ATP has been known for decades as a major form of molecular energy currency, generated by glycolysis, respiration, or other reactions, and used to provide energy for a myriad of other biochemical processes. However, in recent years it has become apparent that ATP also fulfills a role as an extracellular signaling molecule. ATP is secreted by fusion of ATP-containing intracellular vesicles with the cell membrane, but the machinery responsible for filling these vesicles has, until now, been a mystery. In a recent issue of PNAS, Yoshinori Moriyama and his coworkers at Okayama University have identified an ATP transporter present in secretory vesicles that fulfills all of the requirements of the elusive vesicular ATP transporter, which they have named VNUT (1).
When a nerve cell sends a signal to an adjacent cell, that signal is almost always a chemical neurotransmitter secreted by a process known as exocytosis. Neurons contain small intracellular compartments, called synaptic vesicles, filled with a neurotransmitter such as serotonin, acetylcholine, or glutamate. When these vesicles fuse with the cell membrane, their contents are released from the cell and are free to activate receptors on the surface of neighboring cells. Researchers have known for many years that along with the neurotransmitter, ATP is also released, and that most synaptic vesicles store this nucleotide, which is also used to power many of the cell's energy-dependent reactions (2). Until now, however, the history of research on ATP storage within synaptic vesicles had been problematic, leading skeptics to doubt that any firm conclusions could be drawn (3). In this novel and incisive series of experiments, Moriyama and coworkers (1) have finally banished the demons of ATP transport by unequivocally demonstrating the transporter's activity in a purified and reconstituted preparation.
ATP was generally recognized to constitute a major portion of the contents of secretory vesicles in early studies of chromaffin granules (4). These intracellular storage organelles are found in cells of the adrenal medulla that secrete the catecholamines epinephrine and norepinephrine. Just as neurons secrete neurotransmitters stored within synaptic vesicles, chromaffin cells secrete epinephrine by fusion of these granules with the cell membrane, releasing the granule contents into the bloodstream. The released epinephrine acts on many targets in the body, initiating the “fight or flight” response that readies our muscles and circulatory system for intense activity.
Because chromaffin granules are large and heavy, they were relatively easy for early neuroscientists to isolate in a form that retained most of their original contents. Analysis of these contents revealed startlingly high concentrations of epinephrine, norepinephrine—and ATP. If all of the vesicle contents were free in solution, their combined concentrations would lead to an osmolality much greater than that of the cytoplasm (5). This realization led to the idea that ATP formed a complex with vesicular catecholamines and that stabilized the high intravesicular concentration (6). Thus, the role assigned to ATP was that of a cofactor allowing high levels of catecholamine storage.
Neurons secrete many neurotransmitters—not only catecholamines, such as dopamine and norepinephrine, but also other amines such as acetylcholine, serotonin, and histamine, and amino acids such as glutamate, glycine, and GABA. For each of these neurotransmitters, there are proteins in the cell and vesicle membrane for storage of the transmitter within the vesicle and detection of the released transmitter. In most cases, inactivation is achieved by a transport protein in the plasma membrane that removes the transmitter from the extracellular space and concentrates it within the cytoplasm (7), where it is further transported into synaptic vesicles (8). This kind of inactivation process leads to recycling and reuse of the transmitter molecule, but it is not universal. For example, inactivation of acetylcholine and neuropeptides occurs through hydrolysis of the transmitters to inactive products.
Moriyama and coworkers have finally banished the demons of ATP transport.
Vesicular transporters are generally nonselective. For example, both isoforms of the vesicular monoamine transporter, VMAT1 and VMAT2, transport serotonin, norepinephrine, and dopamine, and the vesicular inhibitory amino acid transporter, VIAAT, transports both GABA and glycine. Here the exception seems to be VAChT, which transports acetylcholine, and the glutamate transporter isoforms, VGLUT1–3, which are selective for glutamate. It is this family of vesicular glutamate transporters that contained an orphan protein, designated SLC17A9, representing the long-sought ATP transporter.
As early as the late 1950s, ATP release from nerves was observed (2), and the concept of purinergic neurotransmission was proposed in 1972 (9), but there was reluctance to believe that ATP, well known as an intracellular molecule central to the energy metabolism of the cell, could also function as an extracellular signaling molecule. In agreement with this proposal, a group of purinergic receptors were found to respond to ATP, UTP, and adenosine. They consist of three classes. The P1 receptors (now known as A1–3) are activated by adenosine and are G protein-coupled receptors (GPCRs). A second group, the P2Y receptors, are also GPCRs, but respond to ATP, ADP, UTP, and UDP, and a third group, the P2X receptors, are ligand-gated channels that conduct cation currents when they bind ATP (10).
Part of the resistance to ATP as a signaling molecule came from uncertainty about how ATP entered secretory organelles. Despite the high concentrations of ATP in chromaffin granules, efforts to show that vesicles could accumulate ATP were slow to produce convincing demonstrations of transport. In retrospect, studies on ATP accumulation by chromaffin granules as early as 1978 accurately described characteristics of this transport system (11, 12), but there was a problem inherent in any demonstration that ATP was transported. The driving force for transport was ATP itself, acting on a vesicular proton pump (the vesicular ATPase or V-ATPase). It was difficult to distinguish between effects of ATP and inhibitors on the V-ATPase or on the putative ATP transporter.
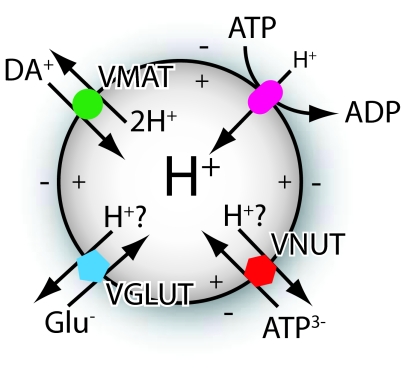
V-ATPase provides the driving force for accumulation of neurotransmitters and ATP within synaptic vesicles and chromaffin granules. Moriyama worked on this enzyme as a postdoctoral fellow in Nathan Nelson's laboratory, where he developed an interest in vacuolar transporters. This enzyme pumps H+ ions into the vesicle, creating an electrical potential (Δψ, inside positive) and a pH difference (ΔpH, inside acidic) (Fig. 1). These constitute two distinct components that are used in different ways by neurotransmitter and ATP transporters. For example, VMAT transports a positively charged amine molecule into the vesicle in exchange for two protons. This 2:1 exchange means that only one net charge leaves the vesicle with the two protons, and the ΔpH is a much more effective driving force than Δψ (13). Because VIAAT transports substrates with no net charge, the exchange of a cytoplasmic GABA or glycine molecule for an intravesicular H+ ion is coupled equally to ΔpH and Δψ (14), but for VGLUT, the involvement of H+ exchange with glutamate is controversial. Influx of a negatively charged cytoplasmic glutamate molecule either by itself, or in exchange for an H+ ion, results in the inward movement of either one negative charge or two negative charges and efflux of one proton, respectively. In both cases, the predominant driving force is Δψ. With VMAT, the unequal contribution of ΔpH and Δψ initially led to the conclusion that only one of the two components, in this case Δψ, contributed (14). However, further investigation suggested the possibility that, rather than transporting each glutamate anion by itself, VGLUT may exchange glutamate with H+ (15).
Fig. 1.
Schematic depiction of how ATP-dependent proton pumping drives vesicular accumulation of dopamine (DA) through VMAT, glutamate through VGLUT, and ATP through VNUT.
Moriyama and coworkers (1) presumed that SLC17A9 might be a transporter for ascorbate or ATP—two anions, like glutamate, whose transporters had never been identified. In this new work, they find that it is a vesicular ATP transporter (VNUT) similar to VGLUT in many ways. It is evolutionarily closely related, belonging to the same SLC17 transporter family, it is stimulated by chloride, as an anion transporter it is coupled predominantly to Δψ, and it is inhibited by Evans blue. Some of these properties had previously been reported (11, 16), but until the present work identifying the transporter, their full importance was not recognized.
One of the most convincing aspects of the present work, which sets it apart from previous studies, is the ability to assay ATP transport without using ATP itself as an energy source. By using purified VNUT reconstituted into lipid vesicles, and artificially imposing Δψ with valinomycin and an inwardly directed K+ gradient, the authors were able to bypass the requirement for V-ATPase and avoid all of the attendant complications that thwarted previous studies. From this work, it is quite clear that Δψ, interior positive, can drive the accumulation of ATP, and also ADP and GTP.
However, the bioenergetic characterization leaves some remaining questions unanswered. In particular, is ATP transported alone, or is it exchanged with H+? The present work showed that ΔpH by itself was ineffective as a driving force, but we would expect Δψ to be a much more effective driving force, and an effect of ΔpH might be undetectable unless Δψ is also imposed. The Cl− requirement also suggests a direction for future studies. If VNUT is incorporated into the proteoliposomes in a random topological orientation and Cl− binds to a site on one side of the membrane, then we might expect that only half of the incorporated VNUT would be activated by extravesicular Cl−. Does intravesicular Cl− activate the other half? Or is a Cl− gradient the critical component, in which case we might expect that intravesicular Cl− might actually inhibit?
To finally tie the identification of VNUT to its role in purinergic signaling, Moriyama and coworkers (1) looked at ATP release from PC-12 cells. These cells, like chromaffin cells, are derived from adrenal medulla and contain secretory vesicles that release their contents (including ATP) by exocytosis when stimulated by depolarization with KCl. The secretory vesicles of PC-12 cells stain with antibodies against VNUT, and the level of VNUT can be knocked down ≈50% with specific siRNA. This knockdown resulted in a decrease in ATP secretion of approximately the same magnitude, demonstrating strong support for its role in filling the vesicles with ATP.
Footnotes
The author declares no conflict of interest.
See companion article on page 5683 in issue 15 of volume 105.
References
- 1.Sawada K, et al. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci USA. 2008;105:5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holton P. The liberation of adenosine triphosphate on antidromic stimulation of sensory nerves. J Physiol. 1959;145:494–504. doi: 10.1113/jphysiol.1959.sp006157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gruninger HA, Apps DK, Phillips JH. Adenine nucleotide and phosphoenolpyruvate transport by bovine chromaffin granule “ghosts.”. Neuroscience. 1983;9:917–924. doi: 10.1016/0306-4522(83)90280-4. [DOI] [PubMed] [Google Scholar]
- 4.Winkler H. The composition of adrenal chromaffin granules: An assessment of controversial results. Neuroscience. 1976;1:65–80. doi: 10.1016/0306-4522(76)90001-4. [DOI] [PubMed] [Google Scholar]
- 5.Njus D, Radda GK. Bioenergetic processes in chromaffin granules: A new perspective on some old problems. Biochim Biophys Acta. 1978;463:219–244. doi: 10.1016/0304-4173(78)90001-0. [DOI] [PubMed] [Google Scholar]
- 6.Kopell WN, Westhead EW. Osmotic pressures of solutions of ATP and catecholamines relating to storage in chromaffin granules. J Biol Chem. 1982;257:5707–5710. [PubMed] [Google Scholar]
- 7.Amara SG. Neurotransmitter transporters: A tale of two families. Nature. 1992;360:420–421. doi: 10.1038/360420d0. [DOI] [PubMed] [Google Scholar]
- 8.Peter D, Liu Y, Brecha N, Edwards RH. The transport of neurotransmitters into synaptic vesicles. Prog Brain Res. 1995;105:273–281. doi: 10.1016/s0079-6123(08)63304-x. [DOI] [PubMed] [Google Scholar]
- 9.Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- 10.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 11.Aberer W, Kostron H, Huber E, Winkler HA. Characterization of the nucleotide uptake of chromaffin granules of bovine adrenal medulla. Biochem J. 1978;172:353–360. doi: 10.1042/bj1720353b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bankston LA, Guidotti G. Characterization of ATP transport into chromaffin granule ghosts. Synergy of ATP and serotonin accumulation in chromaffin granule ghosts. J Biol Chem. 1996;271:17132–17138. doi: 10.1074/jbc.271.29.17132. [DOI] [PubMed] [Google Scholar]
- 13.Kanner BI, Schuldiner S. Mechanism of transport and storage of neurotransmitters. CRC Crit Rev Biochem. 1987;22:1–38. doi: 10.3109/10409238709082546. [DOI] [PubMed] [Google Scholar]
- 14.Maycox PR, Hell JW, Jahn R. Amino acid neurotransmission: Spotlight on synaptic vesicles. Trends Neurosci. 1990;13:83–87. doi: 10.1016/0166-2236(90)90178-d. [DOI] [PubMed] [Google Scholar]
- 15.Tabb J, Kish P, Van Dyke R, Ueda T. Glutamate transport into synaptic vesicles: Roles of membrane potential, pH gradient, and intravesicular pH. J Biol Chem. 1992;267:15412–15418. [PubMed] [Google Scholar]
- 16.Gualix J, Pintor J, Miras-Portugal MT. Characterization of nucleotide transport into rat brain synaptic vesicles. J Neurochem. 1999;73:1098–1104. doi: 10.1046/j.1471-4159.1999.0731098.x. [DOI] [PubMed] [Google Scholar]