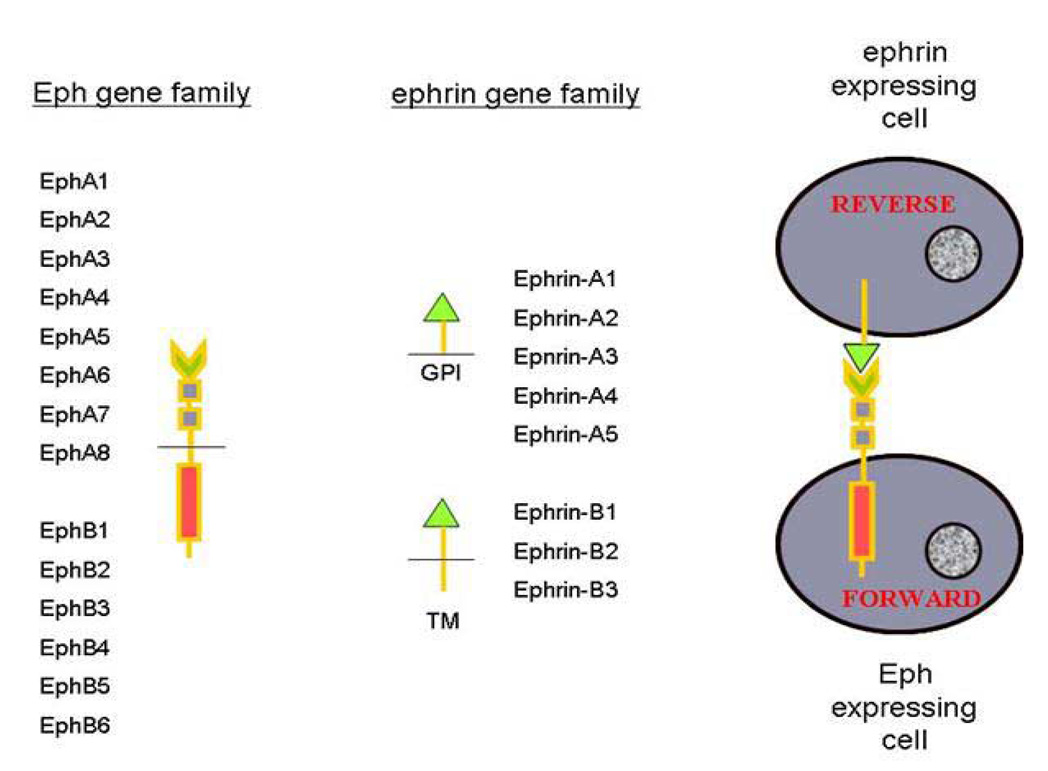
Our research group is investigating a novel genetically engineered murine model manifesting the unanticipated phenotype of hypospadias and genitourinary/anorectal malformations. The Eph and ephrin gene families (Figure 4) are known for their roles in cell–cell signaling, cell sorting32, axonal guidance during neuronal development33,34, delineation of embryonic cellular boundaries35, vasculogenesis36,37 and epithelial–mesenchymal transitions38. The Eph receptors, the largest subclass of receptor tyrosine kinases, and their membrane-bound ephrin ligands are two large highly conserved gene families expressed throughout invertebrates and vertebrates. Both the Ephs and ephrins are subdivided into A and B classes. For the most part, A-class Ephs bind to A-class ephrins and B-class Ephs bind to B-class ephrins, although there are a few exceptions to this rule. To understand their interactions, two cells are depicted in Figure 4, one expressing a B-class Eph molecule and the other expressing a B-class ephrin molecule. Upon activation of the EphB molecule by the ephrin-B molecule, a set of intracellular signals are activated within the EphB-expressing cell, which has been called the “forward” signal. Similarly, upon activation of the ephrin-B molecule by the EphB molecule, the “reverse” signal is activated within the ephrin-B-expressing cell. For ephrin-B2, it is clear that two ligands include EphB2 and EphB3. Thus, these molecules establish a system for cell–cell interactions and bidirectional signaling. Activation of these molecules alters cytoskeletal elements leading to alterations in cell morphology and in the nervous system, and cell–cell adhesive or repulsive events.
Figure 4.
The Eph and ephrin gene families are divided into A and B classes. Protein functional motifs are depicted by shapes; A-class ephrins are bound to the cell membrane by glycosylphosphotidy linositol (GPI) linkage while the B-class ephrins are transmembrane (TM) molecules. On the right, two cells are depicted, an Eph-expressing cell and an ephrin-expressing cell. Activation of the Eph receptor by an ephrin triggers the ‘forward’ signal in the Eph-expressing cell. Simultaneously, the ephrin is activated by the Eph, triggering the ‘reverse’ signal in the ephrin-expressing cell. Thus bidirectional signaling occurs and both gene families act as both receptors and ligands.
In order to better characterize the independent nature of “reverse” signaling, our group created a mutant mouse wherein the intracellular domain of the ephrin-B2 molecule was substituted by a lac Z cassette (ephrin-B2lacZ). The adult male heterozygous mice (Figure 5) manifest hypospadias at approximately 30–40% penetrance, independent of genetic background. Figure 5A demonstrates a normal adult wild-type mouse with a normal penis and tubularized urethra (Figure 5C). In contrast, Figure 5B demonstrates the ephrin-B2lacZ/+ heterozygous hyspospadic adult mouse wherein the anogenital distance is decreased and the penis is hypospadic, with an open urethral plate as indicated in Figure 5D. Given that ephrin-B2 binds to EphB2 and EphB3, adult mice functionally null for both EphB2 and EphB3 (EphB2ki/ki;EphB3Δ/Δ compound homozygotes) also manifested perineal hypospadias and a reduced perineal distance (Figure 6), confirming the key role of this signaling pathway. In these hypospadic mice, there is no sign of insufficient virilization, as judged by prostate and seminal vesicle size.
Figure 5.
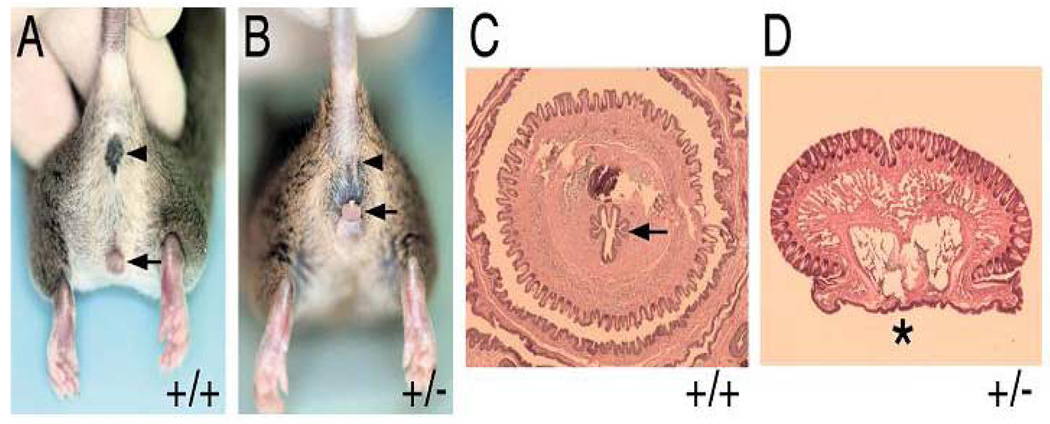
(A) Adult male wild-type (+/+) mouse depicting normal anus (arrowhead) and penis (arrow). (B) Adult male ephrin-B2lacZ/+ heterozygous(+/−) mouse with normal anus (arrowhead), reduced anogenital distance and perineal hypospadias (arrow). (C) Cross section of adult +/+ penis with tubularized urethra (arrow). (D) Cross section of adult-B2lacZ/+ heterozygous (+/−) mouse penis with hypospadias (asterisk). Reproduced with permission39.
Figure 6.
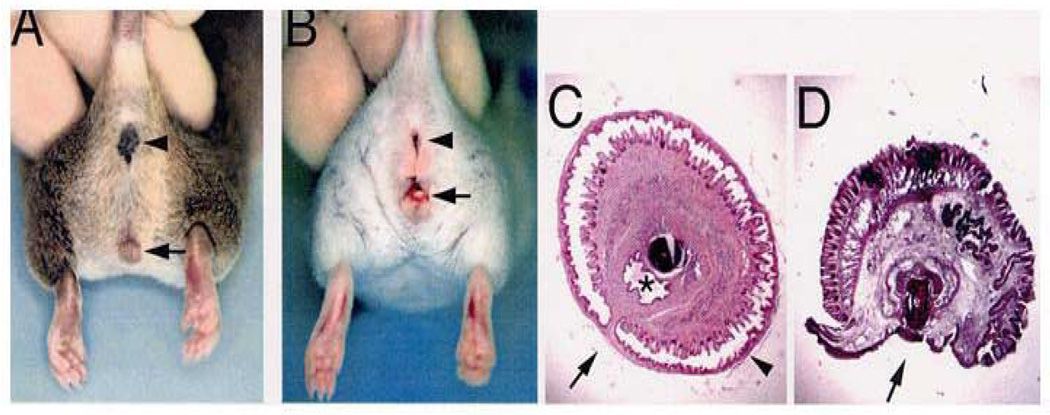
(A) Adult male wild-type (+/+) mouse depicting normal anus (arrowhead) and penis (arrow). (B) Adult male EphB2ki/ki;EphB3Δ/Δ compound homozygote mouse with normal anus (arrowhead), reduced anogenital distance and perineal hypospadias (arrow). (C) Cross section of adult +/+ penis with tubularized urethra (asterisk). (D) Cross section of adult EphB2ki/ki;EphB3Δ/Δ compound homozygous mouse penis with hypospadias (arrow). Reproduced with permission39.
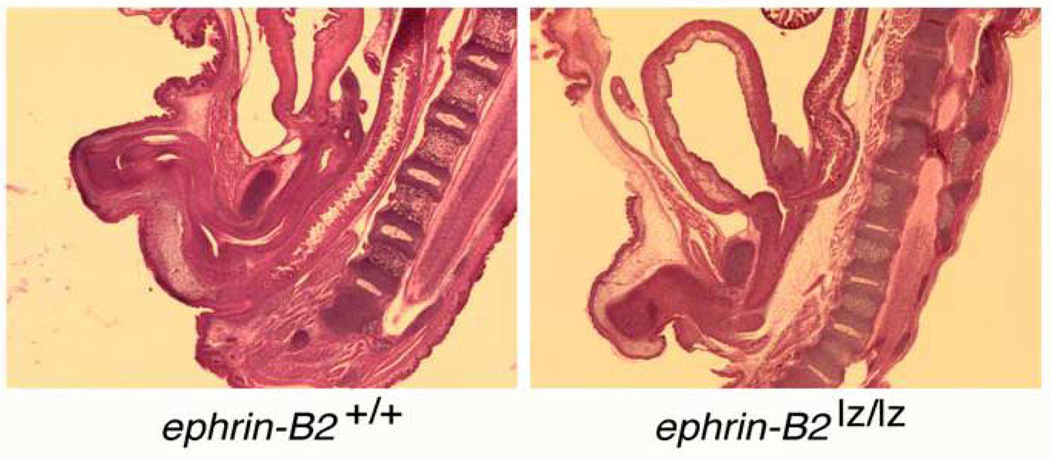
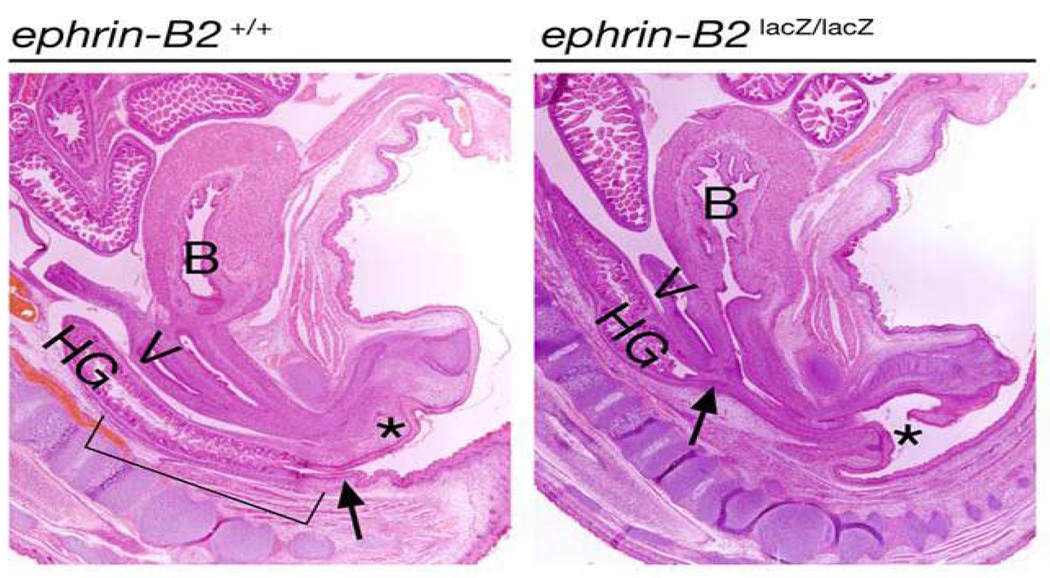
To understand the maldevelopment of the hypospadias in these adult mutant mice, the mutant mouse embryos were studied. In Figure 7, β-gal staining reveals that the ephrin-B2lacZ protein is localized within the endodermal cells of the urethral plate. In these heterozygous mutant mice, urethral tubularization is incomplete, yielding perineal hypospadias. When compared to the wild-type animals as seen in the top panel, the bottom panel also clearly reveals that there is a defect of a persistent cloaca in these males. In the series of images in Figure 8, whole-mount visualization of these same embryos gives another view of the perineal defect at low and high power magnification. The tail has been amputated and is in the upper area of the images. The genital tubercle is then seen coming out towards the viewer in the lower portion of the images. Figure 8A shows a wild-type animal at embryonic day 16, demonstrating complete septation of both the urogenital sinus and the anorectal canal with closure of the perineum in the midline. In comparison, two EphB2χ/χ; EphB3⊗/⊗ mutant littermates are seen in panels 8B and C. These two littermates with identical genotypes show phenotypic variability, with the mouse seen in panel B being less severely affected than the mouse in C, but it is clear that when they are both compared to the wild-type littermate, the perineum has not closed and there is an open cloaca. These data therefore suggest that ephrin-B2 and EphB2/EphB3 are key molecules involved in septation events in the perineum. This concept was further confirmed when we examined the ephrin-B2lacZ/lacZ homozygous mice. As seen in Figure 9, the newborn wild-type male (left panel) demonstrates a normal anorectal canal, normal bladder and completely tubularized urethra. In contrast, on the right, the newborn male ephrin-B2lacZ/lacZ homozygous littermate has a high imperforate anus. The arrow indicates the location of the rectourethral fistula entering at the base of the bladder neck. Female ephrin-B2lacZ/lacZ homozygous mice demonstrate persistent cloaca (Figure 10). This work has recently been published39. Taken together, these murine data reveal the key role of B-subclass Eph/ephrin signaling in normal midline fusion events of the perineum, cloaca and external genitalia, and reveal gene dosage sensitivity, yielding hypospadias, anorectal malformations and cloaca.
Figure 7.
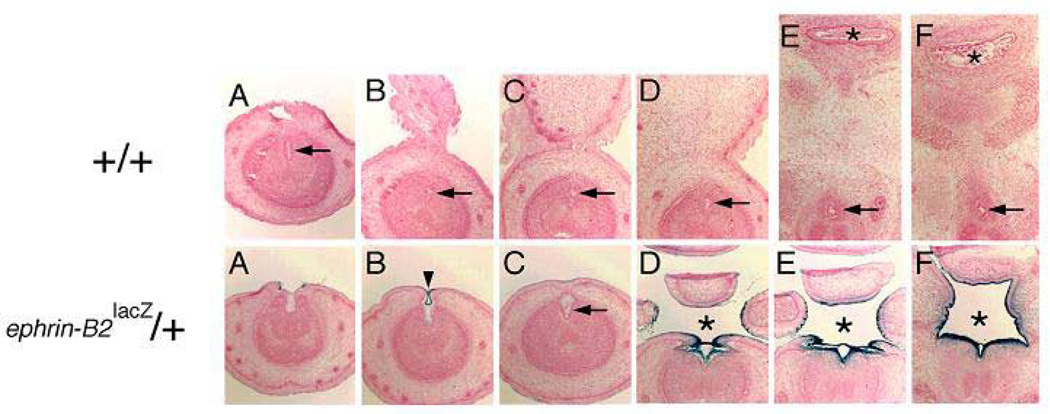
Upper panel: consecutive cross sections of embryonic day 17 (E17) wild-type mouse penis demonstrating tubularized urethra (arrow) and normal septated anus (asterisk). From left to right, the sections progress from the distal penis inward into the perineum. Lower panel: consecutive cross sections of embryonic day 17 (E17) ephrin-B2lacZ/+ heterozygous mouse penis demonstrating incomplete proximal urethral tubularization and perineal closure (asterisk). In some more distal locations, urethral tubularization appeared more normal (arrow and arrowhead). The dark-blue staining indicates the high expression of ephrin-B2lacZ in the tubularizing urethral epithelium, septating epithelium of the cloaca and urogenital sinus (not shown). From left to right, the sections progress from the distal penis inward into the perineum. Reproduced with permission39.
Figure 8.
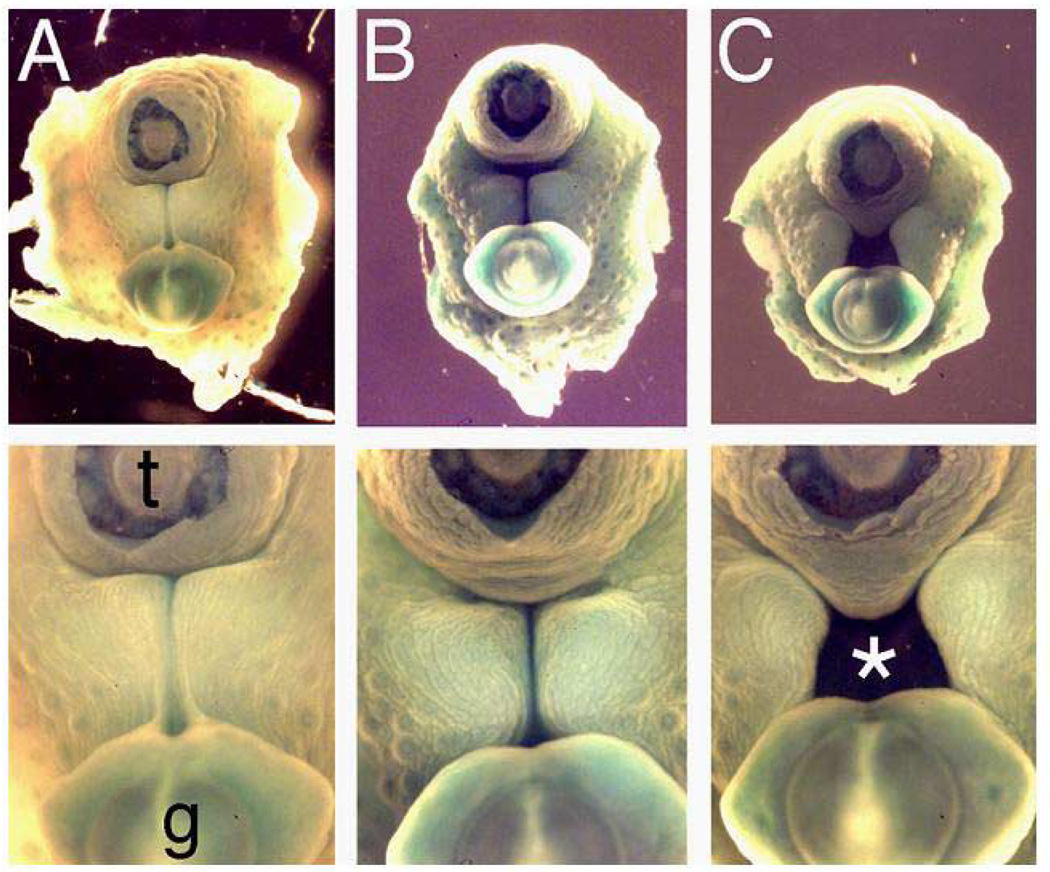
Low power (upper panel) and high power (lower panel) view of the perineum of three E16 littermates with the amputated tail (t) and genital tubercle (g) viewed at top and bottom of photos, respectively. (A) EphB2χi/+;EphB3Δ/Δ mouse demonstrates closed perineum and tubularized urethra. (B and C) Two examples of EphB2χi/χi;EphB3Δ/Δ mice with mild (B) and severe (C) delays in perineal closure and urethral tubularization, with cloaca (asterisk). Reproduced with permission39.
Figure 9.
Left: sagittal section of E18 male wild-type mouse demonstrating normal spine, anorectum and male urethra. Right: sagittal section of E18 ephrin-B2lacZ/+ male with normal spine and high imperforate anus with rectourethral fistula at the bladder neck. Anal dimple is present.
Figure 10.
Left: sagittal section of E18 female wild-type mouse demonstrating normal spine, bladder (B), anorectum–hindgut (HG), vagina (V) and female urethra (asterisk). Right: sagittal section of E18 ephrin-B2lacZ/+ female with normal spine and high persistent cloaca (the arrow). Anal dimple is present. Reproduced with permission39.
To discriminate between EphB2-mediated forward (cell autonomous) and reverse (non-cell autonomous) signaling, a EphB2χ mutation producing a kinase-inactive C-terminally truncated EphB2–β-gal fusion protein was bred to generate EphB2χ/χ; EphB3⊗/⊗ compound heterozygotes. The EphB2χ- encoded EphB2–β-gal fusion protein exhibits normal protein trafficking and ability to stimulate reverse signaling in adjacent ephrin-expressing cells.
Organ Culture System
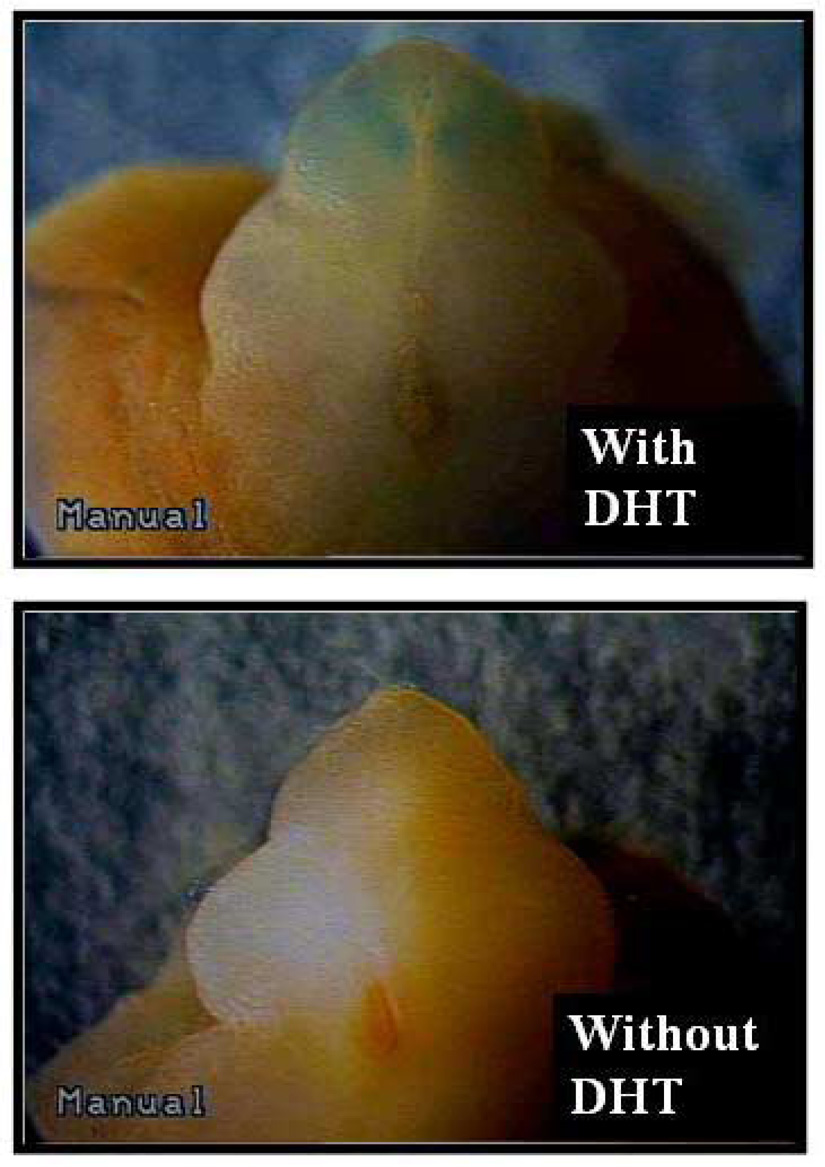
In order to address whether EphB2 might be an androgen-regulated gene, an organ culture system was used wherein embryonic genital tubercles from the EphB2lac Z/+ mice could be grown. In these mice with normal genitalia, EphB2 expression is highlighted by the beta-galactosidase color reaction (blue). Embryonic day 12.5 (E12.5) genital tubercles from females and males were microdissected and grown in culture for 2 days with and without the presence of DHT. As seen in Figure 11, the female genital tubercle responded to DHT exposure with phallic enlargement, urethra closure distally, and increasing EphB2 expression in the urethral plate distal to the closure, all suggesting that EphB2 can be up-regulated by DHT exposure in the genital tubercle40. Thus, EphB2 is a candidate androgen-regulated gene that might mediate the virilized genital phenotypes seen in female pseudohermaphrodites that are exposed to prenatal excess adrenal androgens.
Figure 11.
E12.5 female EphB2lacZ/+ heterozygous genital tubercles were harvested and cultured in media with or without DHT for 2 days. The female genital tubercles responded to DHT exposure with phallic enlargement, urethral tubularization distally and increasing EphB2 expression in the glans and urethral plate distal to closure.
Conclusions
The B-class Eph/ephrin cell–cell signaling pathway is crucial in mouse urethral and anorectal development and EphB2 is a candidate androgen-regulated gene. Many studies are currently underway further exploring the mouse model and the role these molecules may play in human hypospadias and urogenital/anorectal development.
Acknowledgments
This work was funded in part by NIH R01 DK 59164 (Baker, PI), NIH R01 DK 59164-S1 (Baker, PI – Garcia, Mentoree) and a Children’s Medical Center at Dallas Clinical Research Grant (Garcia, PI).
Selcuk Yucel is supported by Akdeniz University Scientific Research and Project Unit.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Paulozzi LJ, Erickson D, Jackson RJ. Hypospadias trends in two US surveillance systems. Pediatrics. 1997;100:831–834. doi: 10.1542/peds.100.5.831. [DOI] [PubMed] [Google Scholar]
- 2.Wilson JD, Griffin JE, George FW, Leshin M. The endocrine control of male phenotypic development. Aust J Biol Sci. 1983;36:101–128. doi: 10.1071/bi9830101. [DOI] [PubMed] [Google Scholar]
- 3.Wilson JD, Griffin JE, Russell DW. Steroid 5 alpha-reductase 2 deficiency. Endocr Rev. 1993;14:577. doi: 10.1210/edrv-14-5-577. [DOI] [PubMed] [Google Scholar]
- 4.Holmes N, Miller W, Baskin L. Lack of defects in androgen production in children with hypospadias. J Clin Endocrinol Metab. 2004;89:2811–2816. doi: 10.1210/jc.2003-032098. [DOI] [PubMed] [Google Scholar]
- 5.Paulozzi L. International trends in rates of hypospadias and cryptorchidism. Environ Health Perspect. 1999;107:297–302. doi: 10.1289/ehp.99107297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharpe R. The 'oestrogen hypothesis - where do we stand now? Int J Androl. 2003;26:2–15. doi: 10.1046/j.1365-2605.2003.00367.x. [DOI] [PubMed] [Google Scholar]
- 7.Fisher J. Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction. 2004;127:305–315. doi: 10.1530/rep.1.00025. [DOI] [PubMed] [Google Scholar]
- 8.Brock WA, Pena A. Urological Implications of Imperforate Anus. AUA Update Series. 1991;X:202–207. [Google Scholar]
- 9.Metts JC, 3rd, Kotkin L, Kasper S, Shyr Y, Adams MC, Brock JW., 3rd Genital malformations and coexistent urinary tract or spinal anomalies in patients with imperforate anus. J Urol. 1997;158:1298–1300. doi: 10.1097/00005392-199709000-00168. [DOI] [PubMed] [Google Scholar]
- 10.Lerone M, Bolino A, Martucciello G. The genetics of Anorectal Malformations: A Complex Matter. Semin Pediatr Surg. 1997;6:170–179. [PubMed] [Google Scholar]
- 11.Christensen K, CM M, H M. An epidemiological study of congenital anorectal malformations: 15 Danish birth cohorts followed for 7 years. Pediatr Perinat Epidemiol. 1990;4:269–275. doi: 10.1111/j.1365-3016.1990.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 12.Kiesewetter W, Chang J. Imperforate anus: A five to thirty year follow-up perspective. Prog Pediatr Surg. 1977;10:111–120. [PubMed] [Google Scholar]
- 13.Spounge D, Baird P. Imperfortate anus in 100,000 consecutive liveborn infants. Am J Med Genet. 1986;2 suppl:151–161. doi: 10.1002/ajmg.1320250619. [DOI] [PubMed] [Google Scholar]
- 14.Smith E. Incidence, frequency of types, and etiology of anorectal malformations. In: Stephens F, Smith E, editors. Anorectal Malformations in Children: Update 1988. New York, NY: Liss; 1988. pp. 231–247. [Google Scholar]
- 15.Kim J, Kim P, Hui CC. The VACTERL association: lessons from the Sonic hedgehog pathway. Clin Genet. 2001;59:306–315. doi: 10.1034/j.1399-0004.2001.590503.x. [DOI] [PubMed] [Google Scholar]
- 16.Mo R, Kim JH, Zhang J, Chiang C, Hui CC, Kim PC. Anorectal malformations caused by defects in sonic hedgehog signaling. Am J Pathol. 2001;159:765–774. doi: 10.1016/S0002-9440(10)61747-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–2772. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- 18.Arsic D, Qi BQ, Beasley SW. Hedgehog in the human: a possible explanation for the VATER association. J Paediatr Child Health. 2002;38:117–121. doi: 10.1046/j.1440-1754.2002.00813.x. [DOI] [PubMed] [Google Scholar]
- 19.Kimmel SG, Mo R, Hui CC, Kim PC. New mouse models of congenital anorectal malformations. J Pediatr Surg. 2000;35:227–230. doi: 10.1016/s0022-3468(00)90014-9. discussion 230-1. [DOI] [PubMed] [Google Scholar]
- 20.Paidas C, Rogers D, Morreale R, Huthcins G. Septation of the anorectal and genitourinary tract in the human embryo: crucial role of the catenoidal shape of the urorectal sulcus. Teratology. 2002;66:144–152. doi: 10.1002/tera.10041. [DOI] [PubMed] [Google Scholar]
- 21.Hynes P, Fraher J. The development of the male genitourinary system. I. The origin of the urorectal septum and the formation of the perineum. Br J Plast Surg. 2004;57:27–36. doi: 10.1016/j.bjps.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Colborn T. The wildlife/human connection: modernizing risk decisions. Environ Health Perspect. 1994;102 Suppl 12:55–59. doi: 10.1289/ehp.94102s1255a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semenza JC, Tolbert PE, Rubin CH, Guillette LJ, Jr, Jackson RJ. Reproductive toxins and alligator abnormalities at Lake Apopka, Florida. Environ Health Perspect. 1997;105:1030–1032. doi: 10.1289/ehp.971051030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warner MR, Warner RL, Clinton CW. Reproductive tract calculi, their induction, age incidence, composition and biological effects in Balb/c Crgl mice injected as newborns with estradiol-17 beta. Biol Reprod. 1979;20:310–322. doi: 10.1095/biolreprod20.2.310. [DOI] [PubMed] [Google Scholar]
- 26.Miyagawa S, Buchanan DL, Sato T, Ohta Y, Nishina Y, Iguchi T. Characterization of diethylstilbestrol-induced hypospadias in female mice. Anat Rec. 2002;266:43–50. doi: 10.1002/ar.10033. [DOI] [PubMed] [Google Scholar]
- 27.McLachlan J. Rodent models for perinatal exposure to diethylstilbestrol and their relation to human disease in the male. In: Herbst AL, Bern HA, editors. Developmental Effects of Diethylstilbestrol (DES) in Pregnancy. New York: Thieme-Stratton, Inc.; 1981. [Google Scholar]
- 28.Kim KS, Torres CR, Jr, Yucel S, Raimondo K, Cunha GR, Baskin LS. Induction of hypospadias in a murine model by maternal exposure to synthetic estrogens. Environ Res. 2004;94:267–275. doi: 10.1016/S0013-9351(03)00085-9. [DOI] [PubMed] [Google Scholar]
- 29.Yucel S, Cavalcanti AG, Desouza A, Wang Z, Baskin LS. The effect of oestrogen and testosterone on the urethral seam of the developing male mouse genital tubercle. BJU Int. 2003;92:1016–1021. doi: 10.1111/j.1464-410x.2003.04511.x. [DOI] [PubMed] [Google Scholar]
- 30.Kim KS, Liu W, Cunha GR, Russell DW, Huang H, Shapiro E, Baskin LS. Expression of the androgen receptor and 5 alpha-reductase type 2 in the developing human fetal penis and urethra. Cell Tissue Res. 2002;307:145–153. doi: 10.1007/s004410100464. [DOI] [PubMed] [Google Scholar]
- 31.Kurzrock EA, Jegatheesan P, Cunha GR, Baskin LS. Urethral development in the fetal rabbit and induction of hypospadias: a model for human development. J Urol. 2000;164:1786–1792. [PubMed] [Google Scholar]
- 32.Mellitzer G, Xu Q, Wilkinson DG. Control of cell behaviour by signalling through Eph receptors and ephrins. Curr Opin Neurobiol. 2000;10:400–408. doi: 10.1016/s0959-4388(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 33.Wilkinson DG. Multiple roles of EPH receptors and ephrins in neural development. Nat Rev Neurosci. 2001;2:155–164. doi: 10.1038/35058515. [DOI] [PubMed] [Google Scholar]
- 34.Knoll B, Drescher U. Ephrin-As as receptors and ephrins in neural development. Nat Rev Neurosci. 2002;25:145–149. doi: 10.1016/s0166-2236(00)02093-2. [DOI] [PubMed] [Google Scholar]
- 35.Kullander K, Klein R. Mechanisms and functions of eph and ephrin signaling. Nat Rev Mol Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- 36.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 37.Gale NW, Baluk P, Pan L, Kwan M, Holash J, DeChiara TM, McDonald DM, Yancopoulos GD. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev Biol. 2001;230:151–160. doi: 10.1006/dbio.2000.0112. [DOI] [PubMed] [Google Scholar]
- 38.Barrios A, Poole RJ, Durbin L, Brennan C, Holder N, Wilson SW. Eph/Ephrin signaling regulates the mesenchymal-to-epithelial transition of the paraxial mesoderm during somite morphogenesis. Curr Biol. 2003;13 doi: 10.1016/j.cub.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 39.Dravis C, Yokoyama N, Chumley MJ, Cowan CA, Silvany RE, Shay J, Baker LA, Henkemeyer M. Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev Biol. 2004;271:272–290. doi: 10.1016/j.ydbio.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 40.Lorenzo AJ, Nguyen MT, Sozubir S, Henkemeyer M, Baker LA. Dihydrotestesterone induction of EPHB2 expression in the female genital tubercle mimics male pattern of expression during embryogenesis. J Urol. 2003;170:1618–1623. doi: 10.1097/01.ju.0000087423.89813.64. discussion 1623. [DOI] [PubMed] [Google Scholar]
- 41.Larsen WJ. Development of the Urogenital System, Human Embryology. 2nd Edition. New York: Churchill Livingstone; 1997. pp. 261–310. [Google Scholar]