Abstract
The clinical success of stem cell therapy for myocardial repair hinges on a better understanding of cardiac fate mechanisms. We have identified small molecules involved in cardiac fate by screening a chemical library for activators of the signature gene Nkx2.5, using a luciferase knockin bacterial artificial chromosome (BAC) in mouse P19CL6 pluripotent stem cells. We describe a family of sulfonyl-hydrazone (Shz) small molecules that can trigger cardiac mRNA and protein expression in a variety of embryonic and adult stem/progenitor cells, including human mobilized peripheral blood mononuclear cells (M-PBMCs). Small-molecule-enhanced M-PBMCs engrafted into the rat heart in proximity to an experimental injury improved cardiac function better than control cells. Recovery of cardiac function correlated with persistence of viable human cells, expressing human-specific cardiac mRNAs and proteins. Shz small molecules are promising starting points for drugs to promote myocardial repair/regeneration by activating cardiac differentiation in M-PBMCs.
Keywords: cardiogenesis, chemical biology, high-throughput screen, myocardial repair
Stem cell therapy and regenerative medicine are promising new frontiers in the treatment of myocardial infarction (MI) and heart failure. Endogenous stem cell repair mechanisms are underpowered for tissue repair in ischemic and nonischemic cardiomyopathies. Thus, there is intense interest in developing therapeutic strategies to enhance the endogenous regenerative potential of adult human myocardium or supply stem/progenitor cells to the injured heart exogenously. Predelivery enhancement of stem/progenitor cell function by promoting cell growth, differentiation, survival, and/or homing with small-molecule drugs or growth factors is an exciting strategy that has been successful in clinical trials of peripheral vascular disease (1, 2). Cytokine-mobilized PBMCs are a universally accessible source of autologous human stem/progenitor cells (3). Despite the uncertainty regarding the cardiogenic plasticity of bone marrow-derived cells, clinical trials have moved forward at a rapid pace. Results from more than a dozen worldwide trials using bone marrow-derived cells in MI and heart failure have demonstrated feasibility, safety, and measurable, albeit modest, clinical benefits, generating considerable optimism for the future of this therapy (4–6). Yet, many basic scientific questions remain unanswered. To fulfill the clinical promise of stem/progenitor cells in cardiovascular repair, it is essential to clarify the roles of cardiomyogenesis, neovascularization, cell fusion (7) and paracrine growth factor secretion (8), all potential contributors to recovery of cardiac function (9).
An enhanced understanding of cell-fate mechanisms should translate into greater clinical success of cardiovascular cell therapy (5, 6). Recent studies indicate that cardiac muscle, vascular smooth muscle, and endothelial cells share a common multipotent progenitor heritage, the cardiovascular master stem cell (10, 11). However, despite decades of intensive investigation using traditional molecular, cellular, and genetic experimental approaches, in vertebrate, invertebrate, and stem-cell models (11), much remains to be learned about what drives cardiovascular fate specification.
Chemical genetics offers a new approach for investigating cardiovascular fate in stem cells (12). High-throughput technology allows rapid screening of hundreds of thousands of synthetic organic chemicals. Bioactive small molecules, selected for highly-specific functions and targeted interactions with proteins, provide versatile experimental probes to explore complex signaling networks and interrogate mechanistic hypotheses. Importantly, they may also serve as platforms for new drugs.
We designed a small-molecule library screen for chemical activators of Nkx2.5, one of the earliest lineage-restricted genes to be expressed in cardiovascular progenitor cells (11). Here, we describe a class of small molecules from this screen called sulfonyl-hydrazones (Shz) that potently induce Nkx2.5 and a subset of other cardiac markers, including myocardin, troponin-I, and sarcomeric α-tropomyosin (SαTM) in a variety of different stem/progenitor cells. Moreover, Shz-pretreated human M-PBMCs displayed cardioregenerative activity as xenografts in injured immunocompromised rat heart. Shz small molecules represent starting points for new drugs to produce cardiomyocytes from human hematopoietic stem/progenitor cells for regenerative repair of injured myocardium.
Results
Cardiogenic Small-Molecule Screen.
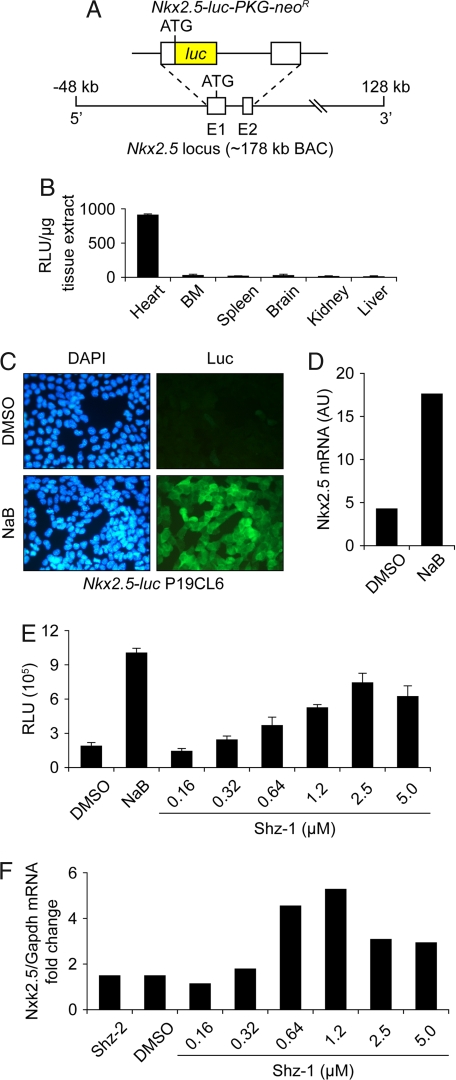
We screened the ≈147,000 compound University of Texas Southwestern chemical library for activators of firefly luciferase (luc), inserted by Escherichia coli recombineering into the Nkx2.5 locus on a 180-kb mouse BAC (Fig. 1A), stably integrated in P19 embryonal carcinoma cells, subclone CL6 (P19CL6) (13). We confirmed that recombinant Nkx2.5-luc BAC function faithfully mirrored the native gene by generating transgenic mice (transgenic Nkx2.5 reporter, TNkxR). We observed luc activity exclusively in adult myocardium of TNkxR mice, using bioluminescence and biochemical assays [Fig. 1B; and see supporting information (SI) Fig. S1A]. Sodium butyrate (NaB) strongly and reliably activated the Nkx2.5-luc transgene (Fig. 1C and Fig. S1B, green line) and native Nkx2.5 (Fig. 1D) and produced spontaneously beating cardiomyocytes in P19CL6 cell cultures (data not shown); we thus concluded that NaB would be a suitable positive control cardiogenic small molecule for our high-throughput screen (HTS). Importantly, dimethyl sulfoxide (DMSO), our chemical library's solvent, at 1.0% concentration did not induce the Nkx2.5-luc BAC reporter in P19CL6 cells above background (Fig. S1B, yellow line). The ≈3,000 strongest positive hits from the primary screen were selected and rescreened by using a dose–response strategy, and more than half of these candidates (≈1,600) remained firmly positive. Using chemoinformatics, we clustered these hits into a top-10 list of families with chemically distinct, synthetically tractable core structural motifs (Scheme S1). Our screen was robust, identifying a diverse group of chemicals, ranging from thiazolidinediones to flavonoids, that regulate Nkx2.5 and/or cardiac fate in P19CL6 cells.
Fig. 1.
Nkx2.5 cardiogenic small-molecule HTS in P19CL6 cells. (A) Schematic of reporter transgene with luciferase inserted by homologous recombination into the Nkx2.5 locus on an ≈178-kb mouse BAC. (B) In vitro luciferase assay of Nkx2.5-luc BAC transgenic mouse confirming cardiac tissue-restricted expression. (C) Nkx2.5-luc BAC transgenic P19CL6 cell line, clone #5-1, with specific induction of luciferase protein (detected by immunostaining) after exposure to 2.5 mM NaB for 3 days (Lower Right), compared with DMSO vehicle control, which showed a lack of luciferase staining (Upper Right). (D) NaB-mediated induction of Nkx2.5 mRNA by real-time RT-PCR in parental P19CL6 cells. (E) Dose-responsive activation of Nkx2.5-luc BAC by Shz-1 in clone #5-1 P19CL6 reporter cells at 48 h after drug exposure. Each data point represents the average + SD of 12 wells from a 96-well plate. (F) Dose-responsive activation of Nkx2.5 mRNA by Shz-1 in P19CL6 cells at 48 h after drug exposure, measured by real-time RT-PCR and normalized to gapdh mRNA levels.
Validation of Sulfonyl-Hydrazone Hits.
As a starting point, we chose to explore in greater detail the Shz small molecules, one of the most promising lead families. We independently identified four structurally distinct biologically active Shz small molecules and, equally important, many biologically inactive variants, a few of which are shown (Fig. S2). To resynthesize library compounds or create Shz variants for structure–activity relationship studies, substituted benzene-sulfonyl hydrazides were condensed with an aromatic aldehyde and isolated as pure crystalline compounds whose structures were verified by using NMR and mass spectroscopy (Fig. S2). The Shz molecules used in this study are shown in Scheme S2.
We confirmed dose-dependent activation of the Nkx2.5-luc BAC transgene in P19CL6 cells (Fig. 1E), with peak activity for Shz-1 in this assay at 2.5 μM. We also confirmed by real-time RT-PCR, with normalization against gapdh mRNA levels, that Shz-1 specifically activated Nkx2.5 in parental P19CL6 cells in a dose-responsive manner (Fig. 1F). In fact, the endogenous Nkx2.5 gene in P19CL6 cells was even more responsive to Shz-1 than the BAC transgene, peaking at 1.2 μM drug concentration (Fig. 1F). The parallel response of the Nkx2.5 BAC and native Nkx2.5 to Shz-1, like the cardiac-specific luc activity in TNkxR mice, provides reassurance that our reporter system is a reliable biosensor of Nkx2.5 gene activation and cardiac fate in P19CL6 cells.
Shz small molecules activated only a limited set of cardiac reporter genes in a subset of cell types. For example, in transient-transfection experiments, Shz small molecules failed to induce the generic CMV promoter/enhancer in P19CL6 cells (data not shown). On the other hand, Shz small molecules activated an Nkx2.5-luc reporter gene (containing ≈3 kb of 5′ regulatory sequence) in P19CL6 cells but not in Cos cells (data not shown). Using in vitro biochemical assays, we confirmed that Shz small molecules are not histone deacetylase (HDAC) inhibitors (data not shown). Finally, to test lineage specificity, we confirmed that Shz small molecules could not activate the neuronal gene program in stem cells, excluding at least one alternative cell fate (data not shown).
Activation of myocardin Gene by Shz Small Molecules.
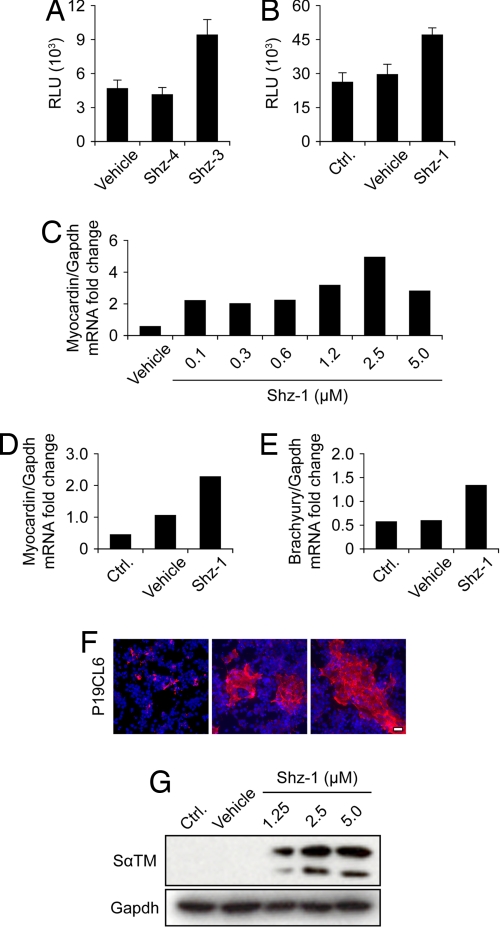
Myocardin is a serum response-factor coactivator that stimulates the transcription of multiple muscle-specific genes during cardiac and smooth muscle development and, therefore, also serves as a very early marker of cardiac progenitor cells (14). We established a second set of stem-cell reporter systems, including a myocardin-luc knockin BAC (generated by E. coli recombineering) integrated as a transgene into the genome of P19CL6 cells (Fig. S3). In addition, we introduced luc by homologous gene recombination directly into the myocardin locus of SM1 mouse ES cells. For the myocardin-luc P19CL6 cells, we tested Shz-3 and Shz-4 (Scheme S2). Whereas Shz-3 strongly activated the myocardin-luc transgene in P19CL6 cells, Shz-4 was inactive (Fig. 2A). This result, in addition to more extensive structure–activity-relationship data, confirmed that small changes in Shz structure could profoundly impact biological activity. Shz-1 specifically activated the myocardin-luc knockin gene in SM1 ES cells (Fig. 2B). Finally, we confirmed that Shz small molecules activated the native myocardin gene in parental P19CL6 cells (Fig. 2C) and SM1 ES cells (Fig. 2D), using real-time RT-PCR with normalization versus gapdh mRNA levels. Thus, like Nkx2.5, the myocardin gene is a sensitive biosensor of chemical cardiogenic signaling.
Fig. 2.
Shz activation of myocardin reporter genes in P19CL6 and mouse ES cells. (A) Activation of myocardin-luc BAC by Shz-3 but not Shz-4 at 2.5 μM in transgenic P19CL6 reporter cells at 48 h after drug exposure. Each data point represents the average + SD of 12 wells from a 96-well plate. (B) Activation of endogenous myocardin tagged by homologous recombination with luciferase in SM1 mouse ES cell genome by Shz-1. Luciferase activity was measured 48 h after removal of LIF and exposure to Shz-1 at 2.5 μM. Each data point represents the average + SD of 12 wells from a 96-well plate. (C) Dose-responsive activation of myocardin mRNA by Shz-1 in P19CL6 cells at 48 h after drug exposure, measured by real-time RT-PCR and normalized to gapdh mRNA levels. (D) Activation of endogenous myocardin mRNA in SM1 ES cells by Shz-1 at 2.5 μM at 48 h after drug exposure and removal of LIF, measured by real time RT-PCR and normalized to GAPDH mRNA levels. (E) Activation of endogenous brachyury-T mRNA in SM1 ES cells by Shz-1 at 2.5 μM at 48 h after drug exposure and removal of LIF, measured by real-time RT-PCR and normalized to gapdh mRNA levels. (F) Expression of sarcomeric α-tropomyosin detected by CH1 mAb in vehicle (Left) or Shz-1 treated (Center and Right) P19CL6 cells at 2.5 μM after 72 h of drug exposure. (Scale bar, 30 μm.) (G) Dose-responsive activation of SαTM in P19CL6 cells by protein blot with CH1 mAb; GAPDH protein levels are shown to document equal loading.
We next tested whether Shz small molecules activated the mesodermal marker, brachyury-T, upstream of Nkx2.5 and myocardin in the normal developmental cascade. Shz small molecules induced expression of brachyury-T mRNA as detected by real-time RT-PCR normalized for gapdh in SM1 mouse ES cells (Fig. 2E). Taken together, these reporter gene and mRNA data establish that Shz small molecules specifically activate brachyury-T and two key early cardiogenic program genes, Nkx2.5 and myocardin, in pluripotent mouse stem cells.
Activation of Sarcomeric α-Tropomyosin Expression by Shz Small Molecules.
We observed that SαTM, a highly-specific protein marker of striated muscle cells and a very early marker of stem-cell cardiogenesis (15), was strongly induced by Shz small molecules in P19CL6 cells. Staining with the CH1 SαTM-specific mAb identified large clusters of cardiac progenitor cells, found exclusively in Shz small-molecule-treated cultures (Fig. 2F). The absence of multinucleated myotubes or MyoD immunostaining, and differentiation in high-serum media, argued against these Shz small-molecule-induced SαTM-positive clusters being skeletal muscle derivatives, a formal possibility with the CH1 mAb (data not shown). We confirmed induction of SαTM in P19CL6 cells by protein blotting (Fig. 2G and Fig. S4). Again, like the stem cell reporter and mRNA assays, Shz-1 activity was already maximal at 2.5 μM. We also observed a rapid and robust induction of SαTM in H9c2 cells, a continuous cell line derived from the embryonic rat ventricular myocardium with selected features of a cardiac progenitor cell (Fig. S5). Indeed, in response to Shz small molecules, SαTM-positive H9c2 cells acquired a cellular morphology strikingly reminiscent of binucleated cardiomyocytes from the newborn rat heart (Fig. S5A). Shz-specific induction of SαTM in H9c2 cells was also confirmed by protein blotting (Fig. S5B).
Collectively, these gene expression studies establish that Shz small molecules can potently activate an important subset of early cardiac genes and phenotypic differentiation in pluripotent stem cells, providing at least an initial chemical trigger for activation of the cardiac genome and cell fate.
Chemical Phenotype of Sulfonyl-Hydrazone-Induced Cardiogenic Progenitors.
Histone modification is one of the earliest hallmarks of stem cell differentiation, and drugs like NaB that target histone-modifying enzymes can trigger stem cell differentiation. NaB induced rapid global modification of histone H3 and H4 but did not induce SαTM in P19CL6 cells (Fig. 3A). In contrast, Shz small molecules induced Sα-TM but did not induce global histone modification (Fig. 3A). We also interrogated the effects of Shz drug exposure on a number of pathways by phosphoprotein blotting. Shz small molecules triggered ERK1/2 pathway (e.g., Elk, Mek, and Raf1) activation in P19CL6 (Fig. S6A) and H9c2 cells (data not shown), suggesting a possible role for MAPK signal transduction in the chemical pathway leading to the genome. The MEK inhibitor, U0126, effectively blocked ERK1/2 phosphorylation in P19CL6 cells (Fig. S6B) but had no effect on the dose-responsive Shz-mediated Nkx2.5-luc BAC reporter activation in P19CL6 (Fig. S6C). Likewise, U0126 failed to attenuate Shz-induced SαTM expression in P19CL6 cells (data not shown). We tested a panel of traditional signal transduction pathway inhibitors and failed to find a blocker of Shz-mediated reporter activation or SαTM induction (16). Thus, Shz small molecules may use an undiscovered pathway to regulate cardiogenic differentiation in stem cells.
Fig. 3.
Comparison of Shz and sodium butyrate and interrogation of known cardiogenic signaling pathways. (A) NaBut induces trimethylation of K27 of histone protein H3 and acetylation of total histone protein H4 but does not induce Sα-TM expression; the converse is true for Shz-1 in P19CL6 cells. (B) Like Shz-1, BMP-2 (50 ng/ml) activated the Nkx2.5-luc BAC in P19CL6 cells. However, the BMP-antagonist Noggin (500 ng/ml) did not significantly block Shz-1-mediated signaling at 48 h. Each data point is the average + SD of 12 wells from a 96-well plate. (C) Representative cell staining demonstrating induction of SαTM by Shz-1 (2.5 μM) (Left), GATA-4 by BMP-2 (50 ng/ml) (Center), and Oct3/4 by FGF-2 (20 ng/ml) (Right) after exposure to agent for 48 h.
We also tested whether activation of Nkx2.5 or other cardiac genes by Shz small molecules involved BMP, FGF, and Wnt pathways, key cardiogenic signaling circuits. The highlights of these functional and biochemical experiments are summarized in (Fig. 3 and Fig. S4). BMP-2 induced Smad-1 phosphorylation (Fig. S4) and activated the Nkx2.5-luc BAC in our reporter stem cells (Fig. 3B), and this activation was attenuated by Noggin, the BMP antagonist. Shz-1, on the other hand, activated Nkx2.5-luc independent of Smad-1 phosphorylation (Fig. S4) and was only weakly blocked by Noggin (Fig. 3B). BMP-2 also induced GATA-4 expression in these cells, but Shz-1 did not (Fig. 3C). At 1 hour, FGF-2 was a much stronger activator than Shz-1 of ERK1/2 phosphorylation in P19CL6 cells (Fig. 3C). FGF-2 also induced Oct3/4 expression in these cells, detected by both immunostaining and protein blot (Fig. 3C and Fig. S4). In contrast to BMP-2 and FGF-2, Shz-1 alone induced Sα-TM, an early phenotypic marker of cardiomyogenesis. Finally, to interrogate whether Shz-1 activated cardiac genes by modulating Wnt signaling, we compared the expression level of total cellular β-catenin in drug-treated versus control P19CL6 cells and found no difference at 1 h (data not shown) or 24 h (Fig. S4). Second, we studied whether Shz-1 could modulate the superTOP-flash (STF) reporter gene system in a stable reporter cell line that directly measures the activity of canonical Wnt pathway. Shz small molecules had no effect at any dose on β-catenin accumulation or on activity of the STF reporter gene in this cell system. Taken together, these results demonstrate that the mechanism of Shz-mediated cardiac fate signaling is distinct from BMP-2, FGF-2, and Wnts and may have a nonredundant, even complementary, functional role in cardiogenesis. By excluding BMP-2, FGF-2, and Wnts, our candidate-based approach to mechanism and target discovery has allowed us to focus on other pathways.
Shz Small-Molecule Enhancement of Human M-PBMCs for Myocardial Repair.
Toward clinical and therapeutic targets, we set out to extend our findings to adult stem/progenitor cells and chose granule colony-stimulating factor (GCSF)-mobilized peripheral blood mononuclear cells (M-PBMCs) as our first test system. We studied the effects of Shz on the growth, differentiation, and survival of human M-PBMCs in a standard culture model and as a cellular inoculum in the cryoinjured rat heart. Using apheresis, we collected GCSF-mobilized PBMCs from anonymous normal donors (“leftover” cells from bone marrow transplants) and cultured unfractionated PBMCs in serum-free chemically defined X-vivo 20, a cell culture media optimized to support growth/survival of human stem/progenitor cells. We tested cells from several donors, independently, with only minor variability in cell yield, composition [≈30–50% monocytes, 1–2% CD34(+), 1–2% side population (by Hoechst dye exclusion), and >95% CD45(+)], and viability. The results of several experiments yielded similar findings. Within the time course of this experiment (3 days drug plus 7 days drug-free media) we observed little, if any, cell proliferation or death in M-PBMC cultures, yet RT-PCR and preliminary gene chip data (not shown) suggest that Shz small molecules induced significant changes in cellular phenotype.
PBMCs from the circulation attach poorly, if at all, to tissue-culture dishes. Yet, when M-PBMCs were treated for the first 3 days after harvest with escalating concentrations of Shz-1, we observed a dose-dependent increase in the number of cells that stably attached to the plate (Fig. 4A and Fig. S7). In contrast, upon gentle washing, few cells attached in vehicle control experiments, even starting from high-density cultures (Fig. S7). We counted the number of attached cells in at least 10 representative low-power fields (lpf) and then averaged these numbers (Fig. 4B). Initially, we observed little proliferation of these cells; a 24-hr pulse of bromodeoxyuridine on day 9 to mark DNA synthesis demonstrated only rare BrdU-positive cells (data not shown). On the other hand, after several weeks in culture in X-vivo 20 without additional additives, we observed limited proliferation of Shz-1-treated human M-PBMCs with the formation of very small cell colonies (data not shown). Therefore, Shz-1 primarily enhanced the attachment and survival rather than the proliferation of a preexisting cell population in cultured human M-PBMCs. Preliminary characterization by cell staining and RNA expression array indicates that the cells induced to attach by Shz small molecules express monocyte/macrophage lineage markers (data not shown). However, all subsequent experiments included the nonadherent cell subpopulation, which is substantially more complex.
Fig. 4.
Small-molecule treatment of human M-PBMCs in vitro. (A) Schematic of human M-PBMCs treatment protocol, 3 days with drug (10 μM) or vehicle control and then 7 days in drug-free media with media changes every 2–3 days. (B) Dose-responsive increase in M-PBMCs attachment/survival with increasing concentration of Shz-1 for the first 3 days or vehicle control; average cell counts on day 10 of at least 10 representative low-power fields (lpf) + SD (C) Activation of cardiac differentiation by Shz small molecules in human M-PBMCs in vitro. M-PBMCs were treated with vehicle or Shz-3 for 3 days, followed by 7 days in drug-free media; the cells were harvested and analyzed by RT-PCR for Nkx2.5, ANP, and gapdh transcripts; adult human heart mRNA was used a positive control. (D) M-PBMCs from a different donor were harvested before treatment (day 0) or treated with Shz-1 for 3 days, followed by 7 days in drug-free media, and then harvested and analyzed with heart muscle control by RT-PCR for cTnI and gapdh transcripts.
Shz-1-treated human M-PBMCs specifically expressed several cardiac marker mRNAs and proteins, demonstrated by RT-PCR and immunostaining, with cells from several donors, using several different Shz small molecules (Figs. 4 C and D and 5C Inset). We observed specific induction of Nkx2.5 and ANP transcripts in human M-PBMCs treated for 3 days with Shz-3 then 7 days in drug-free X-vivo 20 media (Fig. 4C) as well as induction of cTnI transcripts in human M-PBMCs treated, by using the same 3 + 7 day protocol, with Shz-1 (Fig. 4D). We detected cTnI protein after Shz-1 induction in these cells by mAb staining (Fig. 5C Inset), confirming expression of cardiac protein markers.
Fig. 5.
Functional rescue of cryoinjured rat heart by Shz-treated human M-PBMC xenografts. (A) Viable human M-PBMCs pretreated for 3 days with compound Shz-3 (at 5 μM) or vehicle control were injected by needle into the healthy myocardial perimeter of liquid nitrogen probe-mediated transmural burn injury, followed by 7 days in drug-free media. (B) Serial echocardiography was done at baseline (preinjury) and on days 3, 7, 14, and 21 after injury/xenografting, and the fractional shortening was calculated for each animal (n = 4, each group) and was compared with hearts that had received mock injection with media alone (no cells). At days 7, 14, and 21, the difference between Shz-3 small-molecule and vehicle-treated human M-PBMC xenografts was statistically significant, P = 0.00183, P = 0.00023, and P = 0.000238, respectively. (C) IHC of chimeric juxta-burn myocardial tissue from rat injected with in vitro DAPI-stained vehicle (Upper) or Shz-3-treated (Lower) human M-PBMCs by using a mAb that detects α-actinin exclusively in host rat myocardium (human cells evident by DAPI stained nuclei are negative for α-actinin) (Left) versus a human-specific cTnI mAb that exclusively detects viable human drug-induced (cardiac gene-expressing) cells in the needle-track (Lower Right). (Scale bar, 25 μm.) (Inset) Human M-PBMCs treated with drug and immunostained in vitro appear morphologically very similar to their in vivo counterparts. (D) RT-PCR of RNA from chimeric juxta-burn tissue of hearts injected with Shz-3 or vehicle-treated cells under high-stringency conditions that favor amplification of human versus rat Nkx2.5 or cTnI sequences. The control for RNA loading is 18S ribosomal RNA. Human and rat hearts are used as positive and negative controls, respectively.
Given the effects of Shz small molecules on human M-PBMCs in vitro, we tested whether drug-enhanced cells would make suitable xenografts in a rat heart cryoinjury model (Fig. 5A). Unfractionated PBMCs harvested from healthy donors after GCSF-mobilization were treated in vitro with Shz drug for 3 days. The drug was then removed and the cells cultured for an additional 7 days in drug-free media (as in Fig. 4). For the final 12 h, cells to be used for xenograft injection were labeled with a DAPI nuclear stain and then harvested and assayed by trypan blue to assure high viability (>90%). DAPI-labeled human cells pretreated with Shz-3 or vehicle control were then engrafted at three sites, ≈30,000 cells each site, by needle injection into healthy myocardium adjacent to cryoinjury on the anterior left ventricular (LV) wall in athymic nude rats (four rats in each group). An additional control group received injection of media without cells as a sham operative control. All rats survived the burn protocol and cell engraftment. We interrogated cardiac function by serial echocardiography on days 3, 7, 14, and 21 after cryoinjury and cell engraftment and compared these results to baseline echocardiograms (Fig. 5A). At the time of study and data analysis, the echocardiographer was blinded to the treatment group. At 3 days, after successful recovery from surgery, the standardized cryoinjury had caused a ≈15–20% decrease in overall LV fractional shortening, highly consistent from animal to animal. By 21 days, rats that had received myocardial injection of media alone showed no appreciable change in their LV function (Fig. 5B and Fig. S8A). Rats that had received vehicle-treated cells showed only modest improvement in LV function (Fig. 5B). In contrast, rats that had received Shz-3-enhanced cells demonstrated a significant improvement in LV function at 7 days and returned to normal preinjury contractile function levels by day 21 (Fig. 5B). The difference in contractile function between rats that had received Shz-3- or vehicle-treated cells was significant by day 7 after injury (P = 0.00183) and remained significant on day 14 (P = 0.00023) and day 21 (P = 0.00024) (Fig. 5B). Thus, Shz drug-enhanced cells conferred a significant improvement in cardiac function after cryoinjury but control/vehicle-treated cells did not.
To show that the improvement in LV function after engraftment of drug-treated human cells was associated with survival of human xenograft cells in the rat myocardium, we double-immunostained chimeric myocardium with a human-specific cTnI mAb and a panspecies α-actinin mAb to identify host cardiomyocytes (Fig. 5C). Despite exhaustive exploration of microscope slides from control hearts, we failed to identify any surviving xenografts of vehicle-treated human cells by DAPI signal at 1 month, and a representative negative-control image is shown (Fig. 5C Upper). On the other hand, colonies of Shz-3-treated human cells, whose nuclei were clearly marked by DAPI staining, could be definitively identified by using a human-specific cTnI mAb in the needle track and invading adjacent myocardium (Fig. 5C Lower). Importantly, the mutually exclusive species-specific staining pattern obtained with these two muscle mAbs (cTnI → human and α-actinin → rat) strongly argues against fusion between human cells and host rat myocardium as a predominant mechanism, if it occurs at all in this system (Fig. 5C).
To provide additional molecular evidence that Shz small-molecule-treated human cells were still viable and expressing human cardiac genes in chimeric myocardium, we assayed for rare human-specific transcripts (of highly conserved cardiac mRNAs) amidst abundant rat cardiomyocyte transcripts (Fig. 5D and Fig. S8B). First, we did high-stringency RT-PCR using conditions optimized to preferentially amplify the human versus rat Nkx2.5 or cTnI cDNAs (Fig. 5D). For both Nkx2.5 and cTnI, a PCR band was more abundant in chimeric myocardium from rats that had been injected with Shz-3-treated cells than their counterparts that had been injected with cells treated with vehicle alone (Fig. 5D). Thus, although we could not identify vehicle-treated cell islands by microscopic analysis (Fig. 5C Upper), a small number of these cells must have survived as grafts in the injured rat heart, detectable only by ultrasensitive RT-PCR (Fig. 5D). We further cloned and sequenced RT-PCR products generated from the chimeric myocardium of rats injected with Shz small-molecule-treated human M-PBMCs and identified human cDNA sequences from this chimeric tissue (Fig. S8B), providing strong evidence that human cells were viable and synthesizing cardiac mRNAs 1 month after engraftment into the injured rat heart. Thus, the cell-mediated functional recovery enhanced by drug was associated with increased expression of human cardiac mRNAs by xenograft M-PBMCs in the injured rat heart.
Discussion
We report a successful chemical library screen for activators of the early cardiac genome in pluripotent stem cells and characterize a promising family of small molecules that can both promote cardiac regenerative therapies and serve as mechanistic tools to gain insight into the biology that underlies cell-fate decisions in stem cells. The Shz small molecules were among the strongest inducers of the Nkx2.5 BAC reporter gene system to emerge from our primary screen of almost 150,000 compounds using genetically engineered P19CL6 pluripotent stem cells. We tested Shz small molecules in a number of confirmatory stem cell cardiac reporter gene assays and validated their bioactivity by demonstrating specific induction of cardiac mRNAs and proteins. Most remarkably, Shz small molecules activated the human cardiac genome in adult M-PBMCs, one of the most clinically viable sources of stem cells for cardiac therapy. Shz small molecules enhanced the differentiation of M-PBMC-derived cells, which expressed a subset of cardiac genes. Engraftment of drug-induced human M-PBMCs improved cardiac function in a rat myocardial cryoinjury model significantly better than control M-PBMCs treated with vehicle alone. We observed a direct correlation between Shz drug-induced expression of cardiac mRNAs, successful engraftment of cTnI (+) human cell colonies, and recovery of function in cryoinjured rat hearts. We conclude that in this model: (i) Shz drug pretreatment enhances successful engraftment of human cells that express cardiac genes and proteins, and (ii) successful engraftment of Shz-treated human cells is required for efficient recovery of cardiac function. Thus, from a chemical screen based on one of the earliest genes known to be involved in cardiac fate, we have identified a class of small molecules that can enhance the cardioregenerative potential of adult human PBMCs.
We designed a chemical screen to recover small molecules targeting Nkx2.5, a signature gene of the cardiovascular master stem cell (10, 11, 17). In this regard, our chemical screen was fundamentally different from previous cardiogenic small-molecule screens that targeted the 5′ regulatory sequences of two markers of late cardiogenic differentiation, α-myosin heavy chain (α-MHC) (18) or atrial natriuretic factor (ANF), neither of which have known functional roles in cardiac fate. Another distinctive element of our screen was the use of an artificial chromosome, a chromatin domain encompassing all of the transcriptional and epigenetic regulatory sequences necessary for cardiac-specific expression of the Nkx2.5 gene. The α-MHC screen identified ascorbic acid (vitamin C), and the ANF screen identified cardiogenol, a diaminopyrimidine, as compounds that enhanced the efficiency of producing spontaneously beating cardiomyocytes from pluripotent stem cells (19). Of note, our screen identified one family (Scheme 1, 2-aminobenzoxazoles) that contained an aminopyrimidine ring with possible functional homology to cardiogenol.
To explain how successful engraftment of even small amounts of Shz-treated human cells can promote recovery of contractile function, future studies will need to determine the relative roles of paracrine versus contractile repair mechanisms. We speculate that the Shz small-molecule-induced sarcomeric α-tropomyosin-positive P19CL6 cell clusters (Fig. 2F) are colonies of “cardioblasts,” committed to the cardiac cell fate yet still highly proliferative. It remains for future studies to isolate, subclone, and characterize the growth and differentiation properties of these Shz drug-induced cardiac progenitors and determine the additional chemical signals necessary to drive these precursors toward functional and terminal cardiac differentiation. Preliminary pharmacokinetic studies in mice indicate that high plasma concentrations of Shz-1 can be achieved after single dose i.v. administration, even accumulating in myocardium, and lacking obvious organ system toxicity (data not shown). Thus, as the first agent identified in a small-molecule screen capable of enhancing myocardial regeneration with human M-PBMCs, the Shz class of compounds has significant clinical potential, either for ex vivo pretreatment of stem cells or systemic administration.
Materials and Methods
Assay Development.
An Nkx2.5-luc transgene was constructed by replacing the two coding sequence exons of Nkx2.5 locus from the ATG with the coding sequence of luc (from pGL3-basic vector, Promega) in an ≈180,000-bp-long BAC (Fig. 1A). The recombinant BAC DNA was introduced into pluripotent P19CL6 cells (13) by using Lipofectamine-2000 (Invitrogen), and neoR clones were selected and tested for chemically inducible luc activity with sodium butyrate (NaB). A clonal stem cell line (#5-1) with low basal and at least 4-fold-higher NaB-inducible luciferase activity compared with vehicle DMSO control (Z′ value of ≈0.7 in 384-well-plate format) was chosen for the HTS.
HTS for Chemical Inducers of Nkx2.5-luc in P19CL6.
Approximately 147,000 unique compounds were screened by using clone #5-1 P19CL6 Nkx2.5-luc cells in 384-well white plates. To ensure pluripotency at the time of compound screening, each P19CL6 #5-1 batch was prescreened for uniform high-level Oct3/4 expression by immunofluorescence cell staining. Cells were plated by using an automated dispenser at 1,200 cells per well in 70 μl of media per well in 10% fetal calf serum MEMα media. Parallel plating onto clear-bottom plates was done to ensure viability and appropriate cell density for large-scale screens. On day 3, 0.7 μl of library compounds at 5 μM in pure DMSO (1% final DMSO concentration) was dispersed robotically (384-pin array Biomek FX high-precision robot), and the plates were incubated for 48 h before measuring luc activity.
Pretreatment of M-PBMCs and Myocardial Injury.
Human M-PBMCs cells were cultured in X-vivo 20 media with 5 or 10 μM Shz or vehicle (vol/vol equivalent DMSO control) for 3 days, then washed and resuspended in fresh X-vivo 20 media for 7 days. The cells were either harvested in TRIzol (Invitrogen) for RNA isolation, washed with PBS and fixed on chamber slides for immunohistochemistry, or trypsinized (0.05% trypsin) and counted for in vivo delivery. Athymic Harlan nude rats (8–10 weeks) lacking cell-mediated immunity were used. After baseline echocardiography, myocardial cryoinjury was induced by applying a supercooled probe to the epicardial surface of the left ventricular anterior wall. Human M-PBMCs were injected at three sites circumferentially around the cryoinjury zone.
For additional methods, please refer to SI Text.
Supplementary Material
Acknowledgments.
We thank Steven McKnight and Michael Roth for providing helpful advice for the HTS screen and chemistry support; Lisa Cooper and Ling Zhang for technical assistance; Lawrence Lum [University of Texas Southwestern (UTSW)] for sharing Wnt3a-expressing cells; Ning Liu (UTSW) for myocardin reporter cells; Sean Goetsch for graphic support; R. Schwartz (Texas A&M, College Station, TX), R. Kitsis (Albert Einstein University, New York), and I. Komuro (University of Tokyo, Tokyo, Japan) for materials; and Alice Chang for editorial assistance. This work was supported in part by the Reynolds Foundation, UTSW, and the National Institutes of Health. Additional grant support was provided by the Ellison Medical Foundation, the Welch Foundation, and the Haberecht Wildhare-Idea Program.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711507105/DCSupplemental.
References
- 1.Sasaki K, et al. Ex vivo pretreatment of bone marrow mononuclear cells with endothelial NO synthase enhancer AVE9488 enhances their functional activity for cell therapy. Proc Natl Acad Sci USA. 2006;103:14537–14541. doi: 10.1073/pnas.0604144103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dimmeler S, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wojakowski W, Kucia M, Kazmierski M, Ratajczak MZ, Tendera M. Circulating stem/progenitor cells in stable ischemic heart disease and acute coronary syndromes—relevant reparatory mechanism? Heart. 2008;94:27–33. doi: 10.1136/hrt.2006.103358. [DOI] [PubMed] [Google Scholar]
- 4.Rosenzweig A. Cardiac cell therapy—mixed results from mixed cells. New Engl J Med. 2006;355:1274–1277. doi: 10.1056/NEJMe068172. [DOI] [PubMed] [Google Scholar]
- 5.Assmus B, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. New Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 6.Schachinger V, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. New Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 7.Nygren JM, et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 8.Dell'Era P, et al. Fibroblast growth factor receptor-1 is essential for in vitro cardiomyocyte development. Circ Res. 2003;93:414–420. doi: 10.1161/01.RES.0000089460.12061.E1. [DOI] [PubMed] [Google Scholar]
- 9.Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: The scientific foundations of cardiac repair. J Clin Invest. 2005;115:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moretti A, et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 11.Garry DJ, Olson EN. A common progenitor at the heart of development. Cell. 2006;127:1101–1104. doi: 10.1016/j.cell.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 12.Ding S, Schultz PG. A role for chemistry in stem cell biology. Nat Biotechnol. 2004;22:833–840. doi: 10.1038/nbt987. [DOI] [PubMed] [Google Scholar]
- 13.Hara-Ohkubo A. Differentiation of beating cardiac muscle cells from a derivative of P19 embryonal carcinoma cells. Cell Struct Funct. 1996;21:101–110. doi: 10.1247/csf.21.101. [DOI] [PubMed] [Google Scholar]
- 14.Pipes GC, Creemers EE, Olson EN. The myocardin family of transcriptional coactivators: Versatile regulators of cell growth, migration, and myogenesis. Genes Dev. 2006;20:1545–1556. doi: 10.1101/gad.1428006. [DOI] [PubMed] [Google Scholar]
- 15.Muthuchamy M, Pajak L, Howles P, Doetschman T, Wieczorek DF. Developmental analysis of tropomyosin gene expression in embryonic stem cells and mouse embryos. Mol Cell Biol. 1993;13:3311–3323. doi: 10.1128/mcb.13.6.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bush E, et al. A small molecular activator of cardiac hypertrophy uncovered in a chemical screen for modifiers of the calcineurin signaling pathway. Proc Natl Acad Sci USA. 2004;101:2870–2875. doi: 10.1073/pnas.0308723101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu SM, et al. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–1150. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi T, et al. Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation. 2003;107:1912–1916. doi: 10.1161/01.CIR.0000064899.53876.A3. [DOI] [PubMed] [Google Scholar]
- 19.Wu X, Ding S, Ding Q, Gray NS, Schultz PG. Small molecules that induce cardiomyogenesis in embryonic stem cells. J Am Chem Soc. 2004;126:1590–1591. doi: 10.1021/ja038950i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.