Abstract
Considerable research has been directed toward identifying the mechanisms involved in biosilicification to understand and possibly mimic the process for the production of superior silica-based materials while simultaneously minimizing pollution and energy costs. Molecules isolated from diatoms and, most recently sponges, thought to be key to this process contain polyamines with a propylamine backbone and variable levels of methylation. In a chemical approach to understanding the role of amine (especially propylamine) structures in silicification we have explored three key structural features: (i) the degree of polymerization, (ii) the level of amine methylation, and (iii) the size of the amine chain spacers. In this article, we show that there are two factors critical to their function: the ability of the amines to produce microemulsions and the presence of charged and uncharged amine groups within a molecule, with the latter feature helping to catalyze silicic acid condensation by a proton donor/acceptor mechanism. The understanding of amine–silicate interactions obtained from this study has enabled the controlled preparation of hollow and nonporous siliceous materials under mild conditions (circumneutral pH, room temperature, and in all aqueous systems) possibly compatible with the conditions used by biosystems. The “rules” identified from our study were further used predictively to modulate the activity of a given amine. We believe that the outcomes of the present contribution will form the basis for an approach to controlling the growth of inorganic materials by using tailor-made organic molecules.
Keywords: bioinspired materials, biosilica, hollow particles
The commercialization of siliceous materials has generated a multibillion pound industry with the value of precipitated silica products alone estimated to have reached 1.4 billion pounds by the year 2006 (1). This value largely depends on the ability to produce silica for specific applications through control of surface chemistry and morphology during the condensation and aggregation process. Industrial processes involved in the production of high-value siliceous materials typically involve harsh conditions of high temperature and acidity or basicity, environmentally damaging waste streams, and often toxic silicate precursors. These processes also generally exert poor chemical and morphological control over the materials produced. In comparison, natural silica production in organisms such as sponges, diatoms, and radiolaria occurs under biologically benign conditions and produces silica of exquisite form and function, all from a monosilicic acid source of only a few parts per million in concentration (2). Considerable research has been directed toward identifying the mechanisms involved in the biosilicification process to understand and possibly mimic the process to allow us to produce superior materials without the attendant pollution and high-energy usage (3, 4).
Studies of the processes associated with the formation of siliceous diatom shells (thecae) have identified two main types of organic components intimately associated with the shell matrix that have been isolated by dissolution of the silica with buffered hydrofluoric acid. One is a group of (poly)peptides with propylamino-functionalized lysine side chains named silaffins (5–8). The other major organic component is a series of polyamines having propylamine backbones of varying length and levels of methylation on the amine nitrogens giving primary to quaternary amine species (9, 10). In vitro, these polyamines have been shown to rapidly precipitate silica spheres of several hundreds of nanometers in diameter from hydrolyzed tetramethoxysilane solutions. The biosynthesis of these polyamines is thought to be an extension of the existing spermidine/spermine route where ornithine is the primary precursor, a view that has been reinforced by the observation that the addition of an ornithine decarboxylase inhibitor in a culture of the diatom Thalassiosira pseudonana significantly impairs the formation of siliceous valves in this species (11). It is of particular note that polyamines with a propylamine backbone have also been identified from two different sponge species (12) where differences in chain length, presence of a butyl separated amine (present for diatom polyamines reported to date), and degree of methylation have been noted (10, 13). These recent findings suggest that the use of polyamines in the formation of controlled silica structures may be more widespread than previously thought.
Previous work by our group and others has shown that short alkylamines other than propylamines can also induce rapid precipitation of silica spheres from silicic acid systems, a property that appears to be related to the number of amine units present (14, 15). The use of highly methylated propylamines by diatoms or nonmethylated propylamines by sponges could therefore be a serendipitous use of an already existing biosynthetic precursor route, or perhaps there is something specific about the chemical nature of the propylamines that makes them ideal for use in the building of structures in these species. Many experimental studies have attempted to identify the effects of short amines on silicification but these studies have generally focused on a single structural characteristic of the amines in isolation, such as chain length or degree of polymerization (16–23). Here, to more fully assess the role of amine structure in silicification, we have looked at three key structural features: (i) the degree of polymerization, (ii) the level of amine methylation, and (iii) the length of the amine chain spacers. For degree of polymerization we used a commercially available homologous series of ethyleneamines (Fig. 1a) and synthesized a homologous series of propylamines (Fig. 1b). For amine chain spacers we also obtained two naturally occurring polyamines, spermidine and spermine (Fig. 1c), and for methylation level studies we acquired a set of N,N′-(bis-3-aminopropyl)-1,3-diaminopropanes (Fig. 1d) with levels of methylation from 0 to 4. Their effects on a model silica-precipitating system, using dipotassium Tris(1,2-benzenediolato-O,O′)silicate as the monosilicic acid precursor (19, 24), were explored in terms of condensation kinetics and morphological control, and the role of their key variable structural characteristics was assessed.
Fig. 1.
Polyamines used in this study. (a) Ethyleneamines. (b) Propylamines. (c) Natural amines. (d) Different levels on methylation on C3N3.
Results and Discussion
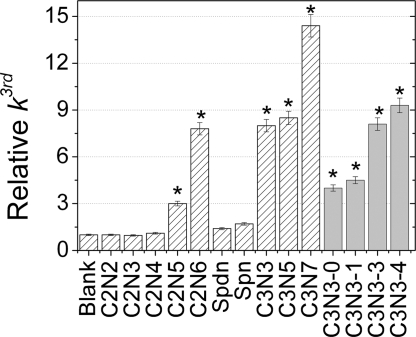
The condensation of monosilicic acid from supersaturated solutions at circumneutral pH progresses by distinct stages: dimerization, trimerization, oligomerization to finally form primary silica particles of a few nanometers in diameter, and slow aggregation to form either a continuous gel or a floc (25). The molybdenum blue method was used to monitor early condensation kinetics (19, 24). This method is sensitive to mono- and dimeric silicic acid and, as a consequence, the trimerization stage is observed as an apparent third-order kinetic region because of the loss of mono- and dimeric silicic acid species from solution. Where this process dominates, a linear relationship between [Si(OH)4]2− and condensation time exists. Here, we specifically present results from this kinetic region to compare the catalytic effects of amine-containing additives in the chosen reaction system and identify additives that generate a greater than threefold increase in the third-order rate constant as being kinetically active.
No significant rate enhancement was observed for the naturally occurring polyamines (SPDN, SPN) or the shorter ethyleneamines (C2N2–4). However, all of the propylamines (C3N3, C3N5, C3N7) and the longer-chain ethyleneamines (C2N5 and C2N6) were found to significantly influence condensation rates with a relative rate enhancement of 14 times the blank being recorded for C3N7, which is the maximum reported to date (Fig. 2). Increasing the methylation levels in the C3N3 species tended to increase the third-order rate constant in line with the degree of methylation, the greater methylation causing more pronounced rate increases (Fig. 2).
Fig. 2.
The effect of the presence of polyamines and the degrees of methylation of C3N3 species at pH 6.8 on the relative k3rd for the silicifying system. All rate constants are relative to the blank at pH 6.8. Kinetically active species are marked with *. Data for ethyleneamines, spermidine, and spermine are replotted from figure S5 of ref. 14, with permission from the Royal Society of Chemistry.
The materials prepared in the presence of the ethyleneamines showed a general tendency to aggregate into clumps with increasing polymerization level but still produced materials that appeared to be mesoporous (see gas adsorption data described below) with the exception of the longest, C2N6, which, like the propylamines, produced nonporous silica spheres (14) (Fig. 3). The extent of methylation had little effect on sphere size with all C3N3 species producing spheres of ≈200–300 nm (Fig. 3). Transmission electron microscopy (TEM) analysis of the silica produced in the presence of the kinetically active amines provided evidence for the formation of hollow silica particles (Fig. 3). The hollow particles were 200–300 nm in size irrespective of the amines used with a central void of ≈50–100 nm. Silicification performed in the presence of the least methylated propylamine precipitated silica spheres that appeared to be coated with porous small-particle silica (Fig. 3c). Syntheses of similar hollow silica spheres have been reported in the literature but these have typically been formed by templating of condensed silica particles on to preformed spheres (26–29) or condensed in situ onto polymer droplets stabilized by surfactants (30–35) under strongly acidic or strongly basic conditions. Additionally, emulsions of either oil in water, in which the precursor acts as the oil droplets (36), or water in oil, in which aqueous hydrolyzed tetra ethoxy silane solutions act as the dispersed phase, have been used to generate hollow spheres (37). Results presented here provide evidence of hollow silica particle formation at circumneutral pH and room temperature and in aqueous media using small additives. It is worth noting that a mixture of solid and hollow particles was observed for all of the samples, with the ratio of hollow-to-solid particles depending on the number of amines per molecule and the amine-to-amine separation. For example, in the case of propylamine additives, because the number of amines per molecule increased from 3 to 7, the amount of hollow particles increased from 0% to 47% (Fig. 3k). Similarly, when the number of hollow particles produced in the presence of C2N6 (20%) was compared with those formed in the presence of C3N5 or C3N7 (33% and 47%, respectively), propylamines were found to be more efficient in generating hollow particles (see below for discussion).
Fig. 3.
Transmission electron micrographs of post-heat treatment (650°C) silica produced in the presence of kinetically active polyamine species at pH 6.8. (a and b) Blank. (c) C3N3–0. (d) C3N3–1. (e) C3N3–3. (f) C3N3–4. (g) C3N5. (h) C2N6. (i and j) C3N7. (Scale bars: 500 nm, except 100 nm for b and j.) (k) The percentage of hollow particles produced in the presence of selected amines.
The kinetically active species were also found to exert structural control on the isolated silicas as determined by gas adsorption/desorption analysis. The typical high surface area mesoporous material observed for materials prepared in the presence of nonactive species was replaced by nonporous materials formed in the presence of kinetically active amines (Fig. 4 a and b) (14). Of the kinetically active species only the least methylated C3N3-0 showed any evidence of mesoporosity for the material produced (Fig. 4b), which could be because of the presence of the small silica particulate coating present on the 200- to 300-nm particles as shown in Fig. 3c. On heat treatment, porosity generated by the removal of entrained organic material from the pores provided evidence for singularly microporous silicas with adsorption occurring only at low partial pressures (Fig. 4 b and c). The absence of mesopores is indicative of a structural change in the silica produced that correlates with rate enhancement. Silica formed by the model blank silicification system is a result of a condensation pathway that generates primary particles of 2–4 nm in size, with aggregation of these primary particles producing interparticle voids that are observed as mesopores. The absence of such voids in the materials formed where the early condensation stages showed enhanced rates suggests that the condensation and assembly of the material occurs as a single stage with a continuous silica phase being produced. This would be the case where the silica forms in the polyamine microemulsion droplets as has been suggested to occur for biosilica formation in diatoms where polyamines are present (38).
Fig. 4.
Porosity and surface area of silica condensed in the presence of polyamines. (a) Surface area. (The data for the C2 series are replotted from ref. 14, with permission from the Royal Society of Chemistry.) (b) Surface area of silica pre- and post-heat treatment. (c) A comparison of pore size distribution between a typical blank mesoporous material with that obtained from nonmesoporous silica produced in the presence of C3N7, both before and after heat treatment.
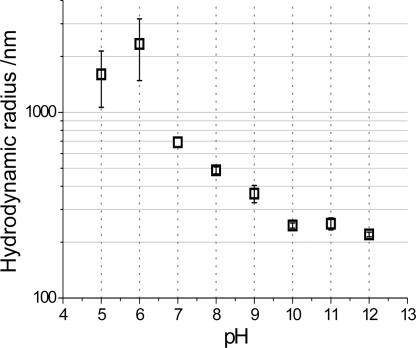
The possibility that the propylamines studied herein form microemulsions was investigated by photon correlation spectroscopy of C3N7 in aqueous media in the absence of silicon. Under basic conditions (pH > 7), the formation of stable droplets was observed (Fig. 5). On decreasing the pH of the system to ≤7, the errors associated with the measurements (which represent the polydispersity and the stability of the droplets) increase, and the droplets become more tenuous and eventually dissipate. This observation provides a rationale for the mechanistic change manifested as kinetic and morphological changes for the siliceous materials formed as the pH is increased from acidic to circumneutral. A similar trend was observed for the other kinetically active amines [supporting information (SI) Fig. S1]. The stability of the amine droplets under consideration reduced as the number of amines per molecule decreased and/or the amine spacing decreased. For example, at ∼pH 7.5, the droplets of C3N7 were moderately stable (error, 55%), whereas the droplets of C3N3 were highly unstable (error, 100%). Similarly, when the stability of amine droplets was compared between C3N7 and C2N6 reaction systems at pH 8, the errors were 22% and 93%, respectively. It is interesting to note that the amine droplet stability in solution follows a trend that is similar to the percentage of hollow particles generated (Fig. 3k), i.e., both phenomena depend on the number of amines per molecule and on the amine–amine separation. This could mean that the stable amine emulsions produce hollow particles, whereas the amines that remain free in solution generate solid particles.
Fig. 5.
Microemulsion droplet size determined by dynamic light scattering on solutions of C3N7 in the absence of silicon. Solutions were 30 mM with respect to nitrogen, with ionic strength adjusted to 60 mM by the addition of potassium chloride to be comparable to that in the model condensing system.
Clearly, the ability of the polyamines to produce microemulsions is critical to their function, but also crucial is the presence of charged and uncharged amine groups that can then help catalyze the condensation of the silicic acid species by a proton donor/acceptor mechanism (Scheme 1) (14, 39). The microemulsions supply a localized water-reduced environment, thus providing the forward-driving force for the proton donor/acceptor condensation mechanism. The presence of charged groups would generally preclude the formation of sufficient hydrophobic domains to allow the entropic formation of microdroplets. However, the amine proximity in these polyamines results in resistance to charging of neighboring amine sites, a condition that is enhanced on increasing the level of polymerization with a concomitant increase in hydrophobicity.
Scheme. 1.
A schematic representation of the microemulsion formation from some amines leading to hollow silica particles.
To correlate the above-mentioned effects of the amines with both the extent of charge and hydrophobic nature of the amines, the amines were analyzed by using the SPARC online pKa tool (http://ibmlc2.chem.uga.edu/sparc). SPARC uses relatively simple algorithms based on chemical structure theory to estimate ionization pKa values. Treating any uncharged amines as hydrophobic in nature it was found that the overall hydrophobic content of the amines used in this study increased with increasing degree of polymerization (Fig. 6).
Fig. 6.
Plot of molecular charge vs. hydrophobic nature of amines at pH 7. Kinetically active species are shown encased in balloons.
Increasing polymerization levels was found to increase resistance to charging because of localized charge density effects, and for a given polymerization level, the order of charging at a given pH was ethyleneamines < propylamines < natural C3/4 amines. The significance of this effect can be demonstrated by the observation that at pH 7 C2N2 contains amines that are statistically 75% charged, whereas in C2N6 these amines are only 45% charged. Also of significance is that, although the propylamines were found to be easier to protonate because of the greater separation of the potential charge centers and therefore carry more charge per molecule, they still exhibited more hydrophobic character than their ethyleneamine counterparts for the same polymerization levels (Fig. 6) and, therefore, have a greater ability to form stable droplets.
By increasing the methylation levels on the amines, despite increasing the inductive effect and producing increased basicity in the gas phase or in apolar solvents, the state is different in protic (in this case H2O) and aprotic polar solvents where a reduction in the solvation energy of the conjugate acid results in lower stabilization levels and a consequent decrease in basicity (41). Hence, charge levels will be lower at any given pH when tertiary amines are compared with secondary ones. This, in conjunction with the increased alkyl functionality, results in enhanced hydrophobicity. Hence, the stabilization of droplet formation by increased methylation of the C3N3 species accounts for the increased condensation rates observed, and the residual mesoporous nature of the silica condensed in the presence of the least methylated of these indicates the limit of the observed mechanistic change. That no mechanistic change was observed for the two naturally occurring polyamines used, even though they contain more carbon chain with respect to amine groups, is a function of the increased ease of protonation of the nitrogen atoms because of the greater amine separation and a consequent reduction in their hydrophobic character (Fig. 6).
Having established that the balance between hydrophobicity and charge of a given amine is crucial for their observed control over reaction rates and product morphologies, one could ask: Is it possible to predict the activity of a given amine under a set of conditions? Could one tailor an amine architecture for a desired activity? We sought to answer these questions as follows. For a given amine, the protonation level will depend on the pH; thus, by varying the system's pH, the hydrophobicity-to-charge balance could be shifted, thereby modulating the activity of that amine. This hypothesis was explored first by performing calculations using SPARC at a range of pH values from 5.6 to 7 for propylamines and ethyleneamines (Fig. 7a). For a given amine, the ratio of the hydrophobic-to-charged nature decreases as the pH is reduced and, therefore, the ability of the amine to enhance kinetics and to produce hollow particles should diminish. This hypothesis was validated by collecting third-order rate constant data for silicification experiments conducted in the presence of amines under a range of pH values (Fig. 7b). The rate enhancement was found to be pH-dependent with a shared onset pH >6 for all of the C3 species added and for C2N6. A step change in the rate constant from pH 6 to pH 6.5 is evident and is marked by an arrow in Fig. 7b. The absence of a significant kinetic effect below pH 6 explains the observations of Behrens et al. (42) who studied a series of polydiaminoethanes and polydiaminopropanes with polymerization levels of 2–9 and 2–13, respectively and found no kinetic effect exerted at pH 5.5. The change in the balance between the hydrophobic and charged nature of the C2 and C3 series from pH 6 to pH 6.6 corresponding to this step change is marked in Fig. 7a for C2N6 and C3N7. In other words, by regulating either reaction conditions or amine architecture, we were able to control the rates of silica formation. The control over the morphology of the silicas generated in the presence of the amines was also achieved by varying the pH of the system. The nature of the material changed from a continuous gel with aggregate sizes ≈50–70 nm at pH 5.7, similar to blank samples, to distinct spherical particles of sizes ≈200–300 nm at pH 7 (Fig. 8). It is interesting to note that the morphology and kinetic effects were observed to have approximately the same onset pH (between 6 and 6.5), suggesting a shared mechanistic cause. Again, we have successfully shown that by tailoring reaction conditions or amine architecture, we were able to control silica morphology.
Fig. 7.
Structure–property relation of amines over the pH range 5.6–7. (a) The variation in the charged and hydrophobic nature of propylamines and ethyleneamines as a function of pH. (b) Relative k3rd for propylamines and ethyleneamines as a function of pH. The arrows show the change in the charged and hydrophobic nature of C2N6 and C3N7 from pH 6 to pH 6.6 (a) that is associated with the change in kinetic activity (b).
Fig. 8.
Scanning electron micrographs of silica produced in the presence of blank at pH 7 (a) and C3N7 at a condensation pH of 5.7 (b), 6.2 (c), 6.5 (d), and 7 (e). (Scale bars: 5 μm.)
The key to the ability of polyamines to enhance condensation rates of monosilicic acid solutions is in their ability to form microemulsions while carrying a positive charge, thereby providing a water-free microenvironment where the removal of water from condensation reactions provides the driving force for the proton donor/acceptor condensation mechanism. Increased levels of amine polymerization enhance this ability by both limiting the levels of charging of the amines providing the alternate charged and uncharged species required and by increasing the hydrophobic nature of the molecules. Increasing methylation levels on the molecules further limits the basicity of the amines while providing an increased hydrophobic nature. The effects observed with relatively small chain length C3-containing compounds is also observed for polyamines containing C2 spacers, but only at greater chain lengths where the conditions of charge and hydrophobicity are optimized for microemulsion formation. Amine separation provides a mechanism for controlling charge levels through increasing localized charge density, causing resistance to protonation as the interamine distance is reduced. At separation lengths longer than C3, the amines are unable to provide stable conditions for droplet formation because of the ease of charging of the amine functional groups in the molecule.
From this study it is apparent that in terms of the ability to enhance silica condensation rates and direct structural control, the propylamines have an optimized structure. It is clear, however, that varying the degree of polymerization and extent of methylation can modify reactivity. The consequent effects on both rate of production and material morphology are used to good effect by both diatoms and sponges to generate impressively controlled biosilica structures (2, 43).
Much can be done to enhance the ability of other polyamines to rapidly condense silica for commercial applications, for example, by providing more extensive amine alkylation and increasing the polymerization level. Our findings also open up the possibility to develop other active systems for silicification based on the same principles of water-free microenvironments (oil in water emulsions) or reverse water in oil systems where the hydrophobic phases contain proton donor/acceptor species. Furthermore, the outcomes of this work can be used to tailor the ability of other organic “additives” to modulate precipitation and dissolution of inorganic materials for commercial applications.
Materials and Methods
The homologous propylamines were synthesized and characterized in accordance with published procedures (16). Homologous ethyleneamines, spermidine (SPN), spermine (SPDN), and N,N′-(di-3-aminopropyl)-1,3-diaminopropanes (C3N3-0, C3N3-1, and C3N3-4) were purchased from Sigma Aldrich and used without further treatment. Dipotassium Tris(1,2-benzenediolato-O,O′)silicate was purchased from Sigma Aldrich and recrystallized from methanol before use. Reagents for the molybdenum blue assay [ammonium molybdate·4H2O, oxalic acid, 4-methylaminophenol sulfate, sodium sulfite, hydrochloric acid (35%), and sulfuric acid (98%)] were purchased from Sigma Aldrich, Fisher, or Acros Chemicals and used without further treatment.
We prepared 30 mM solutions of monosilicic acid by the dissociation of dipotassium Tris(1,2-benzenediolato-O,O′)silicate with 2 M hydrochloric acid. The polyamines were added at a maintained Si/N ratio of 1:1 to compare the effects of the amine functionality. The level of acid addition was predetermined to take into account the basicity of the polyamines added to give a final pH of 6.8 ± 0.05, unless otherwise stated. The rate of condensation was determined by the well established molybdenum blue spectrometric assay method (19, 24, 25). Photon correlation spectroscopy (PCS) of amine microemulsions was carried out by using a Coulter N4 plus photon correlation spectrometer with a He–Ne (632.8 nm) laser supply. Polyamine solutions were prepared at 30 mM relative to amine content in filtered deionized water and pH adjustments made with dilute hydrochloric acid. Ionic strength was maintained throughout at 0.06 molal by the use of potassium chloride. All PCS measurements were carried out in a 1-cm cuvette at an angle of 90° and at 293 K. Measurements obtained were averages of the data collected over intervals of 5 min.
The siliceous products were isolated by centrifugation, water washing (three times), and lyophilizaton. For scanning electron microscopy (SEM; JEOL JSM-840A) analysis the lyophilized samples were placed on a double-sided sticky carbon tape mounted on aluminum sample holders and then sputter-coated with gold. Samples for transmission electron microscopy (TEM; JEOL 2010) were prepared by dipping grids in ethanolic suspensions of lyophilized powders followed by drying under air. Nitrogen gas adsorption/desorption (Quantasorb NOVA 3200e) used Brunauer, Emmett, Teller (BET) (44) analysis to determine the surface areas and Barrett, Joyner, Halenda (BJH) (45) analysis for the porosity measurements. Low partial-pressure adsorption is assumed to be caused by microporosity, but because of limitations of the nitrogen gas adsorption technique, actual pore dimensions could not be determined (40). Samples were heat-treated to remove organic content. The SPARC online pKa tool was used to predict theoretically the protonation behavior of amine molecules under consideration, which was compared with experimentally derived pKa data for the amines used. Agreement between the SPARC theoretical data and the experimentally derived potentiometric data was 2% for all systems studied (data not shown).
See SI Text for further details.
Supplementary Material
Acknowledgments.
This work was supported by the Air Force Office of Scientific Research (C.C.P.), the Royal Society's Joint Project Grants (C.C.P. and V.V.A.), and the Presidium of the Russian Academy of Sciences Project 10.3 (V.V.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.A. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710809105/DCSupplemental.
References
- 1.Notch Consulting Group. World Market for Precipitated Silica. Amherst, MA: Notch Consulting Group; 2006. [Google Scholar]
- 2.Müller WEG, editor. Silicon Biomineralization. Berlin: Springer; 2003. [Google Scholar]
- 3.Perry CC. Silicification: The processes by which organisms capture and mineralize silica. Rev Minerol Geochem. 2003;54:291–327. [Google Scholar]
- 4.Patwardhan SV, Clarson SJ, Perry CC. On the role(s) of additives in bioinspired silicification. Chem Commun. 2005;9:1113–1121. doi: 10.1039/b416926c. [DOI] [PubMed] [Google Scholar]
- 5.Kröger N, Deutzmann R, Sumper M. Polycationic peptides from diatom biosilica that direct silica nanosphere formation. Science. 1999;286:1129–1132. doi: 10.1126/science.286.5442.1129. [DOI] [PubMed] [Google Scholar]
- 6.Kröger N, Deutzmann R, Sumper M. Silica-precipitating peptides from diatoms—The chemical structure of silaffin-1A from Cylindrotheca fusiformis. J Biol Chem. 2001;276:26066–26070. doi: 10.1074/jbc.M102093200. [DOI] [PubMed] [Google Scholar]
- 7.Poulsen N, Sumper M, Kröger N. Biosilica formation in diatoms: Characterization of native silaffin-2 and its role in silica morphogenesis. Proc Natl Acad Sci USA. 2003;100:12075–12080. doi: 10.1073/pnas.2035131100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wenzl S, Deutzmann R, Hett R, Hochmuth E, Sumper M. Quaternary ammonium groups in silica-associated proteins. Angew Chem Int Ed. 2004;43:5933–5936. doi: 10.1002/anie.200461236. [DOI] [PubMed] [Google Scholar]
- 9.Kröger N, Deutzmann R, Bergsdorf C, Sumper M. Species-specific polyamines from diatoms control silica morphology. Proc Natl Acad Sci USA. 2000;97:14133–14138. doi: 10.1073/pnas.260496497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sumper M, Brunner E, Lehmann G. Biomineralization in diatoms: Characterization of novel polyamines associated with silica. FEBS Lett. 2005;579:3765–3769. doi: 10.1016/j.febslet.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Frigeri LG, Radabaugh TR, Haynes PA, Hildebrand M. Identification of proteins from a cell wall fraction of the diatom Thalassiosira pseudonana—Insights into silica structure formation. Mol Cell Proteomics. 2006;5:182–193. doi: 10.1074/mcp.M500174-MCP200. [DOI] [PubMed] [Google Scholar]
- 12.Matsunaga S, Sakai R, Jimbo M, Kamiya H. Long-chain polyamines (LCPAs) from marine sponge: Possible implication in spicule formation. ChemBioChem. 2007;8:1729–1735. doi: 10.1002/cbic.200700305. [DOI] [PubMed] [Google Scholar]
- 13.Sumper M, Lehmann G. Silica pattern formation in diatoms: Species-specific polyamine biosynthesis. ChemBioChem. 2006;7:1419–1427. doi: 10.1002/cbic.200600184. [DOI] [PubMed] [Google Scholar]
- 14.Belton D, Patwardhan SV, Perry CC. Spermine, spermidine and their analogues generate tailored silicas. J Mater Chem. 2005;15:4629–4638. [Google Scholar]
- 15.Noll F, Sumper M, Hampp N. Nanostructure of diatom silica surfaces and of biomimetic analogues. Nano Lett. 2002;2:91–95. [Google Scholar]
- 16.Annenkov VV, Patwardhan SV, Belton D, Danilovtseva EN, Perry CC. Step-by-step full synthesis of propylamines derived from diatom silaffins and their activity in silicification. Chem Commun. 2006:1521–1523. doi: 10.1039/b515967a. [DOI] [PubMed] [Google Scholar]
- 17.Coradin T, Livage J. Effect of some amino acids and peptides on silicic acid polymerization. Colloids Surf B. 2001;21:329–336. doi: 10.1016/s0927-7765(01)00143-6. [DOI] [PubMed] [Google Scholar]
- 18.Coradin T, Durupthy O, Livage J. Interactions of amino-containing peptides with sodium silicate and colloidal silica: A biomimetic approach of silicification. Langmuir. 2002;18:2331–2336. [Google Scholar]
- 19.Belton D, Paine G, Patwardhan SV, Perry CC. Towards an understanding of (bio)silicification: The role of amino acids and lysine oligomers in silicification. J Mater Chem. 2004;14:2231–2241. [Google Scholar]
- 20.Belton D, Patwardhan SV, Perry CC. Putrescine homologues control silica morphogenesis by electrostatic interactions and the hydrophobic effect. Chem Commun. 2005:3475–3477. doi: 10.1039/b504310g. [DOI] [PubMed] [Google Scholar]
- 21.Delak KM, Sahai N. Amine-catalyzed biomimetic hydrolysis and condensation of organosilicate. Chem Mater. 2005;17:3221–3227. [Google Scholar]
- 22.Delak KM, Sahai N. Mechanisms of amine-catalyzed organosilicate hydrolysis at circum-neutral pH. J Phys Chem B. 2006;110:17819–17829. doi: 10.1021/jp062054m. [DOI] [PubMed] [Google Scholar]
- 23.Robinson DB, Rognlien JL, Bauer CA, Simmons BA. Dependence of amine-accelerated silicate condensation on amine structure. J Mater Chem. 2007;17:2113–2119. [Google Scholar]
- 24.Harrison CC, Loton N. Novel routes to designer silicas: Studies of the decomposition of (M+)2 Si(C6H4O2)3·xH2O—Importance of M+ identity of the kinetics of oligomerization and the structural characteristics of the silicas produced. J Chem Soc Faraday Trans. 1995;91:4287–4297. [Google Scholar]
- 25.Iler RK. The Chemistry of Silica. New York: Wiley; 1979. [Google Scholar]
- 26.Caruso F, Caruso RA, Mohwald H. Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating. Science. 1998;282:1111–1114. doi: 10.1126/science.282.5391.1111. [DOI] [PubMed] [Google Scholar]
- 27.Caruso F, Caruso RA, Mohwald H. Production of hollow microspheres from nanostructured composite particles. Chem Mater. 1999;11:3309–3314. [Google Scholar]
- 28.Ding XF, et al. A novel approach to the synthesis of hollow silica nanoparticles. Mater Lett. 2004;58:3618–3621. [Google Scholar]
- 29.Tsai MS, Li MJ. A novel process to prepare a hollow silica sphere via chitosan-polyacrylic acid (CS-PAA) template. J Non-Cryst Solids. 2006;352:2829–2833. [Google Scholar]
- 30.Fowler CE, Khushalani D, Mann S. Facile synthesis of hollow silica microspheres. J Mater Chem. 2001;11:1968–1971. doi: 10.1039/b104879c. [DOI] [PubMed] [Google Scholar]
- 31.van Bommel KJC, Jung JH, Shinkai S. Poly(L-lysine) aggregates as templates for the formation of hollow silica spheres. Adv Mater. 2001;13:1472. [Google Scholar]
- 32.Jan JS, Lee SJ, Carr CS, Shantz DF. Biomimetic synthesis of inorganic nanospheres. Chem Mater. 2005;17:4310–4317. [Google Scholar]
- 33.Zhu YF, et al. Preparation of novel hollow mesoporous silica spheres and their sustained-release property. Nanotechnology. 2005;16:2633–2638. [Google Scholar]
- 34.Bauer CA, Robinson DB, Simmons BA. Silica particle formation in confined environments via bioinspired polyamine catalysis at near-neutral pH. Small. 2007;3:58–62. doi: 10.1002/smll.200600352. [DOI] [PubMed] [Google Scholar]
- 35.Venkatathri N. Synthesis of mesoporous silica nanosphere using different templates. Solid State Commun. 2007;143:493–497. [Google Scholar]
- 36.Wang QB, Yan L, Yan H. Mechanism of a self-templating synthesis of monodispersed hollow silica nanospheres with tunable size and shell thickness. Chem Commun. 2007:2339–2341. doi: 10.1039/b701572k. [DOI] [PubMed] [Google Scholar]
- 37.Yang LM, Wang YJ, Luo GS, Dai YY. A new ‘pH-induced rapid colloid aggregation’ method to prepare micrometer-sized spheres of mesostructured silica in water-in-oil emulsion. Microporous Mesoporous Mater. 2006;94:269–276. [Google Scholar]
- 38.Sumper M. A phase separation model for the nanopatterning of diatom biosilica. Science. 2002;295:2430–2433. doi: 10.1126/science.1070026. [DOI] [PubMed] [Google Scholar]
- 39.Kröger N, Sumper M. In: Biomineralization: From Biology to Biotechnology and Medical Application. Baeuerlein E, editor. Weinheim, Germany: Wiley-VCH; 2000. [Google Scholar]
- 40.Groen JC, Peffer LAA, Perez-Ramírez J. Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater. 2003;60:1–17. [Google Scholar]
- 41.Safi B, Choho K, De Proft F, Geerlings P. Theoretical study of the basicity of alkyl amines in vacuo and in different solvents: A density functional theory approach. Chem Phys Lett. 1999;300:85–92. [Google Scholar]
- 42.Behrens P, Johns M, Menzel H. In: Handbook of Biomineralization: Biomimetic and Bioinspired Chemistry. Beherns P, Baeuerlein E, editors. Weinheim, Germany: Wiley-VCH; 2007. pp. 3–18. [Google Scholar]
- 43.Simpson TL, Volcani BE. In: Silicon and Siliceous Structures in Biologic Systems. Simpson TL, Volcani BE, editors. New York: Springer; 1981. p. 1. [Google Scholar]
- 44.Brunauer S, Emmett PH, Teller E. Adsorption of gases in multimolecular layers. J Am Chem Soc. 1938;60:309–319. [Google Scholar]
- 45.Barrett EP, Joyner LG, Halenda PP. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J Am Chem Soc. 1951;73:373–380. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.