Abstract
The spatial distribution of plant defenses within a leaf may be critical in explaining patterns of herbivory. The generalist lepidopteran larvae, Helicoverpa armigera (the cotton bollworm), avoided the midvein and periphery of Arabidopsis thaliana rosette leaves and fed almost exclusively on the inner lamina. This feeding pattern was attributed to glucosinolates because it was not evident in a myrosinase mutant that lacks the ability to activate glucosinolate defenses by hydrolysis. To measure the spatial distribution of glucosinolates in A. thaliana leaves at a fine scale, we constructed ion intensity maps from MALDI-TOF (matrix assisted laser desorption/ionization-time of flight) mass spectra. The major glucosinolates were found to be more abundant in tissues of the midvein and the periphery of the leaf than the inner lamina, patterns that were validated by HPLC analyses of dissected leaves. In addition, there were differences in the proportions of the three major glucosinolates in different leaf regions. Hence, the distribution of glucosinolates within the leaf appears to control the feeding preference of H. armigera larvae. The preferential allocation of glucosinolates to the periphery may play a key role in the defense of leaves by creating a barrier to the feeding of chewing herbivores that frequently approach leaves from the edge.
Keywords: antiherbivore defense, MALDI-imaging, plant natural products
Many plant natural products appear to serve as defenses against herbivores because of their toxicity or deterrence in artificial diets or when added as a supplement to plant material (1, 2). However, to evaluate the actual defensive role of these substances in planta, it is necessary to estimate the amount that a potential herbivore would encounter during feeding. Although determining the amount of plant tissue ingested during a feeding bout is relatively straightforward, measuring the quantity of specific defense products in that tissue is not. Nearly all studies to date have quantified the levels of defensive metabolites at the level of the whole plant or organ (3). Little is known about the localization of antiherbivore defenses in individual tissues or parts of organs even though this may be critically important to the behavior of small herbivores and to the effectiveness of the defense.
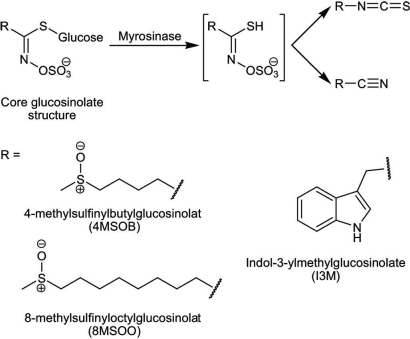
One of the most extensively studied classes of antiherbivore chemical defenses in plants is the glucosinolates, a group of sulfur-rich, amino acid-derived metabolites combining a β-d-glucopyranose residue linked via a sulfur atom to an N-hydroxyamino sulfate ester (4) (Fig. 1). Glucosinolates are widespread in the order Capparales, which includes vegetables (cabbage, cauliflower, and broccoli), spice plants supplying condiments (mustard, horseradish, and wasabi), and the model species, Arabidopsis thaliana (5, 6). Upon insect feeding or mechanical disruption, glucosinolates are hydrolyzed by an endogenous glucohydrolase activity known as myrosinase, and the released aglycone rearranges to form isothiocyanates, nitriles, and other products (7) (Fig. 1). Nearly all of the defensive properties of glucosinolates can be attributed to the toxicity and deterrence of these hydrolysis products (4). To avoid premature hydrolysis and autotoxicity, glucosinolates and myrosinase are stored in separate cells or cellular compartments in the plant (8), but these compartments cannot be too far apart or they would not mix together and react efficiently after herbivore damage. Despite the importance of glucosinolate and myrosinase localization in the activation of this defense system, little is known about their locations within individual leaves, stems, or other organs and how this may influence patterns of herbivory.
Fig. 1.
Structures of A. thaliana glucosinolates identified in this study and scheme for myrosinase-catalyzed hydrolysis of glucosinolates to isothiocyanates and nitriles.
For tissue- or organ-level localization studies, investigators must employ an analytical technique that is sensitive enough for small samples yet specific enough for the compounds of interest. Considering the widespread occurrence of natural products in plants, relatively few suitable histochemical (9), immunocytochemical (10), or spectroscopic techniques (11) have been developed for fine-scale localization in plant tissues. Recently, spectrometric imaging techniques have become available that are capable of mapping metabolite distribution in biological samples with cellular-like resolution (12, 13). Among these is MALDI-TOF (matrix assisted laser desorption/ionization-time of flight) mass spectrometric imaging that was introduced by Caprioli in 1997 (14). The sample is sprayed with a matrix, and the ions of interest are desorbed from the tissue by using a conventional MALDI source. The laser position over the target is gradually changed in steps over a predetermined x, y-grid, and the final ion image is plotted by using three-dimensional coordinates with x and y axes for positions and the z axis for the intensity of the particular ion (15). Diverse analytes have been characterized by MALDI-TOF imaging including drugs, peptides, and proteins in animal tissues (16), and herbicides (17) and peptides (18) in plants. However, despite several very recent reports on MALDI imaging of sugars in plants (19, 20), the distribution of secondary natural products in intact plant tissue has not been determined by using mass spectrometric imaging. Moreover, in most cases the distribution of compounds determined by mass spectrometric imaging has not been validated by using independent methods.
Here, we report the fine-scale, spatial distribution of glucosinolates in leaves, as determined by MALDI-TOF imaging of A. thaliana, and relate this distribution to the pattern of herbivory caused by larvae of the lepidopteran, Helicoverpa armigera (the cotton bollworm). The glucosinolate distribution was confirmed independently by using HPLC and compared with the spatial distribution of myrosinase in the same species. Feeding experiments with H. armigera revealed that the relative abundance of glucosinolates in the inner vs. the peripheral part of the leaf is significant for insect preference and antiherbivore defense.
Results
H. armigera Larvae Avoid the Midvein and Leaf Periphery When Feeding on A. thaliana Leaves.
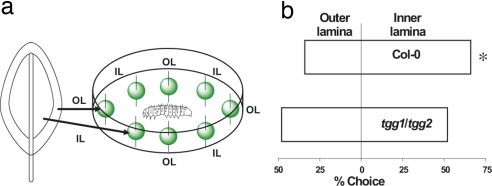
Many small herbivores do not feed uniformly on all parts of the leaf but forage preferentially on specific parts (21). To study this phenomenon and determine its link to the distribution of plant defenses, we began by making extensive observations of the feeding behavior of first- and second-instar larvae of H. armigera, a generalist feeder, on mature rosette leaves of A. thaliana. Larvae were found to consistently avoid the periphery of the leaf and the midvein and feed almost exclusively on the inner lamina (Fig. 2). When second-instar H. armigera were offered a choice between disks cut from the outer lamina (periphery) and inner lamina, they fed significantly more on the inner lamina (Fig. 3). This preference could be a result of the inner lamina having an increased nutrient content, reduced toughness, or lower levels of glucosinolate defenses.
Fig. 2.
Feeding pattern of H. armigera larvae on mature rosette leaf of A. thaliana. Ten second-instar larvae were allowed to feed for 5 h. This photograph is representative of five trials. Photograph by Danny Kessler.
Fig. 3.
Feeding preference of second-instar H. armigera larvae for regions of A. thaliana leaves. (a) Bioassay arena in a 10-mm Petri dish equipped with leaf disks from outer lamina (OL) and inner lamina (IL). Leaf disks and caterpillar are not shown to scale. (b) Bioassay results on A. thaliana wild type (n = 30) and tgg1/tgg2 mutant line (n = 25). For wild-type leaves, larvae preferred to feed on disks removed from the inner over the outer lamina (binomial test, P = 0.001). There was no significant preference for the corresponding leaf discs from the tgg1/tgg2 mutant (binomial test, P = 0.764), which has the two principal myrosinase genes knocked out and is thus unable to activate the hydrolysis of glucosinolates.
To determine the role of glucosinolates in this behavior, we repeated the choice test with leaves of the A. thaliana tgg1/tgg2 mutant line (22), which is knocked out in the genes encoding foliar myrosinases and is thus unable to hydrolyze glucosinolates. Such plants should be identical to wild-type A. thaliana but lack the ability to mobilize an active glucosinolate defense. Larvae of H. armigera showed no preference for leaf disks cut from the outer versus the inner lamina of tgg1/tgg2 leaves (Fig. 3) suggesting that glucosinolate content is responsible for the choice of feeding site on wild-type plants.
MALDI-TOF Imaging Shows the Highest Glucosinolate Concentrations in Midvein and Leaf Periphery.
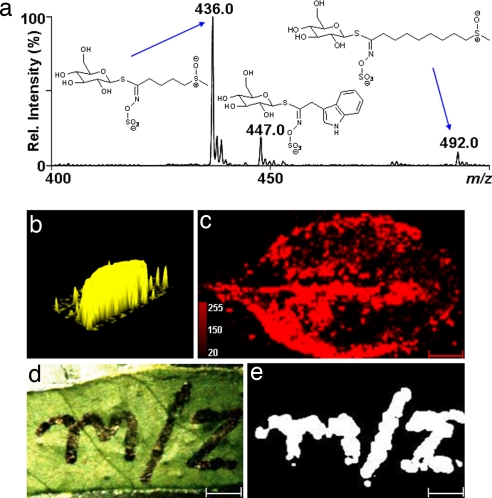
We investigated the localization of glucosinolates in A. thaliana leaves by axial MALDI-TOF imaging of intact leaves that were sprayed on one side with a matrix by using a commercial air brush that provided a very uniform deposition as confirmed by measurement of a matrix ion from an actual leaf sample after coating with matrix (Fig. 4b). Analytes on the leaf maintained their relative position during matrix application and sample evacuation before MALDI measurement (Fig. 4 d and e). Negative ion analysis was selected because it should be a more suitable technique for desorption/ionization of anionic species like glucosinolates than positive ion analysis and should yield a spectrum with less noise. The experimental protocol used was very sensitive, yielding mass spectra with very clear signals for the M− ions of glucosinolates from very low amounts of sample. As little as 60 pg of authentic glucosinolate standards provided readily detectable peaks (signal-to-noise ratio of at least 5). The three glucosinolates detected in A. thaliana leaves were 4-methylsulfinylbutylglucosinolate (4MSOB, glucoraphanin, m/z 436, M−), indol-3-ylmethylglucosinolate (I3M, glucobrassicin, m/z 447, M−) and 8-methylsulfinyl-octylglucosinolate (8MSOO, glucohirsutin, m/z 492, M−) (Fig. 4a). These three compounds are reported to be among the major glucosinolates of A. thaliana rosette leaves (Columbia ecotype), together making up >75% of the total glucosinolate composition (23). The identity of these compounds in the MALDI-TOF mass spectra was confirmed by accurate mass measurement and the isotopic pattern of their respective M− ions. Glucosinolates give a characteristic isotopic pattern with unusually intense A + 2 isotope signals because of the presence of two to three sulfur atoms in their structures (Fig. 1). Further confirmation was obtained by comparing CID (collision-induced dissociation) mass spectra of 4MSOB and I3M standards to spectra obtained directly from the leaf [see supporting information (SI) Table S1] acquired by using an atmospheric pressure MALDI source connected to a linear ion trap instrument.
Fig. 4.
Mass spectrometric imaging of A. thaliana leaves. (a) Section (m/z 400–500) of a MALDI-TOF/MS spectrum averaged from 100 consecutive pixels on a leaf sprayed on its abaxial side with 9-aminoacridine as a matrix. The molecular peaks at m/z 436.0, 447.0, and 492.0 correspond to M− ions of 4-methylsulfinylbutylglucosinolate (4MSOB), indol-3-ylmethylglucosinolate (I3M), and 8-methylsulfinyloctylglucosinolate (8MSOO), respectively. The spectra were collected in a negative reflectron mode on a MALDI micro MX (Waters). (b) Three-dimensional ion intensity map of the matrix ion [m/z 193.0 ± 0.25 (M − H)−] from measurement of a leaf sample mounted on the MALDI target plate. The image demonstrates that the matrix was deposited homogeneously over the leaf. The spraying area was defined with a paper mask to prevent matrix dispersal during application. (c) Ion intensity map of 4MSOB (m/z 436 ± 0.25) created in ImageJ software from ≈100,000 MALDI-TOF/MS spectra (420 × 252 pixels (w × h), pixel size 200 μm). The ion intensities are displayed in a pseudocolor on an intensity scale of 0–255 shades (see Inset for the color scale). (d and e) To demonstrate that analytes on the leaf are not delocalized during matrix application and the other steps of sample processing, a permanent marker pen with methanol-soluble ink was used to write “m/z” on the leaf. The intensity image of a characteristic ion from the marker ink (m/z 663.8) obtained after coating with the 9-aminoacridine matrix corresponds well to the original ink pattern. The matrix coating and the instrumental acquisition parameters were identical to those used in the other imaging experiments. (Scale bars in c and e: 0.5 cm.)
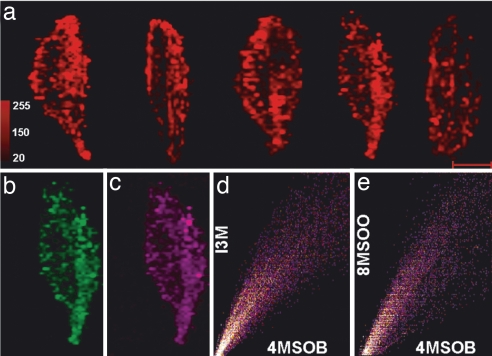
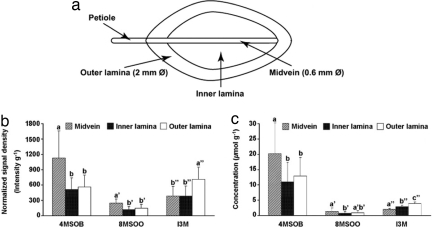
MALDI-TOF spectra were collected from the whole leaf at 200-μm spatial resolution in both x and y directions (≈100,000 mass spectra per leaf), providing a resolution of 2,500 pixels/cm2. Conversion of the ion chromatogram to ion intensity images showed a distinct, nonuniform distribution of glucosinolates within the leaf. Statistical evaluation indicated that the greatest abundance of glucosinolates was in the midvein and peripheral portions of the leaf lamina. Depicted is a series of five leaves of the basic measurement set (Fig. 5a), plus one larger leaf showing the pattern in more detail (Fig. 4c). For 4MSOB and 8MSOO, significantly greater amounts were present in the midvein compared with the outer and inner leaf lamina (Fig. 6a). On the other hand, I3M exhibited greater abundance in the outer lamina than the inner lamina or midvein.
Fig. 5.
Imaging reveals nonuniform glucosinolate distribution in A. thaliana leaves. (a) Five different leaves measured and displayed as in Fig. 2 showing observed 4MSOB ion patterns. (Scale bar: 1 cm.) (b and c) Ion intensity maps of I3M (477 ± 0.25) and 8MSOO (492 ± 0.25), respectively, obtained from mass data measured on the next to last leaf in a. (d) A scatter plot of 4MSOB vs. I3M obtained with the ImageJ applet[36]. The correspondence with a line of slope y = x shows the extent to which the two compounds cooccur. Scales are in false color intensity (0–255) for both x and y axes. (e) A scatter plot of 4MSOB vs. 8MSOO.
Fig. 6.
Comparison of the relative amounts of the three measured glucosinolates in three different leaf regions of A. thaliana leaves defined as in a. Normalized ion intensities were calculated for the midvein, the inner lamina, and the outer lamina areas from mass spectrometric imaging (b) and glucosinolate concentrations were measured by HPLC analysis (c). Error bars represent SEM from five mature leaves from different plants. Bars marked by different letters show significant difference at P < 0.05.
HPLC Analyses Confirm the Pattern of Glucosinolate Distribution.
To validate the mass spectrometric imaging patterns, glucosinolate analysis of leaf parts was conducted by using an established analytical method, HPLC of the desulfated derivatives with UV detection (23). The distribution of glucosinolates observed by means of this technique correlated well with the pattern observed by MALDI-TOF imaging. Larger amounts of 4MSOB and 8MSOO were found in the midvein than in other areas of the leaf, and larger amounts of I3M were found in the outer lamina (Fig. 6c). Relative to the other glucosinolates, the signal for I3M was lower in the HPLC analysis than with MALDI-TOF imaging, probably because of the more facile desorption of aromatic glucosinolates by the UV laser compared with glucosinolates with aliphatic side chains; however, the two methods showed very similar trends. The total amount of the three measured glucosinolates was as high in the midvein (which makes up ≈15% of the total leaf area) as in the remainder of the leaf. To determine whether the glucosinolates were colocalized at a small scale, the x, y positions of their ion intensity images (Fig. 5 a–c) were plotted against each other (Fig. 5 d and e). A linear relationship between 4MSOB and I3M and between 4MSOB and 8MSOO was clearly visible indicating a general occurrence of all three glucosinolates at a fine scale throughout the leaf despite differences among them in relative abundance.
The activation of glucosinolates for plant defense requires hydrolysis by myrosinase. Hence, we also investigated the distribution of myrosinase activity in A. thaliana leaves. An in vitro assay of different leaf parts from 20 leaves showed no significant differences in myrosinase activity among the midvein (4.57 ± 0.844, mean ± SE in nmol of glucose × mg of leaf−1), outer lamina (4.21 ± 0.622) and inner lamina (4.32 ± 0.957) by ANOVA.
Discussion
Ion intensity maps constructed from MALDI-TOF mass spectra demonstrated that glucosinolates have a nonuniform distribution in the leaves of A. thaliana. Of the three major glucosinolates analyzed, two were more abundant in the midvein than the rest of the leaf, whereas one was more abundant on the edge of the leaf than elsewhere. These results were confirmed by HPLC analysis. The protocol used represents an advance over existing mass spectrometric imaging methods by allowing very sensitive measurements of metabolite distribution in an intact leaf in a quantitative manner that can be statistically analyzed and validated by independent methods.
The nonuniform distribution of glucosinolates in A. thaliana leaves appears to explain the feeding preference of H. armigera larvae for specific portions of the leaf. In whole-leaf trials and leaf disk bioassays, larvae avoided feeding on the midvein and the edge of the leaf, the locations with high levels of glucosinolates. Furthermore, this preference hierarchy was not evident during feeding on tgg1/tgg2 mutants, which lack foliar myrosinases and so are unable to activate their glucosinolate defenses. The glucosinolates in A. thaliana leaves are deployed such that the greatest concentrations are present in the midvein and leaf periphery. This pattern may result from various physiological constraints on the synthesis and storage of glucosinolates in certain tissues. For example, the formation of glucosinolates might occur only in vascular bundles because certain biosynthetic enzymes are restricted to this tissue (24, 25). Storage may only be possible in certain parts of the leaf because of the need to avoid autotoxicity to sensitive cells.
The nonuniform distribution of glucosinolates is also likely to have ecological significance because, like other plants, A. thaliana may have been selected to maximize its antiherbivore defenses at sites that are most valuable or vulnerable to attack (26). The midvein is critical to the function of the leaf because damage to this structure would disrupt the transport of water and nutrients and assimilate throughout the leaf. In addition, the integrity of the periphery may also be vital for leaf function because this tissue stabilizes leaf architecture even if there is damage to the inner parts. The high concentration of defenses on the leaf periphery may function as a barrier to insect herbivores that initiate feeding from the leaf edge, a behavior often observed for older instars of H. armigera and other lepidopteran species (F.V., unpublished results).
The distribution of defense compounds in leaves is not often studied at a scale relevant to small herbivores, but in previous work, certain other defense compounds have been localized in peripheral tissues. For example, cyanogenic glycosides are localized in the epidermal cells of sorghum (27), monoterpenes and diterpenes are found in glandular hairs projecting from the epidermis of many members of the Lamiaceae (28), and higher terpenes form a surface resin on leaves of Newcastelia viscida (29). Glucosinolate distribution within leaves was the subject of previous work as well, at a much coarser scale of analysis than in the present study. Wild radish leaves were shown to have higher concentrations of glucosinolates in their lower halves than in their distal halves with a high degree of random variation (30).
The localization of plant glucosinolates at the cellular level is also poorly investigated with the exception of a report that glucosinolates in A. thaliana flower stalks are found in elongated cells just outside the vascular system and adjacent to the phloem (31). As mentioned above, the transcripts of glucosinolate biosynthetic genes have also been associated with vascular tissues (24, 25). Our measurements of high glucosinolate content in the midvein support the association of glucosinolate storage with the vascular system. However, the diffuse localization we observed throughout the leaf lamina suggests that much of the stored glucosinolate pool is not associated with vascular tissue.
An aspect of glucosinolate localization in plants that has been controversial in recent years is whether or not these compounds are found on the leaf surface (32, 33). The presence of glucosinolates on the surface would supply important cues for insect host choice. In our work, we found it necessary to spray many coats of matrix (15 coats) to achieve reproducible imaging, suggesting that glucosinolates in A. thaliana leaves are not very abundant on the surface. When standard glucosinolates were applied directly to the leaf surface, clear spectra were noted after only one to two coatings of matrix. Further information on the vertical distribution of glucosinolates in the leaf was not readily apparent from our results. In contrast to glucosinolates, much more is known about the cell- and organ-level localization of myrosinase. This glucosinolate-activating enzyme is known from all plant organs (4), and in A. thaliana is reported from the phloem parenchyma as well as guard cells (34, 35). Unlike glucosinolates, we found myrosinase activity to be uniformly distributed among the different sectors of the leaf examined.
In summary, MALDI-TOF imaging of A. thaliana leaves has revealed a distinctive pattern of glucosinolate distribution that has important implications for the feeding of a generalist lepidopteran larva. Further investigation of the distribution of glucosinolates and other defensive metabolites in plant tissue should help evaluate their defensive roles and reveal how plants have been selected to deploy defenses for maximum benefit.
Materials and Methods
Detailed descriptions of chemicals, plants, and insects used together with further details on MS imaging, HPLC analyses and myrosinase assays are given in SI Text.
Insect Feeding Experiments.
H. armigera (Lepidoptera: Noctuidae) were grown on an artificial pinto bean-based diet (36) and used at the second-instar stage. Larvae were not fed the previous 12 h before beginning experiments. For whole-leaf tests, single larvae were placed randomly on mature 4-week-old A. thaliana Columbia ecotype or tgg1/tgg2 rosette leaves that had been freshly removed from the plant and placed with the petiole in water. The location of feeding damage was recorded after 5 h. For leaf disk tests, 2-mm-diameter disks of A. thaliana Columbia ecotype or tgg1/tgg2 leaves were removed either from the outer lamina (2-mm-wide strip on periphery) or inner lamina (remaining leaf excluding midvein) by using a hole punch. Plastic Petri dishes were filled with agar (2% wt/vol, 5 mm height) and the disks suspended 2–3 mm above the solidified agar surface by using entomological pins. Four outer and four inner lamina disks from the same leaf were placed alternately along the edge of each Petri dish (Fig. 3a). A single larva was then placed with a random orientation in the center of each dish. After it had fed for 10 consecutive seconds on a single disk, the type of the disk was recorded. Results were analyzed by a binomial test.
Leaf Preparation for Imaging.
Solutions of 9-aminoacridine free base (37) were prepared at 15 mg/ml in HPLC-grade methanol. The leaves were mounted on a MALDI stainless steel LockMass target plate (LM; Waters) by using a double-sided adhesive tape with the abaxial surface of the leaf facing up. After mounting, leaves were spray-coated with 9-aminoacridine by using a commercial airbrush with a 0.2-mm-diameter sprayer jet. The target plate was kept at a 45° angle against a plastic support and the sprayer held at a distance of 13 cm from the plate. This insured that the cone of the spray reaching the target covered the entire leaf. Each coating involved 20–22 seconds of spraying, followed by 5 min of drying. This process was typically carried out 15 times to give maximal signal strength. Mass spectrometric imaging was primarily done with the abaxial side of the leaf up because this side offered a better surface for uniform matrix deposition owing to fewer trichomes; however, the same pattern of glucosinolate distribution was observed when imaging was carried out on the adaxial side.
Mass Spectrometry.
A MALDI micro MX mass spectrometer (Waters) fitted with a nitrogen laser (337-nm, 4-ns laser pulse duration, 10 Hz, and 154 μJ per pulse) was used in a reflectron mode and negative polarity for data acquisitions by using MassLynx version 4.0 software. The x, y coordinates for the imaging acquisition were defined by using proprietary software. The chemical identity of the compounds encountered was confirmed by mass spectrometry on an LTQ ion trap instrument (Thermo Fisher) with an AP-MALDI source equipped with a solid-state NdYAG UV laser (MassTech) and running Target 6 (MassTech) and Excalibur v.2.0 (Thermo Fisher) software for data acquisition.
Construction and Quantification of Mass Spectrometric Images.
Spectral data for the respective molecular ions of the three glucosinolates, 4-methylsulfinylbutylglucosinolate (m/z 436), indol-3-ylmethylglucosinolate (m/z 447), and 8-methylsulfinyloctylglucosinolate (m/z 492), were exported in the software ImageJ (http://rsb.info.nih.gov/ij/) for converting them into two-dimensional ion intensity maps. Ion maps were constructed by using a ± 0.25-Da mass window, because the roughness of the leaf surface could reduce mass accuracy. Each image was first desaturated by using ImageJ software and then divided into regions as in Fig. 6a. The outer lamina was defined as a 10-pixel (2 mm)-wide region around the periphery of the leaf, the midvein as a 3-pixel (0.6 mm)-wide region around the midvein and the remaining leaf was defined as the inner lamina for quantitation. The signal densities (D) of the respective leaf regions (AR in pixels) were integrated, normalized against the dry weight of each leaf (DW in g) by using total leaf area (AT in pixels), and plotted as normalized signal intensities (NSI) according to the following formula: NSI = D/(AR/AT × DW). Results from eight leaves were analyzed by conducting a one-way ANOVA, followed by a Tukey post hoc test using SPSS v15 software. For 4MSOB, the midvein showed a significant difference from the inner (P = 0.007) and outer (P = 0.014) lamina. For I3M, the outer lamina showed a significant difference from the inner lamina (P = 0.015) and midvein (P = 0.014). The 8MSOB showed a similar trend as 4MSOB, with the amount in midvein being significantly different from the inner lamina (P = 0.002) and the outer lamina (P = 0.010). The other comparisons among leaf regions for the three glucosinolates were not significant.
HPLC Analysis.
Leaves were detached from the plant, flash-frozen in liquid nitrogen, and lyophilized. The freeze-dried leaves were dissected into midvein, outer lamina, and inner lamina (Fig. 6a), and each part was weighed before being individually immersed in tubes set up in a 96-well-plate format filled with 1 ml of methanol containing 0.05 μmol of p-hydroxybenzylglucosinolate as an internal standard. Each tube contained three metallic spheres to grind up the leaf material when the plate was agitated at ≈1,000 rpm on a SO-10m paint shaker for 4 min. The ground suspensions were centrifuged at 4,200 × g for 10 min and the supernatants transferred into columns filled with 20 mg of DEAE-Sephadex equilibrated with 800 μl of water, followed by 500 μl of 80% methanol. After 600 μl of sample were loaded, each column was eluted with 500 μl of 80% methanol, 500 μl of water, and 500 μl of 0.02 M Mes buffer (pH 5.2). Next, 25 μl of sulfatase solution [prepared as described (38)] were added to each column and the sample incubated overnight. The resulting desulfoglucosinolates were eluted with 500 μl of water and concentrated for HPLC-DAD (HP-1100 series; Agilent Technologies) analysis as described (3).
Myrosinase Enzyme Assays.
Myrosinase activity was quantified by using a modification of a reported method (3).
Supplementary Material
Acknowledgments.
We thank M. Snell (Waters, Manchester, U.K.) for help with imaging using the MALDI Micro MX instrument; Z. Liu and A. Weber for maintaining the H. armigera and A. thaliana cultures, respectively; the International Max Planck Research School (The Exploration of Ecological Interactions with Molecular and Chemical Techniques) for stipends (to R.S. and F.V.); the program Alβan of the European Union for a stipend (to F.V.); and the Max Planck Society for financial support. We are also grateful to J. Doubský for purification of the free 9-aminoacridine base from the hydrochloride salt and M. Reichelt for advice on glucosinolate HPLC analyses.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711730105/DCSupplemental.
References
- 1.Gershenzon J, Dudareva N. The function of terpene natural products in the natural world. Nat Chem Biol. 2007;3:408–414. doi: 10.1038/nchembio.2007.5. [DOI] [PubMed] [Google Scholar]
- 2.Rosenthal GA, Berenbaum MR, editors. Herbivores: Their Interactions with Secondary Plant Metabolites. 2nd Ed. Vol 1. San Diego: Academic; 1992. [Google Scholar]
- 3.Burow M, Müller R, Gershenzon J, Wittstock U. Altered glucosinolate hydrolysis in genetically engineered Arabidopsis thaliana and its influence on the larval development of Spodoptera littoralis. J Chem Ecol. 2006;32:2333–2349. doi: 10.1007/s10886-006-9149-1. [DOI] [PubMed] [Google Scholar]
- 4.Halkier BA, Gershenzon J. Biology and biochemistry of glucosinolates. Ann Rev Plant Biol. 2006;57:303–333. doi: 10.1146/annurev.arplant.57.032905.105228. [DOI] [PubMed] [Google Scholar]
- 5.Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 6.Reichelt M, et al. Benzoic acid glucosinolate esters and other glucosinolates from Arabidopsis thaliana. Phytochemistry. 2002;59:663–671. doi: 10.1016/s0031-9422(02)00014-6. [DOI] [PubMed] [Google Scholar]
- 7.Bones AM, Rossiter JT. The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry. 2006;67:1053–1067. doi: 10.1016/j.phytochem.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 8.Rask L, et al. Myrosinase: Gene family coevolution and herbivore defense in Brassicaceae. Plant Mol Biol. 2000;42:93–113. [PubMed] [Google Scholar]
- 9.Eisner T, Eisner M, Meinwald J. Technique for visualization of epidermal glandular structures in plants. J Chem Ecol. 1987;13:943–946. doi: 10.1007/BF01020173. [DOI] [PubMed] [Google Scholar]
- 10.Peumans WJ, Hause B, Van Damme EJM. The galactose-binding and mannose-binding jacalin-related lectins are located in different sub-cellular compartments. FEBS Lett. 2000;477:186–192. doi: 10.1016/s0014-5793(00)01801-9. [DOI] [PubMed] [Google Scholar]
- 11.Osbourn AE, Clarke BR, Lunness P, Scott PR, Daniels MJ. An oat species lacking avenacin is susceptible to infection by Gaeumannomyces graminis var. tritici. Physiol Mol Plant Pathol. 1994;45:457–467. [Google Scholar]
- 12.Cooks RG, Ouyang Z, Takats Z, Wiseman JM. Ambient mass spectrometry. Science. 2006;311:1566–1570. doi: 10.1126/science.1119426. [DOI] [PubMed] [Google Scholar]
- 13.Ratcliffe RG, Shachar-Hill Y. Probing plant metabolism with NMR. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:499–526. doi: 10.1146/annurev.arplant.52.1.499. [DOI] [PubMed] [Google Scholar]
- 14.Caprioli RM, Farmer TB, Gile J. Molecular imaging of biological samples: Localization of peptides and proteins using MALDI-TOF MS. Anal Chem. 1997;23:4751–4760. doi: 10.1021/ac970888i. [DOI] [PubMed] [Google Scholar]
- 15.Rubakhin S, Jurchen J, Monroe E, Sweedler J. Imaging mass spectrometry: Fundamentals and applications to drug discovery. Drug Discovery Today. 2005;10:823–837. doi: 10.1016/S1359-6446(05)03458-6. [DOI] [PubMed] [Google Scholar]
- 16.Reyzer ML, Caprioli RM. MALDI-MS-based imaging of small molecules and proteins in tissues. Curr Opin Chem Biol. 2007;11:29–35. doi: 10.1016/j.cbpa.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 17.Mullen AK, Clench MR, Crosland S, Sharples KR. Determination of agrochemical compounds in soya plants by imaging matrix-assisted laser desorption/ionisation mass spectrometry Rapid Commun Mass Spectrom. 2005;19:2507–2516. doi: 10.1002/rcm.2078. [DOI] [PubMed] [Google Scholar]
- 18.Kondo T, et al. A plant peptide encoded by CLV3 identified by in situ MALDI-TOF MS analysis. Science. 2006;313:845–848. doi: 10.1126/science.1128439. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Shrestha B, Vertes A. Atmospheric pressure molecular imaging by infrared MALDI mass spectrometry. Anal Chem. 2007;79:523–532. doi: 10.1021/ac061577n. [DOI] [PubMed] [Google Scholar]
- 20.Robinson S, Warburton K, Seymour M, Clench M, Thomas-Oates J. Localization of water-soluble carbohydrates in wheat stems using imaging matrix-assisted laser desorption ionization mass spectrometry. New Phytol. 2007;173:438–444. doi: 10.1111/j.1469-8137.2006.01934.x. [DOI] [PubMed] [Google Scholar]
- 21.Schiers J, de Bruyn L, Verhagen R. Nutritional benefits of the leaf-mining behavior of two grass miners: A test of the selective feeding hypothesis. Ecol Entomol. 2001;26:509–516. [Google Scholar]
- 22.Barth C, Jander G. Arabidopsis myrosinases TGG1 and TGG2 have redundant function in glucosinolate breakdown and insect defense. Plant J. 2006;46:549–562. doi: 10.1111/j.1365-313X.2006.02716.x. [DOI] [PubMed] [Google Scholar]
- 23.Brown PD, Tokuhisa JG, Reichelt M, Gershenzon J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry. 2003;62:471–481. doi: 10.1016/s0031-9422(02)00549-6. [DOI] [PubMed] [Google Scholar]
- 24.Reintanz B, et al. bus, a bushy Arabidopsis CYP79F1 knockout mutant with abolished synthesis of short-chain aliphatic glucosinolates. Plant Cell. 2001;13:351–367. doi: 10.1105/tpc.13.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuster J, Knill T, Reichelt M, Gershenzon J, Binder S. BRANCHED-CHAIN AMINOTRANSFERASE4 is part of the chain elongation pathway in the biosynthesis of methionine-derived glucosinolates in Arabidopsis. Plant Cell. 2006;18:2664–2679. doi: 10.1105/tpc.105.039339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton JG, Zangerl AR, DeLucia EH, Berenbaum MR. The carbon-nutrient balance hypothesis: its rise and fall. Ecol Lett. 2001;4:86–95. [Google Scholar]
- 27.Kojima M, Poulton JE, Thayer SS, Conn EE. Tissue distributions of dhurrin and of enzymes involved in its metabolism in leaves of Sorghum bicolor. Plant Physiol. 1979;63:1022–1028. doi: 10.1104/pp.63.6.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siebert DJ. Localization of salvinorin A and related compounds in glandular trichomes of the psychoactive sage. Salvia divinorum. Ann Bot. 2004;93:763–771. doi: 10.1093/aob/mch089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dell B, McComb AJ. Glandular hairs, resin production, and habitat of Newcastelia viscida E. Pritzel (Dicrastylidaceae) Aust J Bot. 1975;23:373–390. [Google Scholar]
- 30.Shelton AL. Within-plant variation in glucosinolate concentrations of Raphanus sativus across multiple scales. J Chem Ecol. 2005;31:1711–1732. doi: 10.1007/s10886-005-5922-9. [DOI] [PubMed] [Google Scholar]
- 31.Koroleva OA, et al. Identification of a new glucosinolate-rich cell type in Arabidopsis flower stalk. Plant Physiol. 2000;124:599–608. doi: 10.1104/pp.124.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griffiths DW, et al. Identification of glucosinolates on the leaf surface of plants from the Cruciferae and other closely related species. Phytochemistry. 2001;57:693–700. doi: 10.1016/s0031-9422(01)00138-8. [DOI] [PubMed] [Google Scholar]
- 33.Reifenrath K, Riederer M, Müller C. Leaf surface wax layers of Brassicaceae lack feeding stimulants for Phaedon cochleariae. Entomol Exp Appl. 2005;115:41–50. [Google Scholar]
- 34.Andreasson E, Jorgensen LB, Hoglund AS, Rask L, Meijer J. Different myrosinase and idioblast distribution in Arabidopsis and Brassica napus. Plant Physiol. 2001;127:1750–1763. doi: 10.1104/pp.010334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thangstad OP, et al. Cell specific, cross-species expression of myrosinases in Brassica napus, Arabidopsis thaliana and Nicotiana tabacum. Plant Mol Biol. 2004;54:597–611. doi: 10.1023/B:PLAN.0000038272.99590.10. [DOI] [PubMed] [Google Scholar]
- 36.Joyner K, Gould F. Developmental consequences of cannibalism in Heliothis zea (Lepidoptera, Noctuidea) Ann Entomol Soc Am. 1985;78:24–28. [Google Scholar]
- 37.Albert A, Ritchie B. 9-Aminoacridine. Org Synth Coll. 1955;3:53. [Google Scholar]
- 38.Graser G, Oldham NJ, Brown PD, Temp U, Gershenzon J. The biosynthesis of benzoic acid glucosinolate esters in Arabidopsis thaliana. Phytochemistry. 2001;57:23–32. doi: 10.1016/s0031-9422(00)00501-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.