Abstract
To determine whether adipocyte storage capacity influences the onset and severity of type 2 diabetes and other components of the metabolic syndrome, we made normal and db/db mice resistant to obesity by overexpressing leptin receptor-b on the aP2-Lepr-b promoter. On a 4% diet, these mice have no phenotype, but on a 60% fat diet, they resist diet-induced obesity because constitutive adipocyte-specific overexpression of Lepr-b prevents obesity via the antilipogenic autocrine/paracrine action of leptin on adipocytes. After 8 months on the same 60% fat diet, body fat of transgenic mice was 70% below WT controls. Cardiac and liver fat was elevated in the transgenics, and their hyperinsulinemia was more marked, suggesting greater insulin resistance. The aP2-Lepr-b transgene also prevented obesity in db/db mice; at 10 weeks of age their body fat was half that of the db/db mice. This lack of obesity was attributable to reduced expression of sterol regulatory element binding protein-1c and its target lipogenic enzymes in adipose tissue and a 6-fold increase in Pref-1 mRNA. Severe diabetes was present in transgenics at 4 weeks of age, 10 weeks before db/db controls. Echocardiographic evidence of cardiomyopathy appeared at 10 weeks, weeks before the db/db mice. Histologically, loss of β cells and myocardial fibrosis was present in the transgenic group at least 6 weeks before the db/db mice. These results suggest that the expression level of genes that regulate the adipogenic response to overnutrition profoundly influences the age of onset and severity of diet-induced type 2 diabetes and co-morbidities.
Keywords: adipogenesis, apoptosis, lipotoxicity, Pref-1, leptin
Fifty million Americans now carry the diagnosis of metabolic syndrome (1), a cluster of life-shortening morbidities that includes type 2 diabetes (T2D) (2). The temporal relationship between a change in the caloric environment in the U.S. and the first recognition of the syndrome in 1981 is evidence of a crucial environmental role, whereas individual differences in susceptibility to the chronic caloric excess imply an important genetic input. Whereas many genes may influence the susceptibility to caloric excess, genes of adipogenesis are particularly attractive candidates for two reasons. First, in both rodents and humans, lipids have been shown to accumulate in the organs that are most affected in metabolic syndrome (3, 4). Second, ectopic lipid overload has been demonstrated to disable and destroy normal cardiomyocytes (5) and pancreatic β cells (6) through the process of lipoapoptosis (7). Therefore, it follows that, during sustained overnutrition, the capacity of adipose tissue to store the surplus lipids that might otherwise damage important organs would determine the overflow of lipids into nonadipocytes. Indeed, when adipocytes are deficient, as in generalized congenital lipodystrophy, the metabolic syndrome appears at an earlier age and is more severe than in the obesity-associated counterpart.
To determine the influence of adipogenesis on an environmentally induced form of metabolic syndrome, we have produced a transgenic mouse model in which the adipocytes seem to be normal on a normal diet but do not undergo normal hypertrophy and hyperplasia on a high fat diet. The transgene, the full-length leptin receptor on an aP2 promoter (Lepr-b), prevents the disappearance of adipocyte Lepr-b that normally accompanies overfeeding and that is essential to block the antilipogenic autocrine action of leptin that prevents fat storage (8). We speculated that the persistent constitutive expression of Lepr-b on adipocytes during long-term overnutrition would result in lipid spillover into nonadipose organs and thereby cause manifestations of the metabolic syndrome, such as hepatic steatosis and insulin resistance, to appear.
If, indeed, this scenario were the case, it would imply that the predisposition to metabolic syndrome is inversely related to the adipogenic response to overnutrition. This notion would fit well with the report of Ruderman et al. that “metabolically obese, normal weight” patients with metabolic syndrome tend to be younger than those with overt obesity (9). It would also fit with the demonstration by Kim et al. that further expansion of the fat mass of congenitally obese mice improves their metabolic profile (10). Using this model of restricted adipogenesis, we observed that the level of adipogenesis is a key determinant of both age of onset and severity of the metabolic syndrome in both normal mice and in db/db mice with an inherited predisposition to severe obesity-associated metabolic syndrome caused by a mutation in the leptin receptor-b (Lepr-b) (11). Thus, contrary to popular belief, obesity protects, at least temporarily, against T2D and metabolic syndrome by buffering the effects of overnutrition on ectopic lipid deposition.
Results
aP2-Lepr-b Transgene Increases Ectopic Lipid Deposition During High Fat Feeding.
To determine the influence of adipogenic capacity on the metabolic consequences of overnutrition, normal WT mice and aP2-Lepr-b transgenic mice were placed on a diet containing either 4% or 60% fat for 8 months, and various metabolic parameters were compared. There were no differences in food intake on either diet. On the 4% fat diet, no intergroup differences in body weight or body fat were evident over the 8-month period of observation (Table 1, 4% fat diet). Except for a modest but statistically significant increase in plasma triglyceride levels, none of the metabolic parameters measured differed from WT mice (Table 1, 4% fat diet).
Table 1.
Metabolic parameters of WT and transgenic (tg) (N = 6) mice fed 4% or 60% fat diets for 8 months
| Measurement | 4% fat diet |
60% fat diet |
||||
|---|---|---|---|---|---|---|
| WT | P value(WT vs. tg) | tg | WT | P value(WT vs. tg) | tg | |
| Body weight, g | 36.8 ± 2.6 | NS | 33.2 ± 2.6 | 67.3 ± 1.1 | 0.00008 | 36.2 ± 3.8 |
| Food intake, g | 4.3 ± 0.4 | NS | 4.1 ± 0.4 | 3.6 ± 0.2 | NS | 3.9 ± 0.5 |
| Body fat, g | 5.3 ± 0.4 | NS | 4.8 ± 0.5 | 27.5 ± 0.8 | 0.00002 | 8.4 ± 2.0 |
| Fasting blood glucose, mg/dl | 70.3 ± 9.6 | NS | 82.3 ± 9.1 | 103.5 ± 16.3 | 0.015 | 154.5 ± 4.5 |
| Blood glucose, mg/dl | 146.5 ± 35.5 | NS | 161.7 ± 4.4 | 184.6 ± 30.5 | NS | 189.2 ± 31.8 |
| Insulin, ng/ml | 1.710 ± 0.28 | NS | 1.232 ± 0.087 | 3.927 ± 2.212 | 0.008 | 11.024 ± 0.9 |
| Leptin, ng/ml | 2.32 ± 0.69 | NS | 4.38 ± 2.28 | 216.0 ± 33.3 | 0.015 | 13.75 ± 6.75 |
| TAG, mg/dl | 109 ± 1.3 | 0.01 | 131.7 ± 3.8 | 127.8 ± 39.8 | NS | 105.4 ± 35.1 |
| FFA, mM | 0.142 ± 0.02 | NS | 0.114 ± 0.019 | 0.204 ± 0.006 | 0.09 | 0.255 ± 0.038 |
| Liver weight, g | 1.92 ± 0.15 | NS | 1.86 ± 0.42 | 2.29 ± 0.18 | NS | 3.02 ± 0.49 |
| Heart weight, g | 0.15 ± 0.01 | NS | 0.16 ± 0.013 | 0.15 ± 0.005 | 0.018 | 0.23 ± 0.03 |
| Liver TAG, mg/g | 42.6 ± 3.1 | NS | 49.0 ± 9.1 | 130.62 ± 8.77 | 0.032 | 177.65 ± 15.01 |
| Heart TAG, mg/g | 4.29 ± 1.94 | NS | 3.16 ± 2.55 | 4.17 ± 0.81 | 0.055 | 9.66 ± 2.00 |
| Muscle TAG, mg/g | 14.42 ± 5.23 | NS | 17.32 ± 6.75 | 28.34 ± 3.28 | NS | 23.07 ± 0.77 |
| Pancreas TAG, mg/g | 3.31 ± 0.51 | NS | 4.85 ± 2.28 | 24.39 ± 7.12 | NS | 22.97 ± 2.07 |
NS, Nonsignificant (P > 0.055).
However, on the 60% fat diet for 8 months, marked differences between the two groups appeared despite almost identical food intakes. The body fat of the transgenic mice was 70% less than the WT controls reflecting severe impairment of adipogenesis (Table 1, 60% fat diet). Ectopic triacylglycerol (TAG) deposition was significantly greater in the heart and liver of the transgenic mice. The hearts appeared larger and this increase in size was reflected by a significant increase in organ weight (Table 1, 60% fat diet). However, in skeletal muscle, the differences in TAG content of WT and tg groups, although significantly higher than on a 4% fat diet, did not significantly differ from each other (Table 1, 60% fat diet). Postprandial insulin levels were almost three times higher in the transgenic mice than in controls, despite comparable mild elevation in postprandial glucose concentrations (Table 1, 60% fat diet). This increase is consistent with insulin resistance.
aP2-Lepr-b Transgene Prevents Genetic Obesity in db/db Mice.
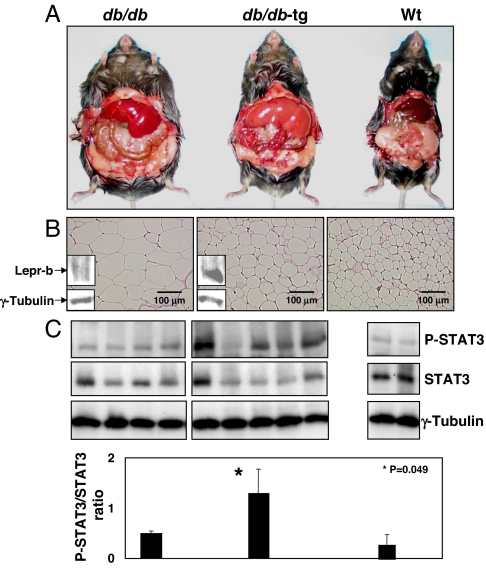
To determine whether the aP2-Lepr-b transgene would also prevent genetic obesity in db/db mice, a well characterized model of hyperphagia, obesity and T2D, we derived transgenic db/db-aP2-Lepr-b (db/db-tg) mice. Lepr-b protein was detected in the fat tissue of db/db-tg mice but not in db/db mice (Fig. 1B).
Fig. 1.
Comparison of 10-week-old db/db, db/db-tg, and normal mice. (A) Exposure of s.c. and visceral fat showing less fat in the db/db-tg mouse. Appearance of both db/db and db/db-tg livers suggests steatosis, which is more marked in the latter. (B) Sections of epididymal fat of db/db, db/db-tg, and WT mice, showing reduced size of db/db-tg adipocytes compared with db/db despite identical food intake (Table 2). Immunoblot for C terminus of mouse Lepr-b is positive in db/db-tg fat but not in db/db fat. (C Upper) Adipose tissue phospho-STAT3, an index of leptin action, in db/db, db/db-tg, and normal mice. (Lower) Bars indicate the mean ± SEM of phospho-STAT3/STAT3 ratios.
Both db/db and db/db-tg mice were fed a chow diet containing 6% fat for 10 weeks and were equally hyperphagic. Their food intake during that period was virtually identical (5.1 vs. 5.3 g/d), exceeding by ≈48% the food intake of normal WT C57BL/6 mice (Table 2).
Table 2.
Metabolic profiles of 10-week-old WT, db/db, and db/db-tg (N = 4) mice fed 6% fat diet
| Measurement | WT | P value (WT vs. db/db) | db/db | P value (db/db vs. db/db-tg) | db/db-tg |
|---|---|---|---|---|---|
| Body weight, g | 30.9 ± 1.0 | 0.0002 | 51.1 ± 3.4 | 0.0007 | 28.5 ± 5.8 |
| Food intake, g | 3.5 ± 0.5 | 0.05 | 5.1 ± 0.5 | 0.836 | 5.3 ± 0.6 |
| Body fat, g | 8.49 ± 1.38 | 0.0001 | 24.38 ± 2.83 | 0.004 | 11.25 ± 4.09 |
| Adipocyte diameter, μm | 17.4 ± 1.4 | 4.2E−13 | 55.3 ± 7.9 | 7.8E−12 | 25.0 ± 4.5 |
| Fasting blood glucose, mg/dl | 77.6 ± 7.9 | 0.059 | 106.4 ± 29.3 | 0.0002 | 271.0 ± 31.2 |
| Blood glucose, mg/dl | 130.0 ± 16.7 | 0.734 | 175.0 ± 42.0 | 0.002 | 574.5 ± 14.5 |
| Urine glucose, mg/dL | negative | negative | >2,000 | ||
| Insulin, ng/ml | 1.849 ± 0.66 | 0.018 | 12.990 ± 5.30 | 0.068 | 5.778 ± 0.742 |
| Leptin, ng/ml | 1.47 ± 0.26 | 0.0014 | 9.19 ± 1.17 | 0.0009 | 3.59 ± 1.46 |
| TAG, mg/dl | 23.2 ± 4.1 | 0.029 | 50.7 ± 5.5 | 0.281 | 62.4 ± 15.1 |
| FFA, mM | 0.166 ± 0.033 | 0.0008 | 0.848 ± 0.198 | 0.229 | 0.694 ± 0.117 |
| Liver TAG, mg/g | 29.2 ± 0.44 | 0.0002 | 59.4 ± 2.65 | 0.097 | 96.9 ± 4.42 |
| Heart TAG, mg/g | 5.2 ± 0.63 | 0.936 | 5.3 ± 0.75 | 0.02 | 7.43 ± 0.75 |
| Muscle TAG, mg/g | 17.2 ± 3.98 | 0.432 | 23.4 ± 7.37 | 0.019 | 49.4 ± 6.58 |
| Pancreas TAG, mg/g | 2.70 ± 0.28 | 0.038 | 23.0 ± 8.89 | 0.065 | 6.83 ± 2.34 |
In contrast to the db/db mice, db/db-tg mice did not become obese. At 10 weeks, their body weight averaged only 55% of the db/db mice (28.5 ± 5.8 g vs. 51 ± 3.4 g; P = 0.0007), and they were not significantly heavier than the normal WT controls (Table 2). The body fat of db/db-tg mice was less than half of db/db mice (Table 2 and Fig. 1A). Microscopically, the adipocyte diameter in the db/db-tg mice averaged 25 ± 5 μm, compared with 55.3 ± 8 μm in the db/db mice (P < 0.0001) (Fig. 1B). The adipose tissue content of P-STAT3, a marker of leptin receptor-mediated action, was increased in the transgenic mice (Fig. 1C), indicating that differences in the adipose tissue of lean db/db-tg mice were likely transduced via the transgenic Lepr-b.
aP2-Lepr-b Transgene Accelerates the Development of Diabetes.
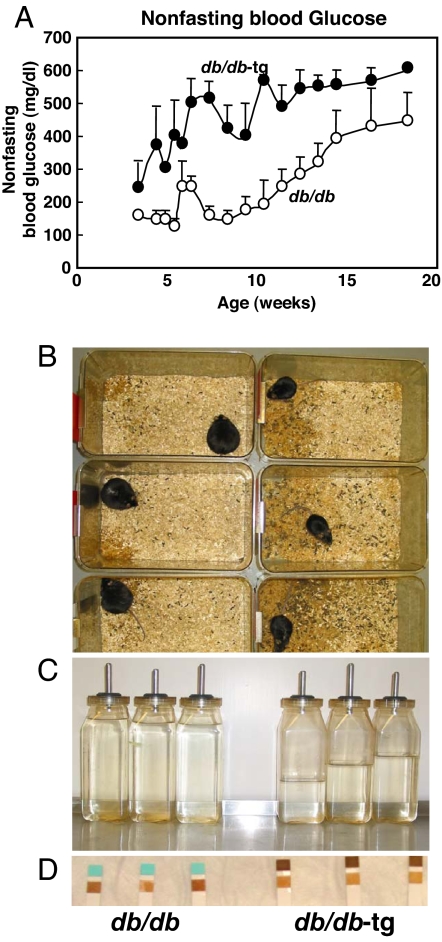
In the db/db mice at 10 weeks of age, ≈6 weeks after the onset of obesity (Fig. 2A), nonfasting blood glucose levels were still relatively normal, averaging 175 ± 42 mg/dl. By contrast, most of the nonobese db/db-tg mice had become overtly diabetic by 5 weeks of age. At 10 weeks of age, all were severely diabetic, with a mean glucose level of 574 ± 15 mg/dl. They were all obviously ill, with severe polyuria (Fig. 2B), polydipsia (Fig. 2C), and glycosuria (Fig. 2D), whereas all db/db mice were still normal clinically at this time point, with a mean nonfasting glucose level of <200 mg/dl (175 ± 42).
Fig. 2.
Clinical evidence that obesity delays the onset of T2D in db/db mice. (A) Comparison of nonfasting blood glucose levels in obese db/db and nonobese db/db-tg mice, demonstrating the later appearance of less severe hyperglycemia in the db/db mice. (B and C) Comparison of the cages and the water bottles of 10-week-old obese db/db and nonobese db/db-tg mice, showing evidence of polyuria and polydipsia only in the latter. (D) Comparison of urine glucose testing in obese db/db and nonobese db/db-tg mice (Keto-Diastix Reagent Strip, Bayer, Elkhart, IN), showing glycosuria only in the latter.
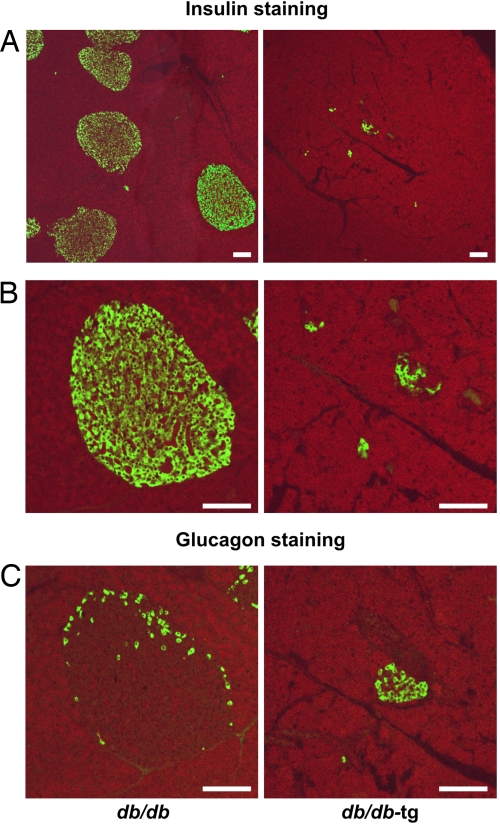
To determine whether the earlier onset and greater severity of T2D in the db/db-tg mice resulted from a greater loss of β cells, their pancreata were stained for insulin and glucagon. At 10 weeks of age, β cells of the db/db-tg mice were profoundly reduced in both number and area (Fig. 3A), and severe derangement of islet organization was apparent (Fig. 3B). By contrast, islet organization in the db/db mice was still perfectly preserved. The volume density of the insulin-positive β cells was 0.053 ± 0.01 in pancreas sections of db/db mice (area evaluated 30.8 mm2, three animals) compared with 0.011 ± 0.007 in the db/db-tg mice (area evaluated 27.4 mm2, three animals) (Fig. 3B).
Fig. 3.
Immunofluorescent staining for insulin and glucagon of pancreas. (A) Low magnification view of pancreatic tissue showing a large number of islets in db/db mice (Left) and a fewer number of small insulin-positive areas in db/db-tg mice (Right). (B) Higher magnification of the cells in A. (C) The normal topography of glucagon-containing α cells in db/db mice (Left) contrasts with disrupted cells in db/db-tg mice (Right). (Scale bars: 100 μm.)
In addition, there were differences in the distribution of glucagon-positive α cells. In db/db mice, they were arrayed peripherally as in normal islets (12), whereas in db/db-tg mice they were found in increased proportion and had lost their characteristic peripheral distribution within the islets (Fig. 3C).
Quantitative Relationship of Adipocyte Lepr-b Expression to Adiposity and Diabetes.
If the aP2-Lepr-b transgene is the cause of the foregoing differences in adipogenesis, ectopic lipid deposition, and glucose metabolism, one would expect to find a relationship between its expression level and the adipogenic capacity and the blood glucose level. To test for this relationship, we divided the db/db-tg mice into high and low expressors of adipocyte aP2-Lepr-b. At 18 weeks of age, the body weight of the high expressors averaged only 27.6 ± 1.3 g, compared with 44.7 ± 1.8 g in the low expressors. Body fat measured 9.1 ± 0.6 g in the high expressors and 13.8 ± 0.8 g in the low group (P = 0.03), evidence that the expression level of this transgene influences adipogenesis.
To determine whether the level of expression of Lepr-b on adipocytes is a factor in the development of diabetes, we compared the nonfasting blood glucose levels and insulin levels in the two groups at 18 weeks of age. In the low expressors, glucose and insulin levels averaged 207 ± 44 mg/dl and 25 ± 7 ng/ml, respectively, compared with 577 ± 30 mg/dl and 15 ± 5 ng/ml in the high expressors (P < 0.05) (data not shown). Although the difference was not statistically significant, the lower insulin levels in the high expressors, despite greater hyperglycemia, are consistent with more severe β cell destruction.
aP2-Lepr-b Transgene Accelerates Lipotoxic Heart Disease of db/db-tg Mice.
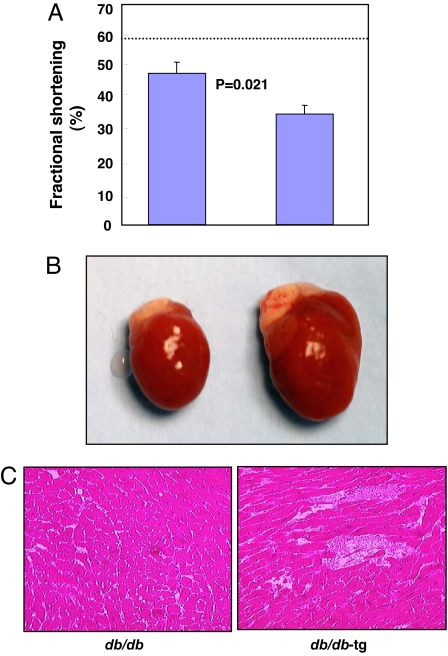
Ectopic fat deposition has been identified in the heart and other organs of obese rodents (3, 13, 14), suggesting that lipotoxic cardiomyopathy may be a genuine disease entity (5, 15). Ectopic fat deposition has also been identified in humans with metabolic syndrome, and, in human obesity, myocardial fat has been implicated in certain cardiac derangements (4). To determine whether there is a relationship between adipogenic capacity and cardiac function, transthoracic echocardiograms were obtained in the nonobese db/db-tg and obese db/db mice at 10 weeks of age. The impairment in systolic function and depression of fractional shortening was greater in the former (Fig. 4A). Postmortem studies at 10 weeks of age showed the hearts of db/db-tg mice to be markedly enlarged (Fig. 4B) and their cardiac TAG content 29% higher than the db/db mice (P = 0.02) (Table 2).
Fig. 4.
Function and gross and microscopic appearance of the heart of 10-week-old db/db and db/db-tg mice. (A) Echocardiographically measured fractional shortening demonstrating greater functional loss in 10-week-old db/db-tg mice. The dotted line marks the normal value of fractional shortening. (B) Gross enlargement of the heart in a db/db-tg mouse. (C) Myocardium of db/db and db/db-tg mice exhibits myofiber disruption and focal fibrosis in the latter.
Morphologic examination of the hearts of db/db-tg mice revealed disruption of their myocardial architecture and replacement of myofibers by fibrous tissue (Fig. 4C). Thus, severe lipotoxic cardiomyopathy occurred at an earlier age in mice with a restricted adipogenic capacity.
Effects of the aP2-Lepr-b Transgene on Lipid Deposition in Other Organs of db/db Mice.
To determine the effect of a reduced adipogenic capacity during overnutrition on ectopic lipid deposition in other organs, we compared the TAG content of the liver, pancreas, and skeletal muscle of db/db and db/db-tg mice. The hepatic TAG content of the db/db-tg mice was 61% higher than in the db/db group [nonsignificant (NS)] and over three times that of normal WT mice. The gastrocnemius muscle TAG content was more than twice that of db/db (P < 0.02), and almost three times the normal value. Pancreatic TAG content was above normal in both db/db and db/db-tg mice but, unexpectedly, was higher in the former. Plasma TAG levels of db/db-tg mice, which were almost three times normal values, were only slightly above those of db/db mice (N.S.) (Table 2).
Expression Profiles in Adipose Tissue.
The ectopic lipid deposition of the db/db-tg mice reflects a diminished capacity of adipocytes to store unoxidized fatty acids, either because of impaired lipogenesis and/or a diminished capacity for hypertrophy and/or proliferation. We therefore determined by quantitative real-time RT-PCR the expression level of relevant genes involved at various levels in these processes (Table 3). The expression of the lipogenic transcription factor, sterol regulatory element-binding protein (SREBP)-1c, was reduced, together with its target enzymes of fatty acid synthesis and esterification, acetyl CoA carboxylase (ACC)-α and -β, fatty acid synthetase, and glycerol phosphate acyl transferase (GPAT) and diacylglycerol acyl transferase (DGAT)-1. Importantly, there was a 6-fold increase in the mRNA of preadipocyte factor (Pref)-1, an inhibitor of adipogenesis that is expressed on preadipocytes but not on adipocytes (16). These results suggest that a block of lipogenesis and adipocyte maturation caused the obesity resistance and ectopic lipid deposition.
Table 3.
Quantitative PCR analysis of mRNAs in the adipocyte tissue of db/db and db/db-tg mice using 36B4 as the invariant control
| Genes | db/db-tg (n = 5) | P value |
|---|---|---|
| AMPKα1 | 0.39 ± 0.23 | 0.049 |
| PPARα | 0.06 ± 0.02 | 0.036 |
| PGC1α | 2.06 ± 0.02 | 0.042 |
| SREBP-1c | 0.62 ± 0.12 | 0.034 |
| Pref-1 | 6.44 ± 0.97 | 0.019 |
| IR | 1.55 ± 0.04 | 0.043 |
| IRS2 | 2.24 ± 0.50 | 0.018 |
| ACO | 2.26 ± 0.84 | 0.028 |
| ACCα | 0.51 ± 0.13 | 0.043 |
| ACCβ | 0.44 ± 0.04 | 0.041 |
| FAS | 0.23 ± 0.12 | 0.005 |
| GPAT | 0.36 ± 0.05 | 0.0003 |
| DGAT1 | 0.35 ± 0.29 | 0.038 |
| Leptin | 0.41 ± 0.02 | 0.028 |
| Resistin | 0.36 ± 0.14 | 0.032 |
| Adiponectin | 0.70 ± 0.09 | 0.025 |
Values in db/db-tg mice are expressed as fold difference from db/db mice. No significant differences were found in the expression of the following genes: AMPKα2, PPARγ, LXRα, RXRα, C/EBPα, C/EBPβ, C/EBPδ, ChREBP, Insig-1, FOXO1, FOXC2, IRS1, CPT1, DGAT2, MCD, SCD, SOCS3, TNFα, IL-6, IL-1β, CRP, MCP-1, SAA-1, UCP1, UCP2, and UCP3.
There was also evidence of increased fatty acid oxidation in adipocytes of the db/db-tg mice, as we had reported (8). The mRNA of the peroxisomal enzyme acyl CoA oxidase (ACO) was increased, and the decreased expression of both ACC isoforms would be expected to reduce malonyl-CoA-mediated inhibition of carnitine palmitoyl CoA transferase (CPT)-1. Further, a 7-fold higher ratio of phosphorylated to total AMP-activated protein kinase (0.7 ± 0.4 vs. 0.1 ± 0.004; P = 0.05) (data not shown) suggested increased mitochondrial oxidation of fatty acids. Finally, expression of peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α, an up-regulator of mitochondrial biogenesis (17), was increased in the fat of db/db-tg mice, presumably expanding its oxidative capacity. Whereas increased fatty acid oxidation could have contributed to the obesity resistance of the db/db-tg mice, it was not sufficient to prevent the ectopic lipid overflow in the db/db-tg mice.
Because of the putative role of inflammation in obesity and metabolic syndrome, we compared various inflammatory markers in the adipose tissue, liver, and muscle of the db/db and db/db-tg mice. At 10 weeks of age, there were no statistically significant differences in IL-1β, IL-6, TNF-α, or macrophage chemoattractant protein-1 (MCP-1) mRNA in the three tissues. Serum amyloid A (SAA-1) mRNA was significantly higher in skeletal muscle of db/db-tg mice (P = 0.026) but not in fat or liver tissue; however, plasma SAA did not differ in the two groups (data not shown). C-reactive protein was increased in the liver of db/db-tg mice (P = 0.05). In contrast to the increase in phosphorylated AMP kinase in the fat tissue of db/db-tg mice, there were no differences in liver or muscle (data not shown). The comparison of expression levels of inflammatory factors appears in supporting information (SI) Table S1.
Discussion
The aim of the present study was to determine the relationship between adipocyte storage capacity and two major components of the metabolic syndrome in rodents, T2D, and lipotoxic cardiomyopathy (14). It has long been accepted that the primary function of adipocytes is to store fuel for distribution to nonadipose tissues in times of need, as during a famine (18). The present study suggests a second function of adipocytes, namely, the compartmentalization into adipocytes of the surplus calories consumed during overnutrition so as to protect nonadipose organs from lipid-induced trauma (3). Indeed, the recent demonstration by Kim et al. (10) that expansion of the fat mass of congenitally obese mice improves their metabolic profile lends further credence to the idea. To test this hypothesis, we studied the effects of preventing adipocyte expansion on the complications of diet-induced obesity in otherwise normal C57BL/6 mice, and in the genetic obesity of db/db mice.
Comparison of WT C57BL/6 controls and the aP2-Lepr-b-tg mice revealed no significant differences in ectopic lipid deposition after 8 months on a 4% fat diet. However, after 8 months of a 60% fat diet, the WT control mice had developed diet-induced obesity, whereas the aP2-Lepr-b-tg mice remained nonobese. The latter mice exhibited greater lipid deposition in the liver and heart. The heart was enlarged and heavier (Table 1, 60% fat diet). Both groups were hyperinsulinemic, but the hyperinsulinemia of the tg mice was approximately three times that of the WT controls, with a similar moderate elevation in nonfasting glucose levels (Table 1, 60% fat diet). But in tg mice, the difference in insulin between the 4% fat diet and the 60% fat diet was 9.8 ng/ml compared with only 2.2 ng/ml in the WT control mice (Table 1). Given the similar levels of hyperglycemia, this difference in insulin was consistent with greater insulin resistance in the tg group.
As expected, comparison of genetically obese WT db/db mice with slender db/db mice expressing the aP2-Lepr-b transgene revealed a far greater impact of restricted adipogenesis. In the former mice, the metabolic syndrome follows the onset of obesity by ≈7 weeks, or ≈12 weeks of age. Blood glucose levels remain <200 mg/dl until that time (Fig. 2A), evidence that their β cells are still delivering enough insulin to meet the demands of rising insulin resistance. By contrast, nonobese db/db-tg mice enjoyed no such prediabetic “honeymoon” period. Some were seriously ill at 4–5 weeks of age, with glucose levels averaging >400 mg/dl, a level not attained by db/db mice until ≈14 weeks of age. All db/db-tg mice exhibited polyuria, polydipsia, glycosuria, and severe hyperglycemia by 10 weeks of age, consistent with the profound depletion of their β cells. Similarly, myocardial dysfunction and dilated cardiomyopathy and echocardiographic evidence of severe functional loss appeared in the db/db-tg mice at 10 weeks of age, whereas at 18 weeks functional loss was still minimal in the db/db group (Fig. 4A).
Given the known lipoapoptotic action of lipid excess on β cells in vitro (7) and in vivo (6) and on cardiomyocytes in vivo (5), a lipotoxic etiology for the observed organ damage seemed plausible, particularly because there was no obvious morphologic evidence of inflammation in the tissues examined and because no increase in either TNF-α, IL-1β, IL-6, or MCP-1 expression was detected in fat, liver, or muscle tissue. However, the increase in expression of SAA-1 in skeletal muscle and CRP in liver (Table S1) makes it impossible to exclude a contributing role of inflammation in the pathogenesis of these disorders (19).
These results may have potentially important clinical implications. First, they imply that getting fat is a premetabolic syndrome manifestation, recognition of which by physicians and patients might encourage more aggressive interventions during the disease-free phase of overnutrition, rather than after the onset of overt disease, as is now customary. Elimination of the caloric surplus in uncomplicated obesity is known to prevent overt T2D in rats (20) and humans (21), evidence that premorbid intervention, long successfully used to prevent coronary artery disease, will also prevent T2D and metabolic syndrome.
Second, there are similarities between the accelerated metabolic syndrome produced transgenically in this study and the syndrome of “metabolically obese, normal weight” patients first described by Ruderman et al. (9). It will be of interest to determine whether their adipocyte storage capacity is subnormal, and, if so, the molecular basis of that subnormal capacity.
Third, the clear relationship between adipogenic capacity and susceptibility to T2D and metabolic syndrome raises the possibility that differences in expression or function of various adipogenic genes may influence susceptibility to diet-induced metabolic syndrome. One gene candidate for such a role is Pref-1, a secreted preadipocyte factor that inhibits adipogenesis and has been shown to exacerbate insulin resistance while preventing diet-induced obesity (H. S. Sul, personal communication). The fact that its expression was 6-fold greater in the db/db-tg mice than in the db/db mice should place it high on the list of genes that increase the ectopic deposition of lipids during chronic overeating and susceptibility for the metabolic syndrome.
Materials and Methods
Animals.
C57BL/6 mice were bred locally. All were housed in individual cages in a temperature-controlled environment with 12-h light/12-h dark cycle. All mice had ad libitum access to water and pelleted mouse chow. Transgenic studies were carried out with C57BL/6 mice.
Animals were killed under anesthesia with pentobarbital sodium. Nonfasting blood samples were obtained from the inferior vena cava. All tissues were rapidly excised, frozen in liquid nitrogen, and stored at −70°C until use. Institutional guidelines for animal care and use were followed. The animal protocol was approved by the Institutional Animal Care and Research Advisory Committee of the University of Texas Southwestern Medical Center at Dallas.
Blood used for glucose determinations was collected in conscious animals by nicked tail vein bleeding between 10:00 and 12:00 a.m. Fasted blood glucose measurements were performed between 10:00 and 12:00 a.m., after food removal at 4:00 p.m. the previous day.
Transgenic Mice Production.
The production of aP2-Lepr-b transgenic mice has been described in ref. 8. Briefly, mouse Lepr-b cDNA was subcloned into an expression plasmid, pSTEC-1-aP2, under the control of aP2 promoter. The resulting pSTEC-1-aP2-Lepr-b construct contained a chimeric intron derived from β-globin intron and IgG intron, and a simian virus 40 polyA addition site required for proper processing of the transgene mRNA in vivo. Transgenic mice were generated by microinjection of purified aP2-Lepr-b DNA fragments into pronuclei of fertilized mouse eggs, which were subsequently transferred into foster mothers. Transgenic founders and offspring were screened by PCR genotyping of genomic DNA prepared from tail biopsies with specific primers.
Generation of aP2-Lepr-b db/db Mice.
Male heterozygous (db/+) mice on the C57BL/6 background (purchased from The Jackson Laboratory) were crossed with aP2-lepr-b transgenic animals that were on the same background. Double heterozygous (aP2-lepr-b db/+) animals were crossed again with db/+ to obtain aP2-lepr-b db/db genotype. Genotyping for db/db was carried out by restriction fragment-length polymorphism analysis using the enzyme RsaI on a 135-bp PCR product generated from genomic DNA isolated from these mice with the primer pair 5′-agaacggacactctttgaagtctc-3′ and 5′-cattcaaaccatagtttaggtttgtgt-3′, with 35 cycles of 94°C for 30 s, 52°C for 45 s, and 72°C for 45 s (22).
Plasma Measurements.
Plasma leptin and insulin were measured by using ELISA kits (Crystal Chem, Downers Grove, IL). Plasma triglycerides were measured by using a glycerol phosphate oxidase-Trinder triglyceride kit (Sigma). Plasma-free fatty acids were measured by using the Wako NEFA kit (Wako Chemical USA, Richmond, VA).
Triacylglycerol (TAG) Content of Tissues.
Mice were anesthetized with pentobarbital sodium. Tissues were rinsed with PBS (pH 7.4), dissected, and placed in liquid nitrogen immediately. Total lipids from tissues were extracted and dried under N2 gas. TG content was assayed as described in ref. 23.
Real-Time Quantitative Polymerase Chain Reaction (RT-QPCR).
Total RNA was extracted from fat tissues by TRIzol isolation method (Life Technologies, Rockville, MD). All PCRs were done in triplicate. mRNA was calculated by using the standard curve method. 36B4 RNA was used as the invariant control. Primer sequences of genes used for quantification of mRNA by RT-QPCR are shown in Table S2.
Immunoblotting.
Total cell extracts prepared from fat tissues of mice were resolved by SDS/PAGE and transferred to a poly(vinylidene difluoride) membrane (Amersham Pharmacia). The blotted membrane was blocked in 1× TBS containing 0.1% Tween (TBST) and 5% nonfat dry milk (MLK) for 1 h at room temperature with gentle, constant agitation. After incubation with primary antibodies anti-phospho-STAT-3 (Tyr-705), anti-STAT-3, anti-phospho-AMPK (Thr-172), anti-AMPK (Cell Signaling Technology, Beverly, MA), anti-serum amyloid A (a gift from P. E. Scherer, University of Texas Southwestern Medical Center at Dallas) or anti-γ-tubulin (Sigma) in freshly prepared TBST-MLK at 4°C overnight with agitation, the membrane was washed twice with TBST buffer followed by incubating with goat anti-rabbit or anti-mouse HRP-conjugated IgG in TBST-MLK for 1 h at room temperature with agitation. The membrane was then washed three times with TBST buffer, and the proteins of interest on immunoblots were detected by using an enhanced chemiluminescence detection system (Amersham Pharmacia).
Islet Morphology.
Pancreata were processed for immunocytochemistry as described in ref. 24 by using insulin or glucagon antibodies. The volume density of immunofluorescent cells was determined by the point counting method of Weibel (25).
Mouse Echocardiography.
Transthoracic echocardiography was performed on 10-week-old mice under ketamine/xylazine anesthesia by using a 12-MHz probe as described (8, 26). Two-dimensional echocardiography in midventricular short-axis view guided left ventricular (LV) end-diastolic and end-systolic dimensions, fractional shortening, and wall thickness measurements.
Statistical Analysis.
Results are presented as means ± SEM and were evaluated by using Student's t test for two groups.
Supplementary Material
Acknowledgments.
We thank J. Shelton and J. Richardson of the Molecular Pathology Core Lab at University of Texas Southwestern Medical Center for their contributions, the Scherer lab for adiponectin measurements, and Han-Yu Wang for help with genotyping. We thank P. Gorden, C. Newgard, N. Ruderman, and P. Scherer for critical review of the manuscript. We thank K. McCorkle for technical assistance with the art work, and P. McCravy for outstanding administrative assistance. This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, the Department of Veterans Affairs Merit Review, the Juvenile Diabetes Research Foundation (R.H.U.), and by the Swiss National Science Foundation (L.O.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801981105/DCSupplemental.
References
- 1.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. J Am Med Assoc. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 2.Reaven G, Abbasi F, McLaughlin T. Obesity, insulin resistance, and cardiovascular disease. Recent Prog Horm Res. 2004;59:207–223. doi: 10.1210/rp.59.1.207. [DOI] [PubMed] [Google Scholar]
- 3.Lee Y, et al. Liporegulation in diet-induced obesity: The antisteatotic role of hyperleptinemia. J Biol Chem. 2001;276:5629–5635. doi: 10.1074/jbc.M008553200. [DOI] [PubMed] [Google Scholar]
- 4.Szczepaniak LS, et al. Myocardial triglycerides and systolic function in humans: In vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn Reson Med. 2003;49:417–423. doi: 10.1002/mrm.10372. [DOI] [PubMed] [Google Scholar]
- 5.Chiu HC, et al. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–822. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee Y, et al. Metabolic mechanisms of failure of intraportally transplanted pancreatic beta-cells in rats: Role of lipotoxicity and prevention by leptin. Diabetes. 2007;56:2295–2301. doi: 10.2337/db07-0460. [DOI] [PubMed] [Google Scholar]
- 7.Shimabukuro M, et al. Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats: Role of serine palmitoyltransferase overexpression. J Biol Chem. 1998;273:32487–32490. doi: 10.1074/jbc.273.49.32487. [DOI] [PubMed] [Google Scholar]
- 8.Wang MY, Orci L, Ravazzola M, Unger RH. Fat storage in adipocytes requires inactivation of leptin's paracrine activity: Implications for treatment of human obesity. Proc Natl Acad Sci USA. 2005;102:18011–18016. doi: 10.1073/pnas.0509001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47:699–713. doi: 10.2337/diabetes.47.5.699. [DOI] [PubMed] [Google Scholar]
- 10.Kim JY, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H, et al. Evidence that the diabetes gene encodes the leptin receptor: Identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 12.Orci L, Unger RH. Functional subdivision of islets of Langerhans and possible role of D cells. Lancet. 1975;2:1243–1244. doi: 10.1016/s0140-6736(75)92078-4. [DOI] [PubMed] [Google Scholar]
- 13.Higa M, et al. Troglitazone prevents mitochondrial alterations, beta cell destruction, and diabetes in obese prediabetic rats. Proc Natl Acad Sci USA. 1999;96:11513–11518. doi: 10.1073/pnas.96.20.11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou YT, et al. Lipotoxic heart disease in obese rats: Implications for human obesity. Proc Natl Acad Sci USA. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Unger RH, Orci L. Diseases of liporegulation: New perspective on obesity and related disorders. FASEB J. 2001;15:312–321. doi: 10.1096/fj.00-0590. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Kim KA, Kim JH, Sul HS. Pref-1, a preadipocyte secreted factor that inhibits adipogenesis. J Nutr. 2006;136:2953–2956. doi: 10.1093/jn/136.12.2953. [DOI] [PubMed] [Google Scholar]
- 17.Wu Z, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 18.Neel JV. Diabetes mellitus: A“thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. 1962;14:353–362. [PMC free article] [PubMed] [Google Scholar]
- 19.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohneda M, Inman LR, Unger RH. Caloric restriction in obese pre-diabetic rats prevents beta-cell depletion, loss of beta-cell GLUT 2 and glucose incompetence. Diabetologia. 1995;38:173–179. doi: 10.1007/BF00400091. [DOI] [PubMed] [Google Scholar]
- 21.Knowler WC, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiedorek FT, Jr, Kay ES. Mapping of PCR-based markers for mouse chromosome 4 on a backcross penetrant for the misty (m) mutation. Mamm Genome. 1994;5:479–485. doi: 10.1007/BF00369316. [DOI] [PubMed] [Google Scholar]
- 23.Danno H, Jincho Y, Budiyanto S, Furukawa Y, Kimura S. A simple enzymatic quantitative analysis of triglycerides in tissues. J Nutr Sci Vitaminol (Tokyo) 1992;38:517–521. doi: 10.3177/jnsv.38.517. [DOI] [PubMed] [Google Scholar]
- 24.Orci L, et al. Hypertrophy and hyperplasia of somatostatin-containing D-cells in diabetes. Proc Natl Acad Sci USA. 1976;73:1338–1342. doi: 10.1073/pnas.73.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weibel ER. Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol. 1969;26:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]
- 26.Kedzierski RM, et al. Cardiomyocyte-specific endothelin A receptor knockout mice have normal cardiac function and an unaltered hypertrophic response to angiotensin II and isoproterenol. Mol Cell Biol. 2003;23:8226–8232. doi: 10.1128/MCB.23.22.8226-8232.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.