Abstract
A new prototype of polymer-derived drug delivery system, the nanoconjugate Polycefin, was tested for its ability to accumulate in tumors based on enhanced permeability and retention (EPR) effect and receptor mediated endocytosis. Polycefin was synthesized for targeted delivery of Morpholino antisense oligonucleotides into certain tumors. It consists of units that are covalently conjugated with poly(β-L-malic acid) (Mw 50,000, Mw/Mn 1.3) highly purified from cultures of myxomycete Physarum polycephalum. The units are active in endosomal uptake, disruption of endosomal membranes, oligonucleotide release in the cytoplasm, and protection against enzymatic degradation in the vascular system. The polymer is biodegradable, non-immunogenic and non-toxic. Polycefin was also coupled with AlexaFluor 680 C2-maleimide dye for in vivo detection.
Nude mice received subcutaneous injections of MDA-MB 468 human breast cancer cells into the left posterior mid-dorsum or intracranial injections of human glioma cell line U87MG. Polycefin at concentration of 2.5 mg/kg was injected via the tail vein. In vivo fluorescence tumor imaging was performed at different time points, 0–180 min up to 24 h after the drug injection. The custom-made macro-illumination imaging MISTI system was used to examine the in vivo drug accumulation in animals bearing human breast and brain tumors. In breast tumors the fluorescence signal in large blood vessels and in the tumor increased rapidly until 60 min and remained in the tumor at a level 6 times higher than in non-tumor tissue (180 min) (p < 0.003). In brain tumors drug accumulated selectively in 24 h without any detectable signal in non-tumor areas. The results of live imaging were corroborated histologically by fluorescence microscopic examination of various organs. In addition to tumors, only kidney and liver showed some fluorescent signal. © 2007 Elsevier Ireland Ltd. All rights reserved.
Keywords: Brain glioma, Breast cancer, EPR effect, Fluorescence imaging, Drug delivery system, Poly(malic acid)
1. Introduction
Targeted delivery is the new momentum in drug tumor treatment allowing increased efficiency, tumor specificity, and reduced systemic toxicity. Nanoconjugates, the covalent complexes of drug releasing molecular machines, offer an excellent control of proper delivery to tumors with minimal side effects, their synthesis following a unit construction system mounting together distinct modules that function in drug delivery. Our emphasis is in the design of a biodegradable, non-toxic and non-immunogenic nanoconjugate. To this end, we introduced a new platform for conjugate synthesis, poly(β-L-malic acid) (PMLA). This polyanion is synthesized by the slime mold Physarum polycephalum, which uses the polymer for stockpiling and trafficking of nucleic acid binding proteins across the giant polynucleate amoeba-like cell, the plasmodium (Fig. 1) [1]. Polycefin has been synthesized for targeted delivery of antisense oligonucleotides (AON) and antibodies into certain tumors and consists of several modules chemically conjugated to PMLA (Fig. 2) [2]. Their functions allow Polycefin for crossing blood-tumor barrier (BTB), attaching to tumor cell surface, endosomal uptake, disrupting endosomal membranes, releasing free AON in the cytoplasm, and protecting against enzymatic degradation in the vascular system. The nanoconjugate is optionaly coupled with a fluorescent dye for tracing its pathway during tumor treatment [2].
Fig. 1.
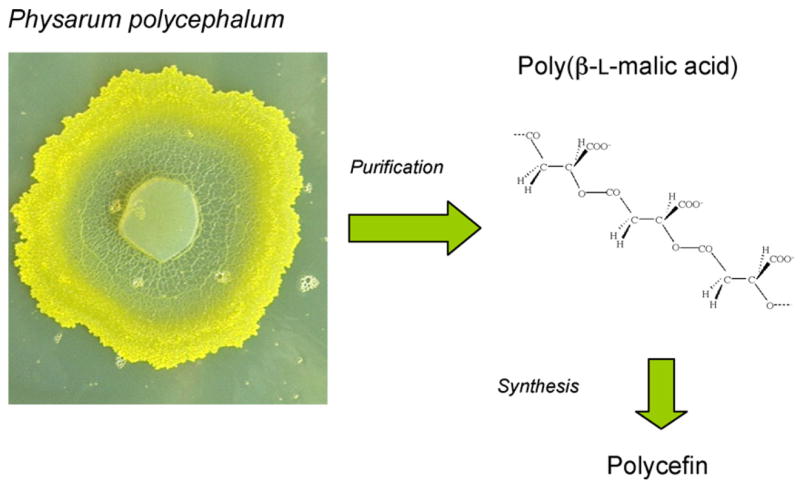
Poly(malic acid) from the slime mold Physarum polycephalum. β-Poly(L-malate) is synthesized and secreted by the plasmodium. The polymer is harvested from the broth of shaken cultures and isolated by ion exchange DEAE-chromatography and ethanol precipitation. From the highly purified β-poly(L-malic acid) Polycefin is synthesized by chemical conjugation of functional groups as described [2].
Fig. 2.
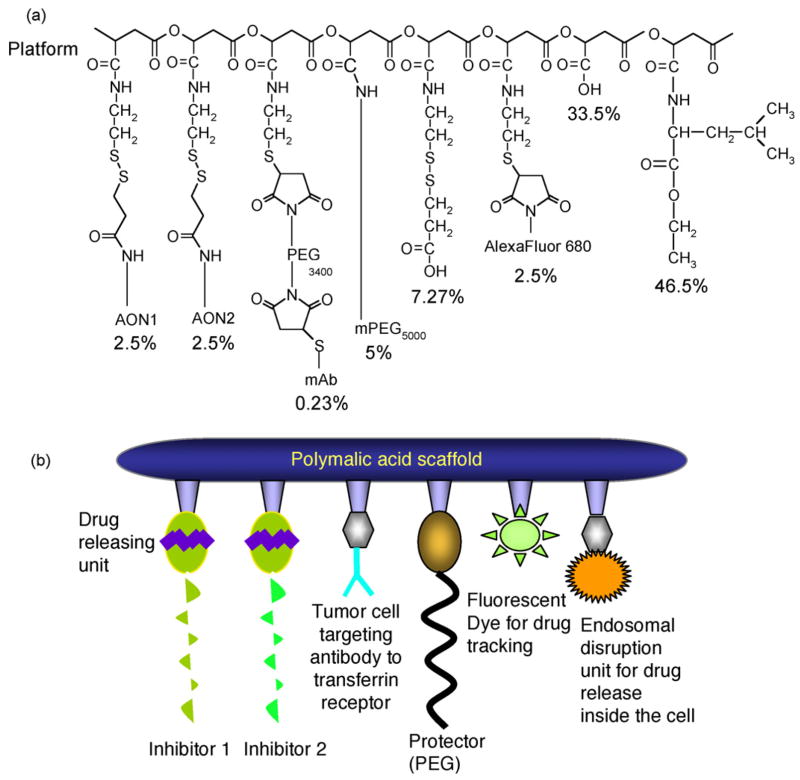
Schematic view of Polycefin. Panel a, schematic formula showing the composition of the nanoconjugate. The functional units (modules) have been chemically conjugated to the carboxyl groups of the PMLA platform [2]. Per cent values refer to total pendant carboxyl residues (100%). AON1 and AON2 are the Morpholino antisense oligonucleotides that target the mRNAs of laminin-8 α4 and β1 chains. The drug releasing unit is the disulfide group. Mouse anti-human TfR mAb targets tumor cells at their cell surface. PEG protects against enzymatic degradation. The fluorescent reporter group, AexaFuor 680, serves in vivo fluorescence imaging. L-Leucine ethylester moieties function in endosomal escape. The groups of 7.27% is the product of sulfhydryl blocking at the end of Polycefin synthesis and has no assigned function. Panel b shows a cartoon of Polycefin with the functional modules described in panel a.
Polycefin was designed to carry more than one drug allowing synergistic effects on tumor cells. This strategy leads to the efficient blocking of tumor cell synthesis of multimeric proteins composed of different polypeptide chains [2]. In particular, brain glioblastomas and invasive ductal breast carcinomas overexpress laminin-8, a laminin isoform of vascular basement membranes in the tumor tissues [3–5]. Laminin-8 represents a trimer of polypeptide chains, α4, β1 and γ1. Simultaneous delivery of the two AONs to mRNAs of laminin-8 chains efficiently blocked their in vitro synthesis and tumor invasion, while delivery of a single one was less effective [6]. Intracranial Polycefin injection into glioma bearing rats blocked angiogenesis by 75% and significantly increased the animal survival rate, p < 0.0004 [14].
To study antitumor activity of Polycefin by systemic treatment entailing large amount of the drug, we changed the model animal species from rats to mice. Accordingly, Polycefin with mouse monoclonal anti-human transferrin receptor (TfR) antibody, instead of mouse monoclonal antibody (OX-26) to rat TfR in the original Polycefin version [2], covalently attached to PMLA was synthesized. It is demonstrated here by fluorescence imaging that this new Polycefin variant accumulates in brain and breast tumors after the drug has been intravenously (I.V.) injected into nude mice. The results of in vivo imaging were corroborated histologically by fluorescence microscopy of multiple organs.
2. Materials and methods
2.1. Poly(β-L-malic acid) production
Poly(β-L-malic acid) (PMLA) was harvested from the culture broth of P. polycephalum M3CVII (ATCC 204388) microplasmodia, grown for 4 days at 25 °C on the medium described by Daniel and Baldwin [7], with the addition of 30 g/L D-glucose and 30 g/L CaCO3 (pH 5.5). When the pH dropped to 4.8, fermentation was terminated by shifts to pH 7.5 and 15 °C. After clearing by centrifugation (500 × g), the supernatant was diluted with one part of 0.05 M Tris–HCl pH 7.5 and pumped at 10 L/h from bottom to top of a 1.5 L column (12 cm diameter, capacity for 10 L of broth) containing Streamline DEAE-cellulose, then washed with 20 mM Tris–HCl buffer (pH 7.5) containing 0.2 M NaCl to remove the yellow pigment, and eluted with 20 mM Tris–HCl buffer containing 0.7 M NaCl from top to bottom. The PMLA containing fraction was adjusted to 0.1 M CaCl2 and poly(malate) Ca2+-salt precipitated with 70–80% (v/v) ethanol at −20 °C. The ice-cold ethanol-washed precipitate was dissolved in a minimum volume of distilled water and PMLA fractionated over Sephadex G25 into portions of 60–90 kDa, 50–60 kDa, and 20–50 kDa (Mw by HPLC-SEC with polystyrene sulfonate as standard). The calcium salt was converted into the acid by passage over Amperlite 120 H+, freeze-dried, and stored at −80 °C. The obtained polymer was white powder, soluble in acetone, showing crystallization during solvent removal under vacuum. It was highly pure with regard to elementary analysis, HPLC-SEC analysis, thin layer chromatography (TLC)/ninhydrin reaction, optical rotation, 200–300 nm wavelength absorbance, infrared absorbance, C13/H1 NMR spectra), and devoid of protein, nucleic acids, polysaccharide, as well as UV-absorbing low molecular compounds [8–12]. PMLA in the 18 L culture broth amounted to 20–25 g. The yield after purification was 50–60%.
2.2. Polycefin synthesis
Polycefin general structure is schematically shown in Fig. 2. The version used here contains mouse anti-human TfR mAb (RVS10) instead of mouse anti-rat TfR mAb (OX-26) in the original version [2]. AlexaFluor 680 C2-maleimide from Molecular Probes-Invitrogen (Carlsbad, CA) was employed as fluorescent reporter [2]. Custom made Morpholino™ (phosphorodiamidate morpholino oligomer) AONs (Gene Tools, Inc., St. Louis, MO) for human laminin-8 α4 and β1 chains had sequences 5′AGCTCAAAGCCATTTCTCCGCTGAC3′ (α4 antisense) and 5′CTAGCAACTGGAGAAGCCCCATGCC3′ (β1 antisense). Mouse monoclonal anti-human TfR antibody RVS10 (Chemicon International, Temecula, CA) was used in this Polycefin variant, which also contained L-leucine ethyl ester instead of previously used valine. The synthesis from poly(β-L-malic acid) (Mw 50,000; Mw/Mn 1.3) followed the protocol previously described [2].
2.3. Animals
Nude mice [Tac:Cr:(MCr)-Foxnnu] received intracranial injections of 5 × 105 U87MG human glioma cells or subcutaneous injections of 5 × 107 epidermal growth factor receptor (EGFR)-positive MDA-MB 468 human breast cancer cells into the left posterior mid-dorsum. For breast cancer model, nude mice received the 17β-estradiol pellet before cancer cell implantation. After manifestation of the tumors, 100 μL of drug solution was intravenously injected via the tail vein at a concentration of 2.5 mg/kg [2,14]. The drug distribution was followed by fluorescence imaging at different time points. These experiments were done with a customized macro-illumination and detection MISTI System for live animals [13].
2.4. Fluorescent tumor imaging
In vivo fluorescence of breast tumor imaging was performed using a customized macro-illumination and detection system similar to that previously described [13]. Uniform excitation light is delivered at a 45° angle through flexible fiber bundles, and imaging is performed using a cooled CCD camera (Hamamatsu ORCA-ER) through a macro lens. The excitation wavelength for the AlexaFluor 680 dye is 660 nm with a 20 nm bandwidth ChromaTech interference filter (Chroma Technology, Brattleboro, VT), and emission wavelength 700 nm with a 30 nm bandwidth ChromaTech interference filter. The distance between the specimen and the lens is 12 cm. Ex vivo brain imaging was performed with the same device using similar excitation/emission parameters that we used for breast tumor in vivo imaging. For these measurements, however, the distance between the specimen and the lens was 6 cm, and the images were recorded with a Princeton Instruments CCD camera (PIXIS 400) cooled at −70 °C. We changed parameters because of the deep tumor location in the organ/brain, not like breast under the skin immediately. The relevant regions of the images were cropped using ImageJ (Version1.34) on a Mac OS X platform for better visualization of the object of interest. The same program was used to determine absolute intensities of the image and mean intensities for regions of interest. For confirmation, the commercial Xenogen IVIS 200 imaging system was used with very similar results (not shown).
2.5. Microscopic distribution of fluorescent drug
Frozen 7 μm-thick sections of different organs were fixed in 2% paraformaldehyde and examined under a Nikon TE2000 microscope equipped with a broadband Hg light source and a Hamamatsu ORCA-ER 1024/1024 camera. Autofluorescence was excited at 480 nm (30 nm bandwidth) and recorded at 535 nm (40 nm bandwidth). AlexaFluor 680 dye is excited at 660 nm (20 nm bandwidth) and recorded at 700 nm (30 nm bandwidth). ChromaTech interference filters were used.
2.6. Statistical analysis of data
Statistical analysis of data was performed using ANOVA (Graph Pad Software, San Diego, CA). p < 0.05 is considered significant.
3. Results
Previous work from our laboratory has demonstrated that Polycefin bearing AON to laminin-8 α4 and β1 chains significantly prolonged animal survival when injected intracranially into rats with transplanted human U87MG glioblastoma multiforme [14]. Our goal is to use Polycefin in the future, conjugated with the appropriate mAbs to tumor cell surface receptors, as a drug for systemic treatment. To this end, the drug accumulation in tumors and its distribution in non-tumor organs were examined. Here, we use the Polycefin version having previous mouse anti-rat TfR mAb (OX-26) [2] substituted by mouse anti-human TfR mAb (RVS10). After I.V. drug injection into mice, fluorescently labeled Polycefin indeed selectively accumulated in brain tumor and was sustained up to 24 h (Fig. 3). Low signals were detected in kidneys and liver (not shown).
Fig. 3.
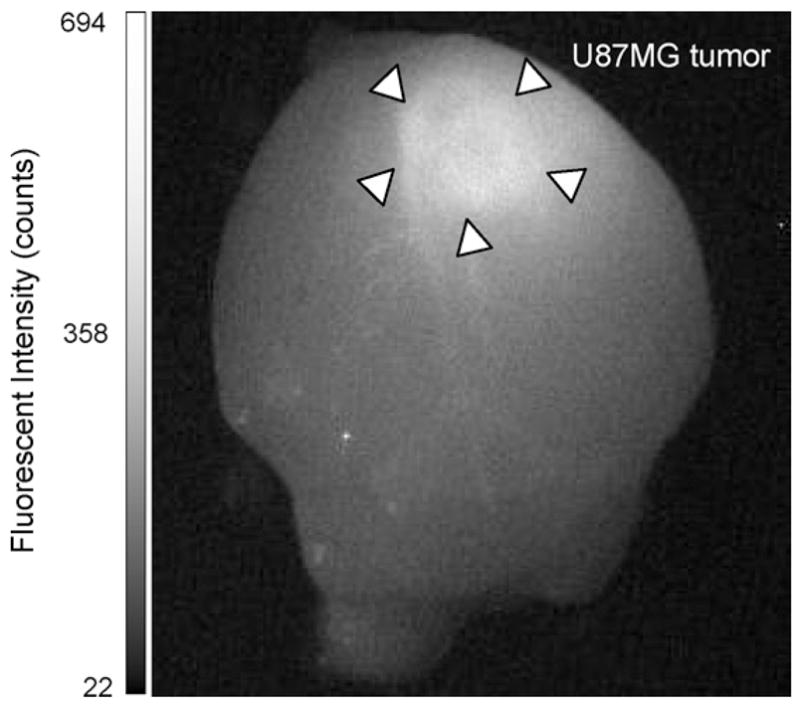
In vivo fluorescence imaging (MISTI) of mouse brain with xenografted human U87MG glioma 24 h after I.V. injection of Polycefin. The tumor area shows distinct fluorescent signal.
When breast tumor-bearing mice were intravenously injected with Polycefin-coupled AlexaFluor 680, a similar result was obtained as for brain tumor fluorescence imaging (Fig. 4). Again, the fluorescence signal was observed in breast tumor, in kidneys and in liver. The time course of fluorescence was measured with the MISTI imaging system by comparing light intensities from the center of the inoculated breast tumor and from an equally sized area of the breast outside the tumor (Fig. 4). In the tumor, the intensity of Polycefin-coupled AlexaFluor 680 increased rapidly until 60 min after drug injection at a level six times higher (p < 0.003) than in the surrounding tissue, and then decreased slowly (10% after 2 h). Control experiments after injection of free AlexaFluor 680 or a Polycefin version without conjugated AlexaFluor 680 gave no signal in tissues. When Polycefin-coupled AlexaFluor 680 conjugate without bound anti-TfR mAb was injected into tumor-bearing mice, only weak fluorescence of the brain tumor was seen (not shown).
Fig. 4.
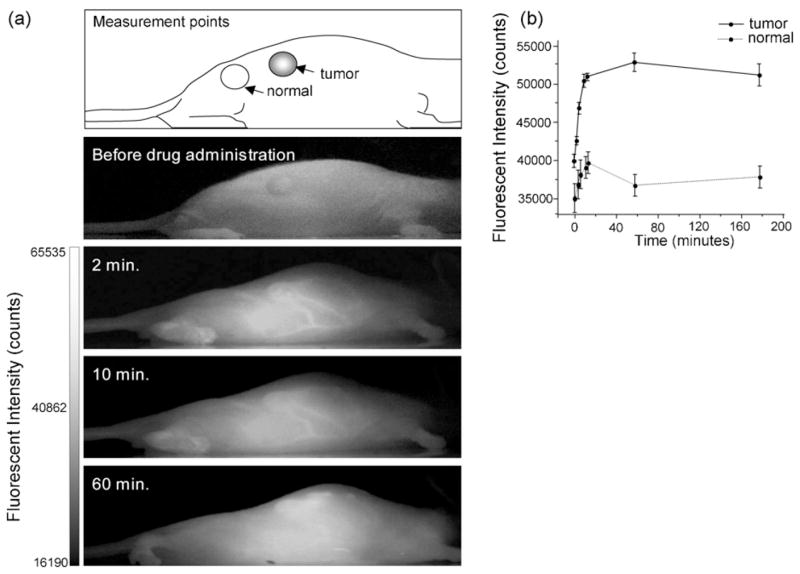
In vivo fluorescence images of mice with xenografted human MDA-MB 468 breast tumor before treatment and in 2, 10, and 60 min after intravenous injection of AlexaFluor 680-labeled Polycefin (a). Fluorescence intensity analysis was performed by selecting small areas within the tumor and normal regions as shown in the top panel. The time course fluorescence intensities in tumor area (solid line) and normal area (dotted line) are shown in graph (b). Polycefin circulated in blood vessels in early phase (2–10 min), but declined after 60 min (a and b). However, the drug kept retaining in tumor tissues even after 60 min (b).
The live imaging results were corroborated by the immunohistological study as shown in Fig. 5. Fluorescence of AlexaFluor 680 was detected on hematoxylin-eosin stained serial sections of heart, lung, normal brain, normal breast, liver, kidney, and breast tumor. Breast tumor accumulated Polycefin significantly more than any normal non-tumor tissue; kidney and liver as drug recycling organs also showed some accumulation (Fig. 5).
Fig. 5.
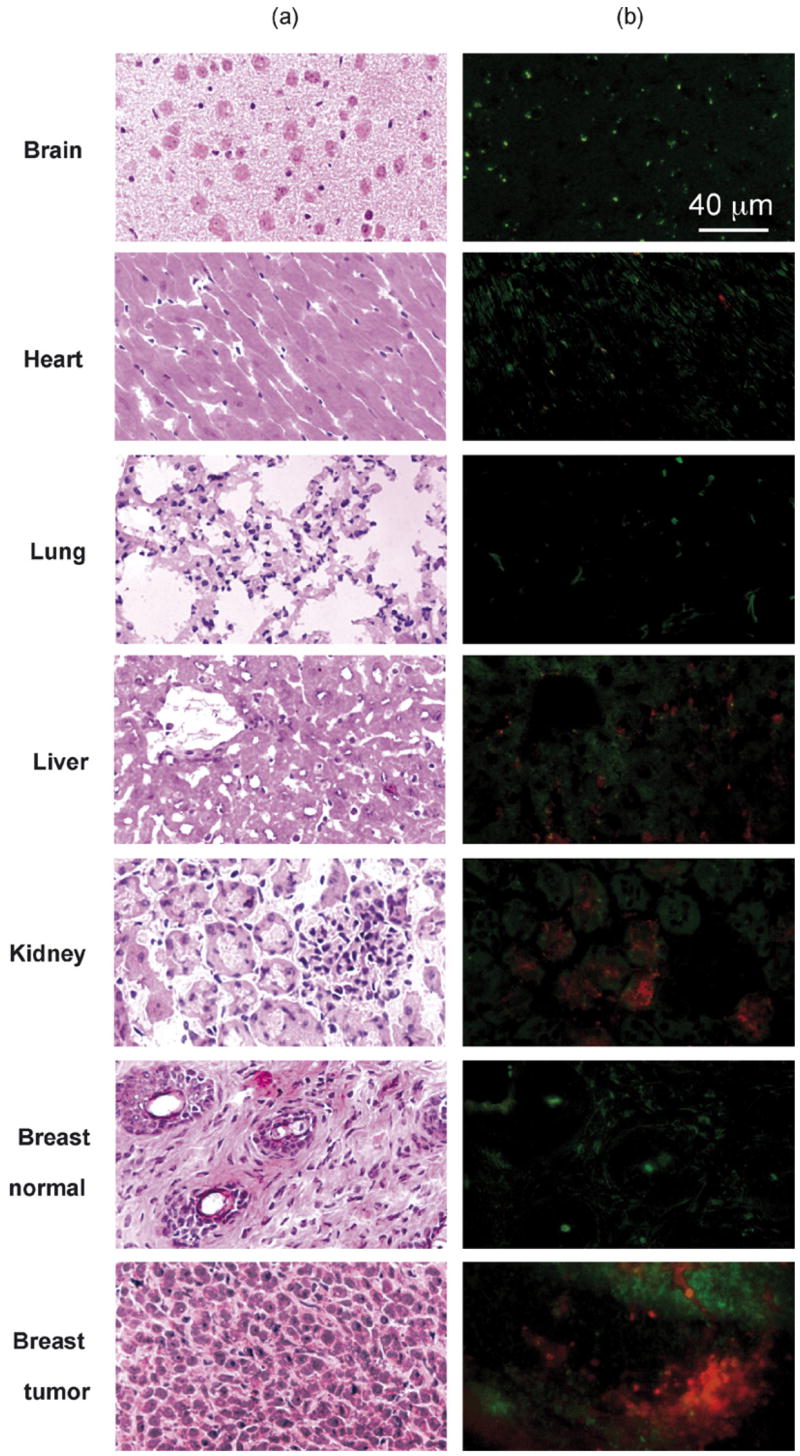
Sections of tissues including brain, lung, heart, liver, kidney, normal breast, and breast tumor were analyzed for the presence of Polycefin after I.V. injection into mice. Serial sections for each corresponding tissue were prepared to detect fluorescence of the conjugate by microscopy. (a) Hematoxylin-eosin staining. (b) Fluorescence of Polycefin. Tissue sections in (a) and (b) are not identical. Red, Polycefin-AlexaFluor 680 fluorescence; green, non-specific autofluorescence. The positive signal was seen 3 h after I.V. administration in implanted breast tumor, normal liver and kidney, whereas it was not detected in other organs, such as heart, lung, brain and normal breast. Accumulation in liver and kidney was expected, because they participate in drug metabolism and elimination.
4. Discussion
To date, various nanoscale drug delivery systems have been developed. Some of them make use of the sustained drug release and the tumor-specific EPR effect. Drugs of this kind are tetramethylindodicarbocyanine [15] mitoxantrone (DHAQ) [16], liposome-encapsulated or polymer conjugates of doxorubicin or paclitaxl [17–20], and HPMA conjugate of O-(chloroacetyl-carbamoyl) fumagillol (TNP-470) [21].
Other nanocarriers target certain tumor tissues by specifically recognizing cell surface molecules such as the folate receptor [22,23], EGFR [22], TfR [24,25] or sulfatide, a glycoshingolipid binding extracellular matrix glycoproteins [26]. All these surface molecules are upregulated in tumor cells; examples of delivered drugs are doxorubicin or AON. Of interest is the targeting of prostate-specific membrane antigen (PSMA) by docetaxel-encapsulated nanoparticles of poly(D,L-lactic-co-glycolic acid)-block-poly(ethylene glycol) PLGA-b-PEG copolymer functionalized with the A10 2′-fluoropyrimidine RNA aptamers that recognize PSMA [27] or the targeting of liposomes containing an amino acid sequence recognized by certain glycosaminoglycans of liver cells [28]. Another interesting approach is cell specific targeting by nanoparticles with attached multivalent small molecules [29] and by peptide-derived biodegradable nanoparticles [30]. Nanocarriers belonging to this group were found suitable for imaging of pancreatic cancer and glioma [31,32].
Another group of nanocarriers, such as prednisolone phosphate [33] and AON to tumor overexpressed proteins [34] encapsulated in liposomes, target tumor markers without additional tumor-specific delivery agents [35].
An emerging class of promising nanocarriers are designed to target both cell surface receptors in the tumor tissue and protein markers overexpressed in tumors providing the highest degree of targeting specificity. Recent examples are DNA polyplex activity with EGFR targeting [36] and prostate cancer-specific delivery of siRNA [37].
Our drug delivery nanoconjugate, Polycefin, is a member of the latter group targeting both TfR on tumor cells of brain and breast and tumor-specific laminin-8 mRNA. In vitro experiments with U87MG glioma cells have shown that Polycefin was rapidly endocytosed after binding to TfR and released from endosomes into cytoplasm followed by inhibition of laminin-8 synthesis [2]. In vivo treatment of human glioma U87MG xenografted into rats has demonstrated that Polycefin treatment inhibited tumor angiogenesis [14]. On that basis, the cellular and molecular targeting properties of Polycefin have been clearly established. The platform used in the design of Polycefin is biodegradable poly(β-L-malic acid) of microbial origin, a polyester with pendant carboxyl groups, which all can serve for covalent conjugation of functional groups, especially of targeting molecules. This multiplicity of attachment sites not only allows to carry multiple systemic drugs but also a variety of targeting moieties, such as several mAbs with different specificities, as well as any other molecular and/or tissue targeting residues. In Polycefin synthesis, multiple targeting molecules are efficiently conjugated to the poly(malic acid) platform at pendant sulfhydryl groups [2] by established chemical methods, in principle allowing the molecular design of various combinations of residues targeting cell surface and intra-cellular molecules that may eventually be tailored and optimized for individual patients [38].
High-resolution imaging analysis molecules showed that Polycefin was systemically available at maximum concentration after 1 h of injection, and was still available after 4 h. The imaging results suggest that Polycefin is a suitable drug with moderate systemic residing time, thus minimizing possible toxic or immunogenic reactions, and prolonged residing times within tumors of brain and breast allowing efficient inhibition of target molecule (here laminin-8) synthesis. Previous pharmacokinetic studies with synthetic radioactively labeled poly(β-D,L-malic acid) indicated rapid urinary excretion of 70% of the injected radioactivity in 1 h and varying degrees of retention in the liver, spleen, kidney, muscle, heart, brain, lung, and intestine [39–41]. Time course of clearance indicated here by whole animal imaging is to some extent comparable to that of the neutral copolymer HPMA (Mw 40,000–78,000) or immunoglobulin IgG (Mw 150,000) [42,43]. This comparison, however, can only be an approximation because of clearance dependence on molecular masses, molecular composition, electrostatic charges, molecular mass heterogeneity, and other molecular parameters.
In conclusion, the results of the imaging experiments show significant and specific accumulation of the nanoconjugate drug delivery system Polycefin in brain and breast tumors. These data favor further development of Polycefin for anticancer treatment.
Acknowledgments
This work was supported by grants from NIH (R01 CA123495 to JYL) and Department of Navy (awards 1435-04-04-GT-41387 and 1435-04-04-CT-73136 to DLF).
References
- 1.Lee BS, Vert M, Holler E. Water-soluble aliphatic polyesters; poly(malic acid)s. In: Doi Y, Steinbüchel A, editors. Biopolymers. 3a. Polyesters, Wiley–VCH; Weinheim: 2002. pp. 75–103. [Google Scholar]
- 2.Lee BS, Fujita M, Khazenon NM, Wawrowsky KA, Wachsmann-Hogiu S, Farkas DL, Black KL, Ljubimova JY, Holler E. Polycefin, a new prototype of multifunctional nanoconjugate based on poly(β-L-malic acid) for drug delivery. Bioconjugate Chem. 2006;17:317–326. doi: 10.1021/bc0502457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ljubimova JY, Fugita M, Khazenzon NM, Das A, Pikul BB, Newman D, Sekiguchi K, Sorokin LM, Sasaki T, Black KL. Laminin-8 association with glial tumor grade, recurrence and patient survival. Cancer. 2004;101:604–612. doi: 10.1002/cncr.20397. [DOI] [PubMed] [Google Scholar]
- 4.Fujita M, Khazenzon NM, Bose S, Sekiguchi K, Sasaki T, Carter WG, Ljubimov AV, Black KL, Ljubimova JY. Over-expression of β1 chain-containing laminins in capillary basement membranes of human breast cancer and its metastases. Breast Cancer Res. 2005;7:411–421. doi: 10.1186/bcr1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ljubimova JY, Fujita M, Khazenzon NM, Ljubimov AV, Black KL. Changes in laminin isoforms associated with brain tumor invasion and angiogenesis. Front Biosci. 2006;11:81–88. doi: 10.2741/1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khazenzon NM, Ljubimov AV, Lakhter AJ, Fujiwara H, Sekiguchi K, Sorokin LM, Virtanen I, Black KL, Ljubimova JY. Antisense inhibition of laminin-8 expression reduces invasion of human gliomas in vitro. Mol Cancer Ther. 2003;2:985–994. [PubMed] [Google Scholar]
- 7.Daniel JW, Baldwin HH. Methods of culture for plasmodial myxomycetes. In: Prescott DA, editor. Methods in Cell Physiology. Academic Press; New York, NY: 1964. pp. 9–41. [Google Scholar]
- 8.Fischer H, Erdmann S, Holler E. An unusual polyanion from Physarum polycephalum that inhibits homologous DNA polymerase-α in vitro. Biochemistry. 1989;28:5219–5226. doi: 10.1021/bi00438a045. [DOI] [PubMed] [Google Scholar]
- 9.Cammas S, Guerin Ph, Girault JP, Holler E, Gache Y, Vert M. Natural poly(L-malic acid): NMR shows a poly(3-hydroxy acid)-type structure. Macromolecules. 1993;28:4681–4684. [Google Scholar]
- 10.Fernandez CE, Mancera M, Holler E, Bou JJ, Galbis JA, Munoz-Guerra S. Low molecular weight poly(alpha-methyl-beta-L-malate) of microbial origin: synthesis and crystallization. Macromol Biosci. 2005;5:172–176. doi: 10.1002/mabi.200400166. [DOI] [PubMed] [Google Scholar]
- 11.Portilla JA, Garcia-Alvarez M, de Ilarduya AM, Holler E, Munoz-Guerra S. Nanostructural complexes of poly(beta-L-malate) and cationic surfactants: synthesis, characterization and structural aspects. Biomacromolecules. 2006;7:161–170. doi: 10.1021/bm050490t. [DOI] [PubMed] [Google Scholar]
- 12.Lee B-S, Vert M, Holler E. Water-soluble aliphatic polyesters: poly(malic acid)s. In: Doi Y, Steinbüchel A, editors. Biopolymers. Wiley–VCH; Weinheim: 2002. pp. 75–103. [Google Scholar]
- 13.Farkas DL. Invention and commercialization in optical bioimaging. Nat Biotechnol. 2003;21:1269–1271. doi: 10.1038/nbt1103-1269. [DOI] [PubMed] [Google Scholar]
- 14.Fujita M, Khazenzon NM, Ljubimov AV, Lee BS, Virtanen I, Holler E, Black KL, Ljubimova JY. Inhibition of laminin-8 in vivo using a novel poly(malic acid)-based carrier reduces glioma angiogenesis. Angiogenesis. 2006;9:183–191. doi: 10.1007/s10456-006-9046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasatsu M, Onishi H, Machida Y. In vitro and in vivo characterization of nanoparticles made of MeO-PEG amine/PLA block copolymer and PLA. Int J Pharm. 2006;317:167–174. doi: 10.1016/j.ijpharm.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 16.Duan Y, Sun X, Gong T, Wang Q, Zhang Z. Preparation of DHQ-loaded mPEG-PLGA-mPEG nanoparticles and evaluation of drug release behaviours in vitro/in vivo. J Mater Sci Mater Med. 2006;17:509–516. doi: 10.1007/s10856-006-8933-3. [DOI] [PubMed] [Google Scholar]
- 17.Heyes J, Hall K, Tailor V, Lenz R, MacLachlan I. Synthesis and characterization of novel poly(ethylene glycol)-lipid conjugates suitable for use in drug delivery. J Control Release. 2006;112:280–290. doi: 10.1016/j.jconrel.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed F, Pakunlu RI, Brannan A, Bates F, Minko T, Discher DE. Biodegradable polymerosomes loaded with both paclitaxel and doxorubicin permeate and shrink tumors, inducing apoptosis in proportion to accumulated drug. J Control Release. 2006;116:150–158. doi: 10.1016/j.jconrel.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Koziara JM, Whisman TR, Tseng MT, Mumper RJ. In vivo efficacy of novel paclitaxel nanoparticles in paclitaxel-resistant human colorectal tumors. J Control Release. 2006;112:312–319. doi: 10.1016/j.jconrel.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Chytil P, Etrych T, Konak C, Sirova M, Mrkvan T, Rihova B, Ulbrich K. Properties of HPMA copolymer-doxorubicin conjugates with pH-controlled activation: effect of polymer chain modification. J Control Release. 2006;115:26–36. doi: 10.1016/j.jconrel.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 21.Satchi-Fainaro R, Puder M, Davies JW, Tran HT, Sampson DA, Greene AK, Corfas G, Folkman J. Targeting angiogenesis with a conjugate of HPMA copolymer and TNP-470. Nat Med. 2004;10:255–261. doi: 10.1038/nm1002. [DOI] [PubMed] [Google Scholar]
- 22.Saul JM, Annapragada AV, Bellamkonda RV. A dual-ligand approach for enhancing targeting selectivity of therapeutic nanocarriers. J Control Release. 2006;114:277–287. doi: 10.1016/j.jconrel.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 23.Leamon CP, Reddy JA, Vlahov IR, Kleindl PJ, Vetzel M, Westrick E. Synthesis and biological evaluation of EC140: a novel folate-targeted Vinca alkaloid conjugate. Bioconjugate Chem. 2006;17:1226–1232. doi: 10.1021/bc060145g. [DOI] [PubMed] [Google Scholar]
- 24.Chiu SJ, Liu S, Perrotti D, Marcucci G, Lee RJ. Efficient delivery of a Bcl-2-specific antisense oligodeoxyribonucleotide (G3139) via transferrin receptor-targeted liposomes. J Control Release. 2006;112:199–207. doi: 10.1016/j.jconrel.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Pun SH, Tack F, Bellocq NC, Cheng J, Grubbs BH, Jensen GS, Davis ME, Brewster M, Janicot M, Janssens B, Floren W, Bakker A. Targeted delivery of RNA-cleaving DNA enzyme (DNAzyme) to tumor tissue by transferrin-modified, cyclodextrin-based particles. Cancer Biol Ther. 2004;3:641–650. doi: 10.4161/cbt.3.7.918. [DOI] [PubMed] [Google Scholar]
- 26.Shao K, Hou Q, Go ML, Wong KP, Li QT. Intracellular drug delivery by sulfatide-mediated liposomes to gliomas. J Control Release. 2006;115:150–157. doi: 10.1016/j.jconrel.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 27.Farokhzad OC, Cheng J, Teply BA, Sherifi I, Ion S, Kantoff PW, Richie JP, Langer R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci USA. 2006;103:6315–6320. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Longmuir KJ, Robertson RT, Haynes SM, Baratta JL, Waring AJ. Effective targeting of liposomes to liver and hepatocytes in vivo by incorporation of Plasmodium amino acid sequence. Pharm Res. 2006;23:759–769. doi: 10.1007/s11095-006-9609-x. [DOI] [PubMed] [Google Scholar]
- 29.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotechnol. 2005;23:1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 30.Costantino L, Gandolfi F, Tosi G, Rivasi F, Vandelli MA, Forni F. Peptide-derivatized biodegradable nanoparticles able to cross the blood–brain barrier. J Control Release. 2005;108:84–96. doi: 10.1016/j.jconrel.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Montet X, Weissleder R, Josephson L. Imaging pancreatic cancer with a peptide-nanoparticle conjugate targeted to normal pancreas. Bioconjugate Chem. 2006;17:905–911. doi: 10.1021/bc060035+. [DOI] [PubMed] [Google Scholar]
- 32.Jia B, Shi J, Yang Z, Xu B, Liu Z, Zhao H, Liu S, Wang F. 99mTc-Labeled cyclic RGDfK dimer: initial evaluation for SPECT imaging of glioma integrin αvβ3 expression. Bioconjugate Chem. 2006;17:1069–1076. doi: 10.1021/bc060055b. [DOI] [PubMed] [Google Scholar]
- 33.Banciu M, Schiffelers RM, Fens MHAM, Metselaar JM, Storm G. Anti-angiogenic effects of liposomal prednisolone phosphate on B16 melanoma in mice. J Control Release. 2006;113:1–8. doi: 10.1016/j.jconrel.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 34.Pakunlu RI, Wang Y, Saad M, Khandare JJ, Starovoytov V, Minko T. In vitro and in vivo intracellular liposomal delivery of anti-sense oligonucleotides and anticancer drug. J Control Release. 2006;114:153–162. doi: 10.1016/j.jconrel.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Torchilin VP, Lukyanov AN. Peptide and protein drug delivery to and into tumors: challenges and solutions. Drug Discov Today. 2003;8:259–266. doi: 10.1016/s1359-6446(03)02623-0. [DOI] [PubMed] [Google Scholar]
- 36.Kloeckner J, Boeckle S, Persson D, Roedl W, Ogris M, Berg K, Wagner E. DNA polyplexes based on degradable oligoethylenimine-derivatives: combination with EGF receptor targeting and endosomal release functions. J Control Release. 2006;116:115–122. doi: 10.1016/j.jconrel.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 37.McNamara JO, II, Andrechek ER, Wang Y, Viles KD, Rempel RE, Gilboa E, Sullenger BA, Giangrande PH. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat Biotechnol. 2006;24:1005–1014. doi: 10.1038/nbt1223. [DOI] [PubMed] [Google Scholar]
- 38.Dancey JE, Chen HX. Strategies for optimizing combinations of molecular targeted anticancer agents. Nat Rev Drug Discov. 2006;5:649–659. doi: 10.1038/nrd2089. [DOI] [PubMed] [Google Scholar]
- 39.Fournié P, Domurado D, Guérin P, Braud C, Vert M, Madelmont JC. In vivo fate of end-chain radiolabelled poly(β-malic acid), a water soluble biodegradable drug carrier. J Bioact Compat Polym. 1990;5:381–395. [Google Scholar]
- 40.Fournié P, Domurado D, Guérin P, Braud C, Vert M, Pontikis R. In vivo fate of repeat-unit-radiolabelled poly(β-malic acid), a potential drug carrier. J Bioact Compat Polym. 1992;7:113–129. [Google Scholar]
- 41.Domurado D, Fournié P, Braud C, Vert M, Guérin P, Simonnet F. In vivo fates of degradable poly(β-malic acid) and of its precursor, malic acid. J Bioact Compat Polym. 2003;18:23–32. [Google Scholar]
- 42.Seymour LW, Miyamoto Y, Maeda H, Brereton M, Strohalm J, Ulbrich KU, Duncan R. Influence of molecular weight on passive tumor accumulation of a soluble macromolecular drug carrier. Eur J Cancer. 1995;31A:766–777. doi: 10.1016/0959-8049(94)00514-6. [DOI] [PubMed] [Google Scholar]
- 43.Ramakrishnan S, Houston LL. Immunological and biological stability of immunotoxins in vivo as studied by the clearance of disulfide-linked pokeweed antivirial protein-antibody conjugates from blood. Cancer Res. 1985;45:2031–2036. [PubMed] [Google Scholar]