Abstract
ActA, a surface protein of Listeria monocytogenes, is able to induce continuous actin polymerization at the rear of the bacterium, in the cytosol of the infected cells. Its N-terminal domain is sufficient to induce actin tail formation and movement. Here, we demonstrate, using the yeast two-hybrid system, that the N-terminal domain of ActA may form homodimers. By using chemical cross-linking to explore the possibility that ActA could be a multimer on the surface of the bacteria, we show that ActA is a dimer. Cross-linking experiments on various L. monocytogenes strains expressing different ActA variants demonstrated that the region spanning amino acids 97–126, and previously identified as critical for actin tail formation, is also critical for dimer formation. A model of actin polymerization by L. monocytogenes, involving the ActA dimer, is presented.
Keywords: actin polymerization, Listeria monocytogenes
Listeria monocytogenes is an intracellular bacterial pathogen that, after entry into the host cell, lyses the phagocytic vacuole and moves intracellularly, using as a driving force for movement a continuous process of actin polymerization at one pole of the bacterium. This phenomenon results in the formation of an actin tail visible behind moving bacteria (for reviews, see refs. 1–3, 21).
Actin polymerization by L. monocytogenes is mediated by ActA (4, 5), a protein with a polar distribution over the bacterial surface with a maximal concentration at one pole (6). This distribution predetermines the site of actin assembly on the bacterial surface and the direction of movement. ActA is a 610-amino acid protein with a N-terminal region (1–234) (ActA.N) that protrudes from the surface, as shown by immunolabeling experiments (6, 7), a central proline-rich repeat domain (235–394) (ActA.P), and a C-terminal hydrophobic end immediately followed by a few positively charged residues that retain the protein in the bacterial membrane (8).
Recently two different experimental approaches were used to demonstrate that ActA is sufficient to induce actin-based motility: (i) by producing ActA in the nonpathogenic species Listeria innocua, we converted this normally nonmotile bacterium into an organism capable of actin polymerization and movement in Xenopus cytoplasmic extracts (9); and (ii) Smith et al. (10) incubated Streptococcus pneumoniae with a recombinant ActA–LytA hybrid protein, which adsorbed and covered the streptococcal surface. The decorated bacteria became able to polymerize actin and move. However, these events only occurred after they had divided to generate a polar distribution of ActA on the streptococcal surface. Thus, both ActA production and its polar distribution are prerequisites for actin assembly and movement.
To gain insight into the molecular function of ActA, we and others investigated the role of various ActA regions. These studies demonstrated that the N-terminal domain of ActA is essential for the actin polymerization process (7, 11, 12). We recently demonstrated by fusing ActA.N to the ω fragment of LacZ that ActA.N is sufficient to confer actin-based motility, because the L. monocytogenes strain producing this chimeric ActA–LacZ protein moved in cytoplasmic extracts (13).
To understand the function of the N-terminal domain, it became important to identify the potential ligands interacting with it. It was recently demonstrated that vasodilator-stimulated phosphoprotein (VASP) (14) and/or its homolog, the mammalian enabled protein MEMA (15), bind to the proline-rich repeats region of ActA (ActA.P), but ligands able to bind to ActA.N have not been identified. Up to now, actin has not been demonstrated to be a direct ligand for the full-length ActA, although experiments with peptides suggested that the region containing amino acids 21–97 of the N-terminal domain can interact with actin (13). To identify ligands that interact with the N-terminal domain of ActA, we used the yeast two-hybrid system. During control experiments, we observed that ActA.N produced a positive signal when tested against itself, suggesting that two ActA molecules may be able to interact. In this work, we present genetic evidence, using the yeast two-hybrid system and biochemical data using cross-linking experiments, that ActA forms dimers at the surface of the bacterium. Moreover, we identified a 30-amino acid stretch in the N terminus of ActA that is needed for dimer formation. Deletion of this region almost completely prevents dimer formation while reducing the efficiency of actin polymerization. These observations suggest that dimerization is needed for actin-based motility. This novel property of ActA will undoubtedly shed some light on the role of ActA.
MATERIALS AND METHODS
Strains and Plasmids.
Saccharomyces cerevisiae strain SFY526 (MATa, ura3-52, his3-200, ade2-101, lys2-801, trp1- leu2-3, 112, canr, gal4-542, gal80- URA3::GAL1-lacZ) was used for two-hybrid analysis. The following yeast two-hybrid system vectors were obtained from CLONTECH: pGBT9 (TRP1, Ampr) for the expression of GAL4-DNA binding domain (DBD) fusion proteins, and pGAD424 (LEU2, Ampr) for the expression of GAL4-activation domain fusion proteins.
The N-terminal (1–234) and the proline-rich repeats domains (229–401) of ActA were amplified by PCR using the following primers: 5′-TTGGATCCCGGGAGCGACAGATAGCGAAGAT-3′, 5′-TTGGATCCTTACGAAGCATTTACCTCTTCAC-3′, 5′-TTGGATCCCGGGTGAAGAGGTAAATGCTTCG-3′, and 5′-AAGGATCCTTAATCTCTTAAATCAGCTAGGC-3′. PCR fragments were digested with SmaI/BamHI and cloned in pGBT9 and pGAD424, which have been digested with the same enzymes producing pGBT9-DBD-ActA.N, pGAD424-activation domain (AD)-ActA.N, pGBT9-DBD-ActA.P and pGAD424-AD-ActA.P, respectively. These plasmids were sequenced to verify the reading frame and absence of mutations using Sequenase 2.0 (United States Biochemical) according to the manufacturer’s protocol.
L. monocytogenes LO28 ΔactA strains producing the following ActA variants, ActA-ΔN, ActA-ΔP, and ActA-ΔC, were described (7). L. monocytogenes LO28 ΔactA strains producing the following ActA variants, ActA-Δ158–231, ActA-Δ126–231, ActA-Δ97–231, ActA-Δ21–97, and ActA-Δ116–122, were described (13).
For the construct ActA-Δ97–126, a fragment encoding part of the N-terminal of ActA (amino acids 126–231) was produced using the following primers: 5′-CCGGTACCATCGGATAGTGAGCTTG-3′ and 5′-GGGTACCGAAGCATTTACCT-3′. This PCR fragment, when digested with KpnI, was cloned into pActA97-231 (13) when digested with the same restriction enzyme and dephosphorylated.
GAL4 Two-Hybrid System.
The GAL4 two-hybrid technique was used to detect protein–protein interactions. For each transformation, yeast strain SFY526 was grown and transformed according to the Matchmaker Two-Hybrid System protocol (CLONTECH). Briefly, the two-hybrid vectors were cotransformed into yeast cells by lithium acetate method, and the transformants were selected on the minus Trp-Leu synthetic dropout synthetic plates for 4 days at 30°C. Filter lift assays were performed for the qualitative measurement of β-galactosidase (β-gal) activity. Transformed yeast colonies were grown in liquid culture and patched onto filters which were layered over minus Trp-Leu synthetic dropout synthetic agar plates overnight at 30°C. Filters were submerged in liquid nitrogen for 10 s and placed onto filters that were presoaked in 5-bromo-4 chloro-3-indolyl β-d-galactoside (X-Gal) solution containing 100 mM phosphate buffer (pH 7.0), 10 mM KCl, and 1 mM MgSO4. Filters were then incubated at 30°C and periodically examined for the appearance of blue colonies.
Quantitative assays were performed on liquid cultures. Individual yeast transformant colonies were inoculated into appropriate synthetic media containing glucose, and incubated at 30°C until an OD600 0.5. β-Gal was assayed using 4-methyl-umbelliferyl β-d-galactopiranoside (MUG) as described by Klarsfeld et al. (20). Fluorescence was measured using a Dynatech apparatus with 365 nm excitation and 450 nm emission wavelengths. Activities were computed as fluorescence units per hour per yeast.
Plasmids pVA3 and pTD1 (CLONTECH), encoding, respectively GAL4-DBD-p53 and GAL4-AD-T antigen fusion proteins, were used as positive controls.
Cross-Linking and Protein Analysis.
Overnight cultures of L. monocytogenes wild-type strain LO28 or L. monocytogenes ΔactA producing different ActA variants were grown on in brain heart infusion broth (Difco) at 37°C with aeration, and then were diluted 20-fold and grown under the same conditions to an OD600 = 1. Bacterial culture was washed, resuspended in appropriate volume of PBS to give an optical value of OD600 = 1.6. One milliliter of each culture was incubated in the absence or presence of the cross-linking reagent at room temperature for 30 min. Dithio-bis(succinimidylpropionate) (DSP) and bis(sulfosuccinimidyl) suberate (BS3) were used at several concentrations from 10 μM to 8 mM, and formaldehyde was used at concentrations raising from 0.018 to 1.8%. The cross-linking reagent was then quenched by addition of 100 mM Tris⋅HCl (pH 7.4), and the bacteria were centrifuged and resuspended in 100 μl SDS sample buffer (without 2-mercaptoethanol for DSP) for analysis by SDS/PAGE and immunoblotting. Thirty-five microliters of total protein extract was separated by SDS/PAGE. Samples were heated to 100°C for 5 min before being loading onto the gel. After electrophoresis, proteins were transferred onto sheets of nitrocellulose using a semi-dry blot apparatus (Schleicher & Schüll), and then detected using affinity-purified polyclonal ActA-specific antibodies directed against a synthetic peptide containing the first 18 residues of mature ActA (A18K). The secondary antibody, horseradish peroxidase-coupled anti-rabbit IgG (Amersham), was used according to manufacturer’s instructions. The nitrocellulose membranes were developed by enhanced chemiluminescence using ECL (Amersham).
RESULTS
Detection of ActA.N–ActA.N Interactions Using the Yeast Two-Hybrid System.
The ActA.N and ActA.P domains of ActA were cloned in the yeast two-hybrid expression vector pGBT9 to encode GAL4–DBD hybrid proteins, and in pGAD424 to encode GAL4–AD hybrid proteins. Production of the hybrid proteins in yeast was verified by immunoblotting of total protein extracts using affinity-purified antibodies raised against the N-terminal domain of ActA (A18K) (12) or against the proline-rich repeats domain of ActA (Y21T) (6) (data not shown).
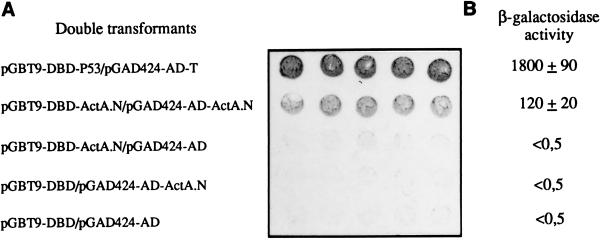
To detect potential interactions, yeasts were cotransformed with plasmids derivatives of pGBT9 and pGAD424 which encoded the same ActA domain. Yeast cotransformed with plasmids pGBT9-DBD-ActA.N and pGAD424-AD-ActA.N activated transcription of the lacZ reporter gene as shown by the filter assay (Fig. 1A), whereas β-gal activity was not detected with yeast transformed with each of the two plasmids separately. These results suggested that ActA.N interacts with itself. The approximate strength of the interaction was estimated by measuring the production of β-gal (Fig. 1B). ActA.N interacts with itself in producing 120 units of β-gal. Under the same conditions, the positive controls encoding GAL4-DBD-p53 and GAL4-AD-T antigen fusion proteins interact producing 1,800 units. The basal level in yeast transformed with the vectors pGBT9 and pGAD424 or in yeast transformed with only one of the recombinant vectors was lower than 0.5 unit. These data suggest that the interaction of the N-terminal domain with itself was significant.
Figure 1.
Qualitative and quantitative analysis of β-gal activity in the yeast two-hybrid system. (A) Filter assay: five independent colonies of cotransformants were patched onto filters layered over synthetic medium lacking Trp and Leu, and incubated overnight at 30°C. Filters were incubated in the X-Gal solution during 30 min for positive control (pGBT9-DBD-p53/pGAD424-AD-T) and 3–4 h for (pGBT9-DBD-ActA.N/pGAD424-AD-ActA.N). (B) β-Gal activity of yeast cotransformed with indicated plasmids was measured using 4-methyl-umbelliferyl β-d-galactopiranoside (MUG) as the substrate. The results represent the average ± SD of triplicate samples from three independent transformants. Activities were computed as [(fluorescence units/hour/yeast) × 100,000].
Surprisingly, yeast transformed with only the plasmid encoding the ActA.P fused to the GAL4–DBD (pGBT9-DBD-ActA.P) showed transcription of the lacZ reporter gene, as demonstrated by the appearance of blue colonies in the β-gal activity assay (data not shown). We were therefore unable to use the two-hybrid system to identify ligands of the proline-rich domain of ActA.
Detection of ActA Dimer Formation on the Bacterial Surface Using Chemical Cross-Linking.
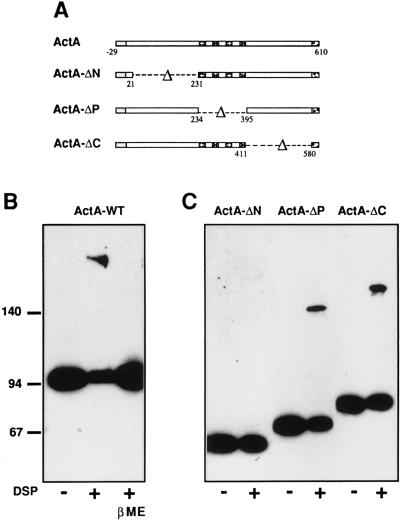
To examine whether ActA forms multimers in vivo, we undertook chemical cross-linking experiments. Three different cross-linking reagents—i.e., formaldehyde, DSP, and BS3—were used (see Material and Methods). Cross-linking was performed on the L. monocytogenes wild-type strain LO28 grown in broth. Total protein extracts were separated by SDS/PAGE and analyzed by immunoblot using anti-ActA affinity purified antibodies (A18K). As shown in (Fig. 2B), DSP treatment generated an additional molecular species that migrated as an ≈190-kDa protein—i.e., twice the apparent molecular size of the native ActA protein, which migrates at 95 kDa. The presence of the 190-kDa band was reversible, as shown by treatment of cross-linked proteins with β-mercaptoethanol before loading onto the gel (Fig. 2B). Generation of the 190-kDa species was optimal when the DSP concentration was 100 μM. Essentially identical profiles were obtained when cross-linking experiments were performed with BS3 or formaldehyde (data not shown). In no case, species of higher apparent molecular size, suggesting formation of a trimer, was detected. Taken together these results revealed that ActA forms a homodimer on the surface of bacteria.
Figure 2.
Detection of cross-linked ActA by SDS/PAGE and immunoblotting. (A) Schematic representation of the ActA protein and the ActA variants. (B) L. monocytogenes LO28 wild-type strain grown in broth was treated with DSP (100 μM) as described. Protein extracts were separated by SDS/PAGE (7%) and analyzed by immunoblotting using anti-ActA affinity purified antibodies (A18K). Treatment of cross-linked proteins with β-mercaptoethanol (5%) before loading reverse the presence of the 190-kDa protein. (C) L. monocytogenes ΔactA producing different ActA variants were grown in broth and treated with DSP (10 μM). Total protein extracts of bacteria expressing ActA-ΔN, ActA-ΔP and ActA-ΔC were separated by SDS/PAGE (7%) and analyzed by immunoblotting using anti-ActA affinity purified antibodies (A18K).
The N-Terminal Region of ActA Is Needed for Dimer Formation.
To identify the domain of ActA needed for dimerization, we performed cross-linking experiments on a L. monocytogenes ΔactA strain expressing different ActA variants (Fig. 2A). Chemical cross-linking, with different concentrations of DSP, on bacteria producing an ActA variant in which the N-terminal domain was deleted (ActA-ΔN) did not produce slower migrating bands (Fig. 2C). In contrast, cross-linking on bacteria producing ActA variants in which the proline-rich repeat (ActA-ΔP) or the C-terminal domain (ActA-ΔC) was deleted generated molecular species that migrated at positions corresponding to twice the apparent molecular size (Fig. 2C) of those obtained with untreated bacteria (Fig. 2C). These results were in agreement with those obtained by the two-hybrid system, and showed that the N-terminal domain of ActA is essential for dimerization and that cross-linking occurs between two N-terminal domains.
Identification in the N-Terminal Domain of ActA of a Critical Region Needed for Dimerization.
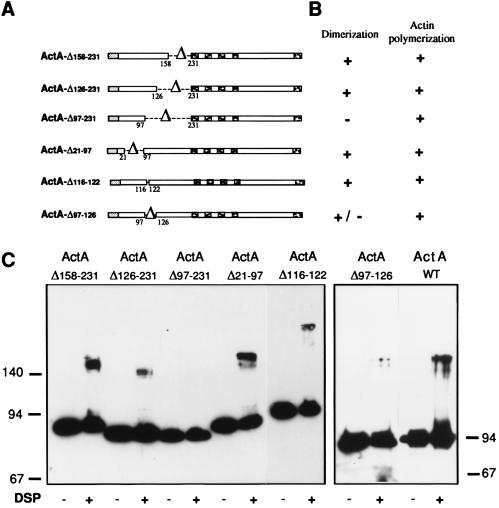
To identify the minimal region of the N-terminal domain necessary for dimerization, cross-linking experiments were performed on several L. monocytogenes ΔactA strains producing ActA variants in which small regions of the N-terminal domain were deleted (Fig. 3A). These strains have recently been used to analyze the ActA N-terminal domain (13). Cross-linking experiments showed that bacteria producing ActA-Δ158–231, ActA-Δ126–231, ActA-Δ21–97, or ActA-Δ116–122 generated molecular species that migrated as proteins of twice the molecular size (Fig. 3C) of the ActA variant produced by untreated bacteria (Fig. 3C). These results indicated that regions 21–97, 116–122, and 126–231 of the N terminus are not essential for dimerization. In contrast, cross-linking on bacteria producing ActA-Δ97–231, which still polymerizes actin (13), did not generate additional bands (Fig. 3C). Taken together these data suggested that the region between amino acids 97–126 is critical for dimerization.
Figure 3.
Identification of the minimal region of ActA necessary for DSP cross-linking. (A) Schematic representation of the ActA variants used for the cross-linking experiments. (B) Properties of the variants. (C) Cross-linking experiments: L. monocytogenes producing different ActA variants were grown in broth and treated with DSP. Total protein extracts of bacteria producing ActA-Δ158–231, ActA-Δ126–231 ActA-Δ97–231, ActA-Δ21–97, ActA-Δ116–122, and ActA-Δ97–126 were separated by SDS/PAGE (9%) and analyzed by immunoblotting using anti-ActA affinity purified antibodies (A18K). Bacteria were treated with 40 μM (ActA-Δ158–231), 10 μM (ActA-Δ126–231, ActA-Δ97–126, and ActA-Δ116–122), and 20 μM (ActA-Δ97–231 and ActA-Δ21–97) of DSP.
To address the precise role of region 97–126, two types of experiments were performed. (i) A deletion spanning amino acids 97–126 was generated. This deletion resulted in bacteria expressing ActA on the surface as shown by immunofluorescence in infected cells (data not shown). These bacteria, as mutant Δ97–231 (13), were able to polymerize actin, which indicated that ActA was expressed on the surface in a nearly native state. However, in these bacteria, in contrast to mutant Δ97–231, a very low level of dimerization was detectable. To test whether region 97–126 of ActA interacts with itself, a DNA fragment encoding amino acids 97–126 of ActA was cloned in the two-hybrid yeast expression vectors pGBT-9 and pGAD424. However, yeasts cotransformed with both plasmids were not able to activate the lacZ reporter gene (data not shown), suggesting either that this region is critical but not sufficient for dimerization or that this region is too small to reconstitute a functional transactivator in the two-hybrid system or that this region, although critical for dimer formation, is not the dimerization site.
DISCUSSION
In the present work, we used the yeast two-hybrid system to provide evidence that the N-terminal domain of ActA interacts with itself. However, detection of interactions by the two-hybrid system does not necessarily indicate that the interactions detected occur in vivo. To detect ActA.N–ActA.N interactions in vivo, chemical cross-linking experiments on the L. monocytogenes wild-type strain LO28 grown in broth were performed. These experiments showed that treatment with cross-linking reagents generated an additional molecular species that migrated as approximately twice the molecular size of the native ActA protein (no higher molecular size species are found), indicating that ActA exists as a homodimer on the surface of the bacteria. When similar experiments were performed on L. monocytogenes ΔactA, which produced different ActA variants, ActA.N was identified as necessary for the dimerization process. Deletions in ActA.N were then analyzed. All these deletions can be considered not to strongly affect conformation because bacteria still retain the capacity to polymerize actin. For most of the deletions, dimer formation was detected, thus restricting a region critical for dimer formation to the region between amino acids 97 and 126 of ActA. However, some dimer formation was still observed in ActAΔ97–126 in contrast to deletion 97–231, suggesting that region 97–126 is not the dimerization site or is not the unique region involved in dimer formation.
The relevance of these results must be discussed keeping in mind the fact that ActA is responsible for the still not understood process of actin polymerization at the rear of intracellular bacteria (1, 2, 3, 21). Recently, we have demonstrated that ActA.N contains all the elements necessary to induce actin tail formation and movement (13), whereas the other two domains, the proline-rich repeats (ActA.P) and the C-terminal domain, are not absolutely essential for this process (7). The fact that ActA.N is also responsible for the dimerization process strongly suggests that dimerization is directly involved in the mechanism that allows continuous actin polymerization. Alternatively, dimerization could indirectly affect the process by simply increasing the affinity of ActA for its cellular ligands.
At present there is no argument to exclude the possibility that dimerization is directly involved in the actin polymerization process. Thermodynamic studies have demonstrated that actin assembly results from the local maintenance of uncapped filament barbed ends that can spontaneously polymerize due to favorable conditions of the host cytoplasm at the bacterium surface (16). ActA itself is the prime candidate to generate uncapped filament barbed ends either by causing nucleation of new filaments or by uncapping or by severing preexisting filaments. Alternatively, ActA could recruit a host factor that would somehow generate uncapped barbed ends. ActA could have in addition an active role in the elongation step. As said above, we have recently shown that ActA.N is sufficient for the process. In addition, we have identified two critical regions in ActA.N (13). The first region (region T) contains a highly positive charged peptide (117-KKRRK-121) and is critical for actin tail formation, as shown by the absence of actin tails in a 116–122 deletion mutant (ActA-Δ116–122). The second region (region C) spans amino acids 21–97 and is necessary for continuous actin polymerization, because deletion of 21–97 leads to the formation of a “discontinuous” actin tail. The experiments reported here have shown that a region essential for the dimerization process (97–126) is also essential for a fully active polymerization process. Indeed deletions 97–126, as with deletions 97–231 and 116–122 (13), affect tail formation. Taken together, these data strongly suggest that dimerization of ActA is necessary or at least plays a critical role in actin polymerization. If each ActA monomer is able to bind monomeric actin, as recently suggested (13), dimerization of ActA could stimulate the polymerization process by bringing one actin monomer close to another, either during the first step of the process (generation of free barbed ends) or during elongation. ActA dimerization may also place two ActA subunits in a configuration such that they can both bind efficiently to F-actin and protect barbed-ends from capping proteins (see model below).
Concerning the possibility that dimerization of ActA could be involved in the interaction of ActA with its ligands, it has been demonstrated that VASP (14) and its homolog MENA (15) bind to the proline-rich repeats of ActA. In addition, the amount of VASP that colocalizes with ActA is proportional to the number of repeats in the proline-rich domain of ActA (17). Because VASP is a tetramer (18), it is tempting to speculate that dimerization of ActA could increase its affinity for VASP. However, one should not exclude the possibility that dimerization could favor recruitment of another unidentified ligand, which could also be a dimer or a tetramer.
An intriguing feature of ActA is its polar distribution on the bacterial surface (6). The mechanism by which polarity is established is unknown but seems to be linked to bacterial division. The possibility that ActA can form a dimer could favor establishment of this polarity if the ActA monomer is relatively mobile in the bacterial membrane. In addition, the ActA dimer may help organize actin filaments, in line with the hypothesis of Smith et al. (17), that the two long repeats located downstream from each proline-rich repeat may function as multimerization motif facilitating the organization of actin filaments in parallel arrays.
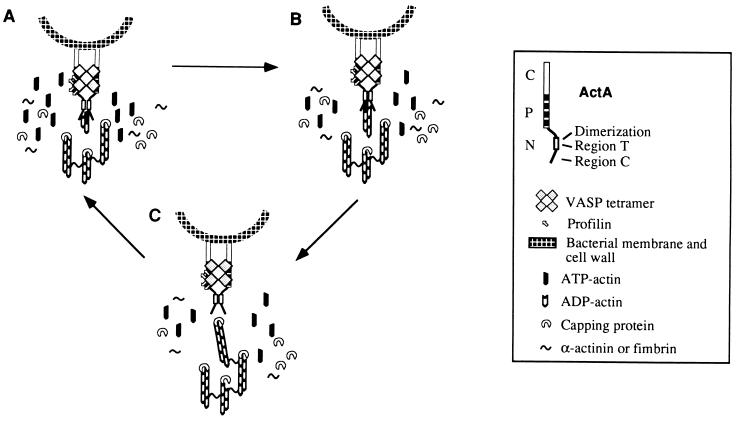
Based on the results presented here and in previous studies (3, 7, 17), we propose a model (Fig. 4) of actin-based motility mediated by ActA dimers divided in three phases. (i) Generation of free barbed ends by nucleation, in which the ActA dimer may be involved either directly, by promoting actin dimer and trimer formation, or indirectly by allowing the recruitment of a host nucleator. Alternatively, the ActA dimer could generate free barbed ends either by displacing directly or indirectly a capping protein or by severing directly or indirectly actin filaments. (ii) Elongation: dimerization may allow two C regions (21–97) to bind one F-actin filament and keep the newly generated barbed-end uncapped, thereby allowing continuous filament elongation. In addition, dimerization may increase the affinity of VASP for the proline rich domain of one or even two ActA dimers. In the latter case VASP may cross-link ActA and participate to an increase in the polar distribution of ActA, in infected cells (see above). VASP binds profilin through its four GlyPro5 sequences (19). Because profilin can attract actin, the VASP–profilin complex bound to the central proline rich region of ActA might present polymerization-competent actin monomers to the N-terminal domain and increase the efficiency of actin polymerization at free barbed-ends. (iii) Release: whenever a filament becomes capped, it is released and rapidly cross-linked to the performed tail. The ActA dimer is thus available to start a new cycle of nucleation.
Figure 4.
Hypothetical model of actin assembly by ActA dimers. The N-terminal domain of ActA contains two regions critical for actin based motility. Both are required for actin tail formation but each has in addition a specific role to maintain the dynamics of the process. Region T (116–122) is necessary for actin tail formation, and region C (21–97) is necessary for continuous actin filament elongation. The region of ActA spanning amino acids 97–126 is necessary for both dimerization of ActA and actin polymerization. (A) Generation of free barbed ends either by nucleation or uncapping or severing. Dimerization of ActA.N could allow ActA either to nucleate actin, or to interact with a host nucleator or to displace a capping protein, or to sever actin filaments. (B) Elongation. Once nucleation has occurred, two ActA monomers bind the actin filament through region 21–97, which keeps the barbed ends uncapped. ActA dimerization favors recruitment of VASP–profilin–actin complexes on the central proline rich region of ActA, bringing polymerization competent actin monomers in the vicinity of the bacterium, resulting in filament elongation. (C) Release. Actin filament elongation continues until capping, release, and cross-linking to the performed tail take place. When the filament is released, the ActA dimer is immediately available for a new cycle of nucleation, elongation, and release.
Dimerization is a novel property of ActA that should help explain how this protein induces actin polymerization. Our results suggest that a region necessary for dimerization is also required for actin comet tail formation and movement. However, whether both phenomena are absolutely linked remains an open question. The key question to address now is what is the dimerization site or what are the dimerization sites. Indeed, region 97–126 is essential for dimerization but may not represent the actual dimerization site(s).
Acknowledgments
We thank K. Ireton for critical reading the manuscript. This study received financial support from the Association pour la Recherche sur le Cancer (Grant CT1164), the Ministere de la Recherche (ACC-SV6), the European Economic Community (Grants BMH4-CT 96-0659 and CHRX-CT 92-0018), and the Pasteur Institute. I.L. is a recipient of a postdoctoral fellowship from the European Economic Community (Training and Mobility of Researchers).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: VASP, vasodilator-stimulated phosphoprotein; DBD, DNA binding domain; BS3, bis(sulfosuccinimidyl) suberate; DSP, dithio-bis(succinimidylpropionate); AD, activation domain; β-gal, β-galactosidase.
References
- 1.Cossart P. Curr Opin Cell Biol. 1995;7:94–101. doi: 10.1016/0955-0674(95)80050-6. [DOI] [PubMed] [Google Scholar]
- 2.Theriot J. Annu Rev Cell Dev Biol. 1995;11:213–239. doi: 10.1146/annurev.cb.11.110195.001241. [DOI] [PubMed] [Google Scholar]
- 3.Lasa I, Cossart P. Trends Cell Biol. 1996;6:109–114. doi: 10.1016/0962-8924(96)81001-4. [DOI] [PubMed] [Google Scholar]
- 4.Domann E, Wehland J, Rohde M, Pistor S, Hartl M, Goebel W, Leimeister-Wächter M, Wuenscher M, Chakraborty T. EMBO J. 1992;11:1981–1990. doi: 10.1002/j.1460-2075.1992.tb05252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. Cell. 1992;68:521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- 6.Kocks C, Hellio R, Gounon P, Ohayon H, Cossart P. J Cell Sci. 1993;105:699–710. doi: 10.1242/jcs.105.3.699. [DOI] [PubMed] [Google Scholar]
- 7.Lasa I, David V, Gouin E, Marchand J, Cossart P. Mol Microbiol. 1995;18:425–436. doi: 10.1111/j.1365-2958.1995.mmi_18030425.x. [DOI] [PubMed] [Google Scholar]
- 8.Vazquez-Boland J-A, Kocks C, Dramsi S, Ohayon H, Geoffroy C, Mengaud J, Cossart P. Infect Immun. 1992;60:219–230. doi: 10.1128/iai.60.1.219-230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kocks C, Marchand J B, Gouin E, d’Hauteville H, Sansonetti P J, Carlier M F, Cossart P. Mol Microbiol. 1995;18:413–423. doi: 10.1111/j.1365-2958.1995.mmi_18030413.x. [DOI] [PubMed] [Google Scholar]
- 10.Smith G A, Portnoy D A, Theriot J A. Mol Microbiol. 1995;17:945–951. doi: 10.1111/j.1365-2958.1995.mmi_17050945.x. [DOI] [PubMed] [Google Scholar]
- 11.Pistor S, Chakraborty T, Walter U, Wehland J. Curr Biol. 1995;5:517–525. doi: 10.1016/s0960-9822(95)00104-7. [DOI] [PubMed] [Google Scholar]
- 12.Friederich E, Gouin E, Hellio R, Kocks C, Cossart P, Louvard D. EMBO J. 1995;14:2731–2744. doi: 10.1002/j.1460-2075.1995.tb07274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lasa I, Gouin E, Goethals M, Vancompernolle K, David V, Vandekerckhove J, Cossart P. EMBO J. 1997;16:1531–1540. doi: 10.1093/emboj/16.7.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakraborty T, Ebel F, Domann E, Niebuhr K, Gerstel B, Pistor S, Temm-Grove C J, Jockusch B M, Reinhard M, Walter U, Wehland J. EMBO J. 1995;14:1314–1321. doi: 10.1002/j.1460-2075.1995.tb07117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gertler F B, Niebuhr K, Reinhard M, Wehland J, Soriano P. Cell. 1996;87:227–239. doi: 10.1016/s0092-8674(00)81341-0. [DOI] [PubMed] [Google Scholar]
- 16.Marchand J B, Moreau P, Paoletti A, Cossart P, Carlier M F, Pantaloni D. J Cell Biol. 1995;130:331–343. doi: 10.1083/jcb.130.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith G A, Theriot J, Portnoy D. J Cell Biol. 1996;135:647–660. doi: 10.1083/jcb.135.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haffner C, Jarchau T, Reinhard M, Hoppe J, Lohmann S H, Walter U. EMBO J. 1995;14:19–27. doi: 10.1002/j.1460-2075.1995.tb06971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reinhard M, Giehl K, Abel K, Haffner C, Jarchau T, Hoppe V, Jockusch B M, Walter U. EMBO J. 1995;14:1583–1589. doi: 10.1002/j.1460-2075.1995.tb07146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klarsfeld A, Goossens P, Cossart P. Mol Microbiol. 1994;13:585–597. doi: 10.1111/j.1365-2958.1994.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 21.Ireton K, Cossart P. Annu Rev Gener. 1997;31:113–138. doi: 10.1146/annurev.genet.31.1.113. [DOI] [PubMed] [Google Scholar]